Abstract
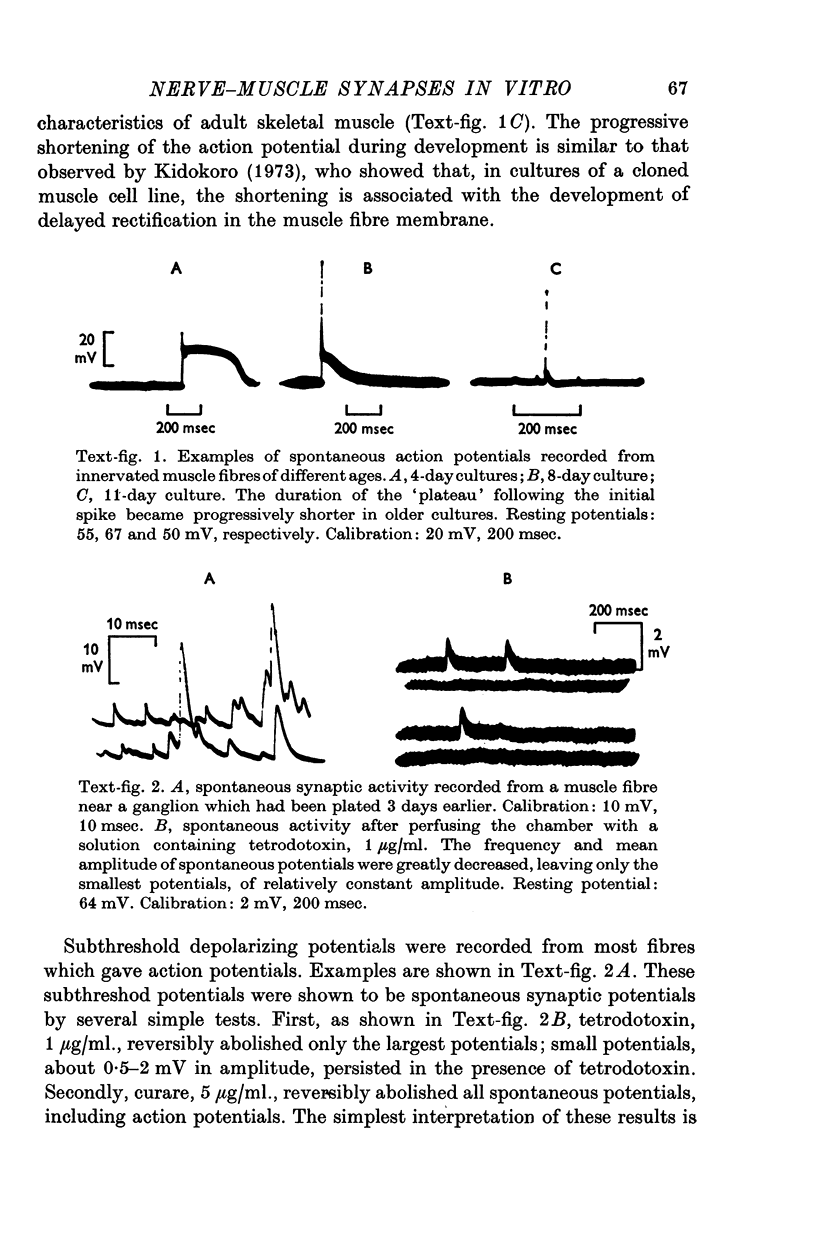
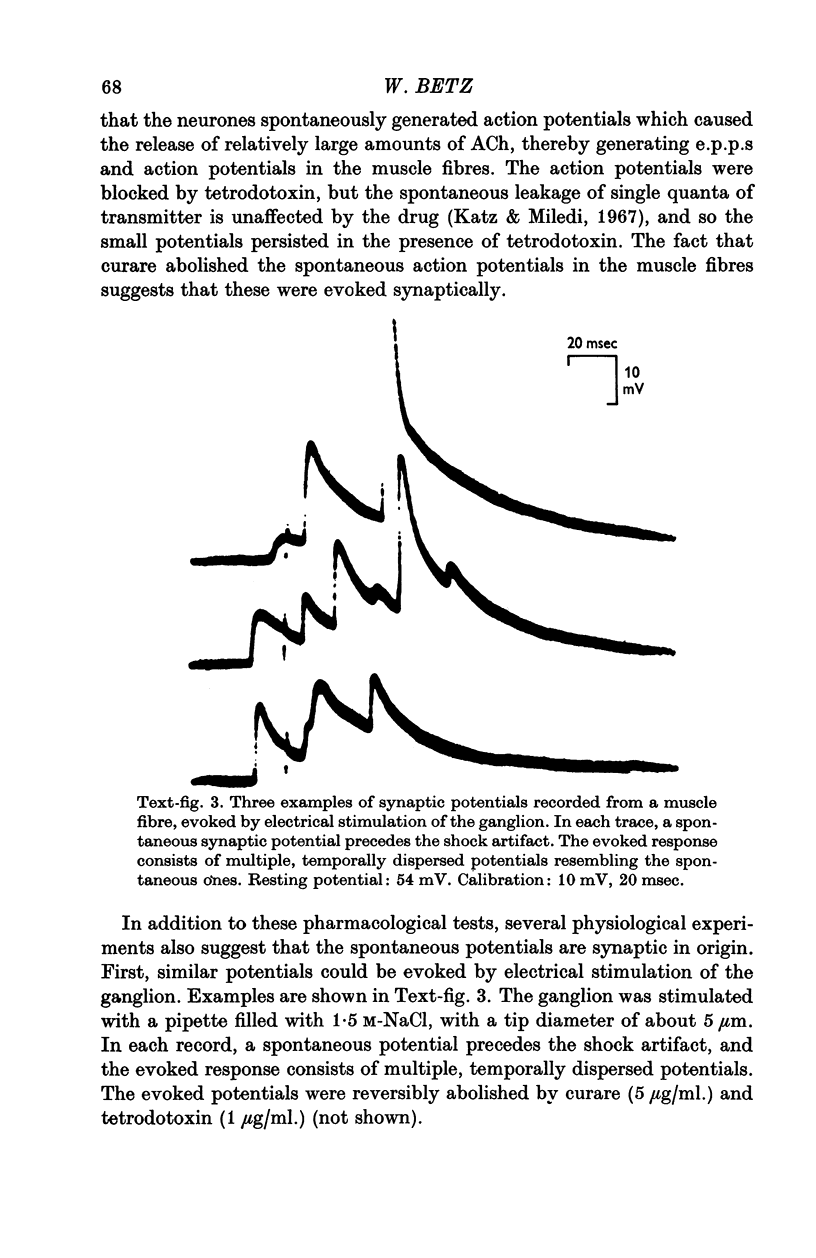
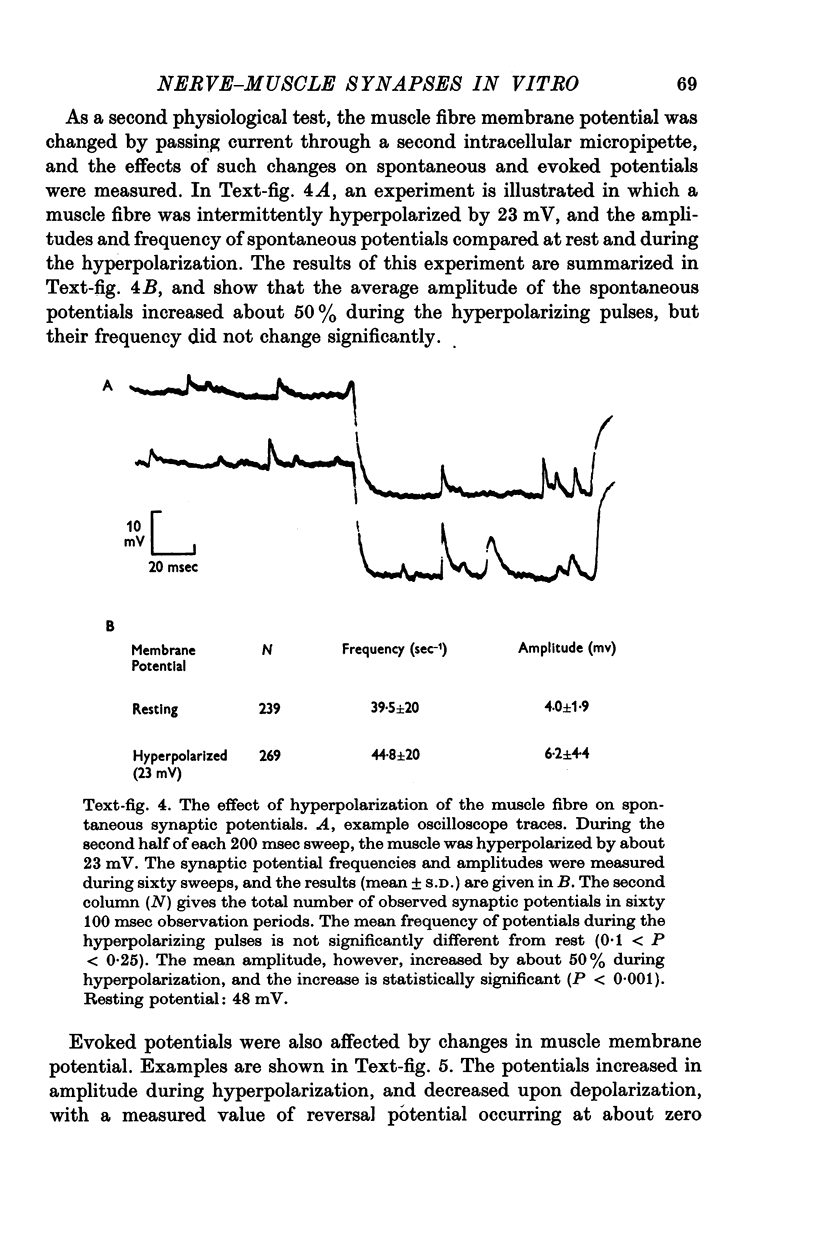
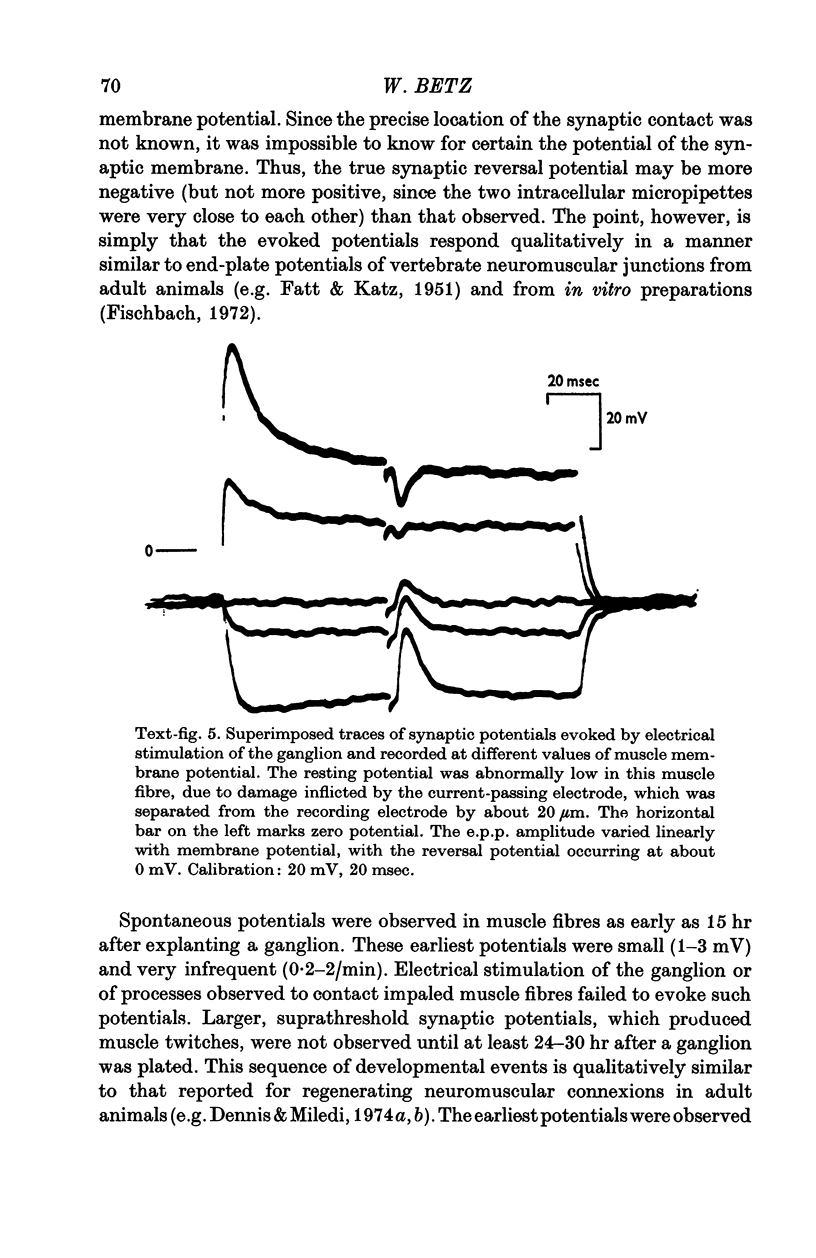
1. Chick embryo ciliary ganglia (explanted) and skeletal muscle (dissociated) were grown together in vitro for up to 3 weeks. Nerve processes sprouted from the ganglia and contacted neighbouring myotubes and striated muscle fibres. 2. Spontaneous action potentials and subthreshold e.p.p.s. were recorded from muscle fibres with intracellular micropipettes. Similar potentials could be evoked by electrical stimulation of the ganglion. The pharmacological effects of curare and tetrodotoxin were identical to those observed at adult vertebrate neuromuscular junctions. 3. The amplitude, but not the frequency, of the spontaneous potentials was affected by changing the muscle fibre membrane potential. The reversal potential of evoked synaptic potentials occurred at a membrane potential of about 0 mV.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantino D., Mugnaini E. Adrenergic innervation of the parasympathetic ciliary ganglion in the chick. Science. 1974 Jul 19;185(4147):279–281. doi: 10.1126/science.185.4147.279. [DOI] [PubMed] [Google Scholar]
- Crain S. M., Alfei L., Peterson E. R. Neuromuscular transmission in cultures of adult human and rodent skeletal muscle after innervation in vitro by fetal rodent spinal cord. J Neurobiol. 1970;1(4):471–489. doi: 10.1002/neu.480010409. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Non-transmitting neuromuscular junctions during an early stage of end-plate reinnervation. J Physiol. 1974 Jun;239(3):553–570. doi: 10.1113/jphysiol.1974.sp010582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehinger B. Adrenergic nerves in the avian eye and ciliary ganglion. Z Zellforsch Mikrosk Anat. 1967;82(4):577–588. doi: 10.1007/BF00337123. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972 Jun;28(2):407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- Hooisma J., Slaaf D. W., Meeter E., Stevens W. F. The innervation of chick striated muscle fibers by the chick ciliary ganglion in tissue culture. Brain Res. 1975 Feb 21;85(1):79–85. doi: 10.1016/0006-8993(75)91009-4. [DOI] [PubMed] [Google Scholar]
- Kano M., Shimada Y. Innervation of skeletal muscle cells differentiated in vitro from chick embryo. Brain Res. 1971 Apr 2;27(2):402–405. doi: 10.1016/0006-8993(71)90271-x. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Development of action potentials in a clonal rat skeletal muscle cell line. Nat New Biol. 1973 Jan 31;241(109):158–159. doi: 10.1038/newbio241158a0. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Selective reinnervation of two cell populations in the adult pigeon ciliary ganglion. J Physiol. 1970 Nov;211(1):203–216. doi: 10.1113/jphysiol.1970.sp009275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synapse formation during embryogenesis on ganglion cells lacking a periphery. J Physiol. 1974 Sep;241(3):715–736. doi: 10.1113/jphysiol.1974.sp010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. QUANTAL COMPONENTS OF THE SYNAPTIC POTENTIAL IN THE CILIARY GANGLION OF THE CHICK. J Physiol. 1964 Dec;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwitt R., Pilar G., Weakly J. N. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971 Jan 22;25(2):317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- Pilar G., Jenden D. J., Campbell B. Distribution of acetylcholine in the normal and denervated pigeon ciliary ganglion. Brain Res. 1973 Jan 30;49(2):245–256. doi: 10.1016/0006-8993(73)90421-6. [DOI] [PubMed] [Google Scholar]
- Pilar G., Vaughan P. C. Electrophysiological investigations of the pigeon iris neuromuscular junctions. Comp Biochem Physiol. 1969 Apr;29(1):51–72. doi: 10.1016/0010-406x(69)91725-3. [DOI] [PubMed] [Google Scholar]
- Robbins N., Yonezawa T. Developing neuromuscular junctions: first signs of chemical transmission during formation in tissue culture. Science. 1971 Apr 23;172(3981):395–398. doi: 10.1126/science.172.3981.395. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Fischman D. A., Moscona A. A. Formation of neuromuscular junctions in embryonic cell cultures. Proc Natl Acad Sci U S A. 1969 Mar;62(3):715–721. doi: 10.1073/pnas.62.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]





