Abstract
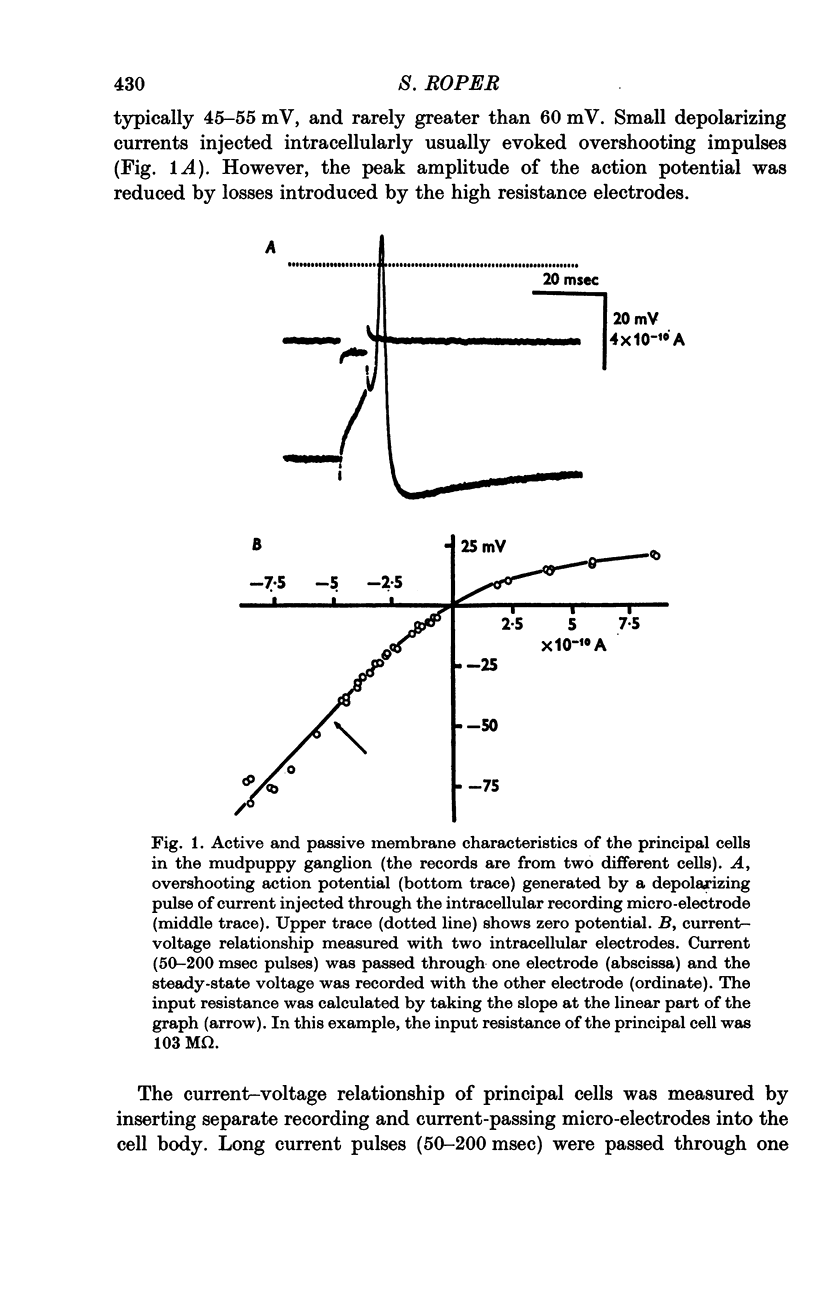
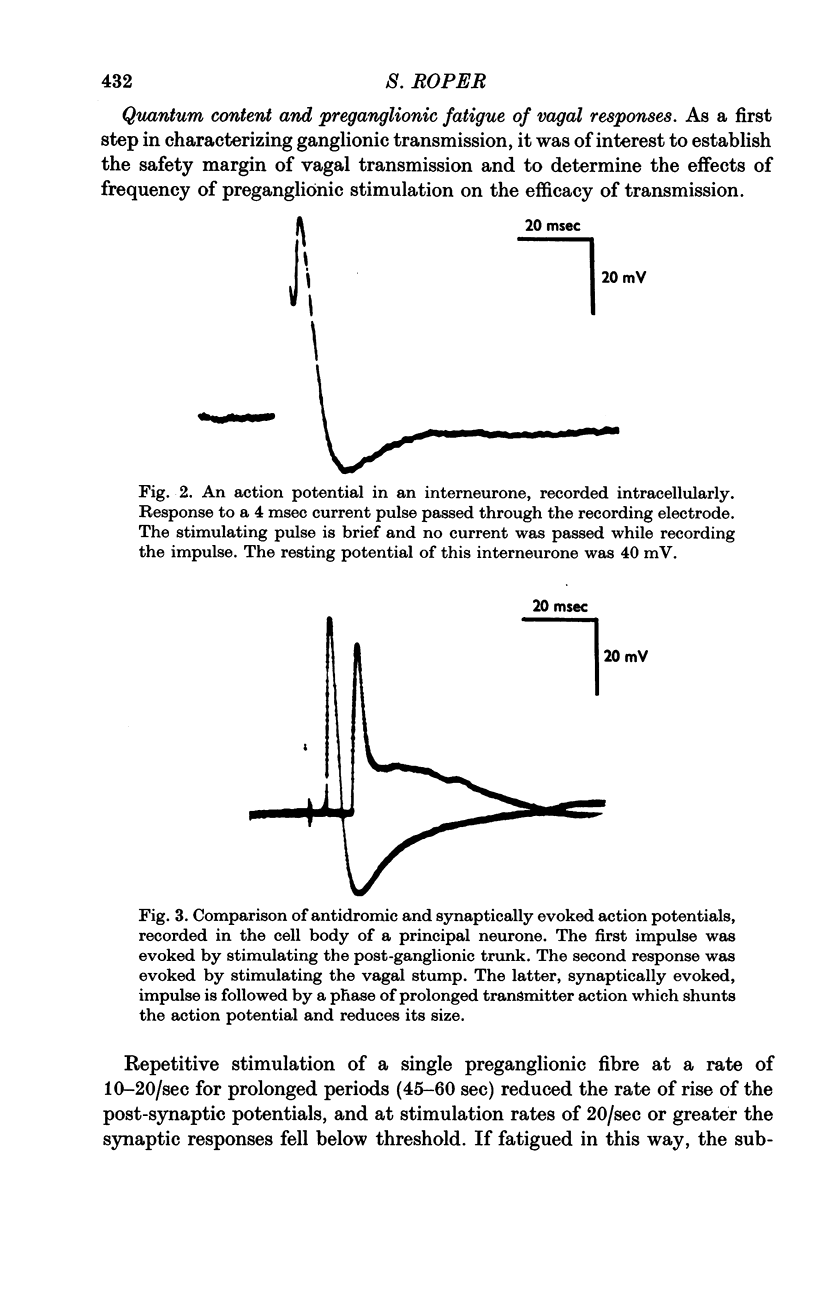
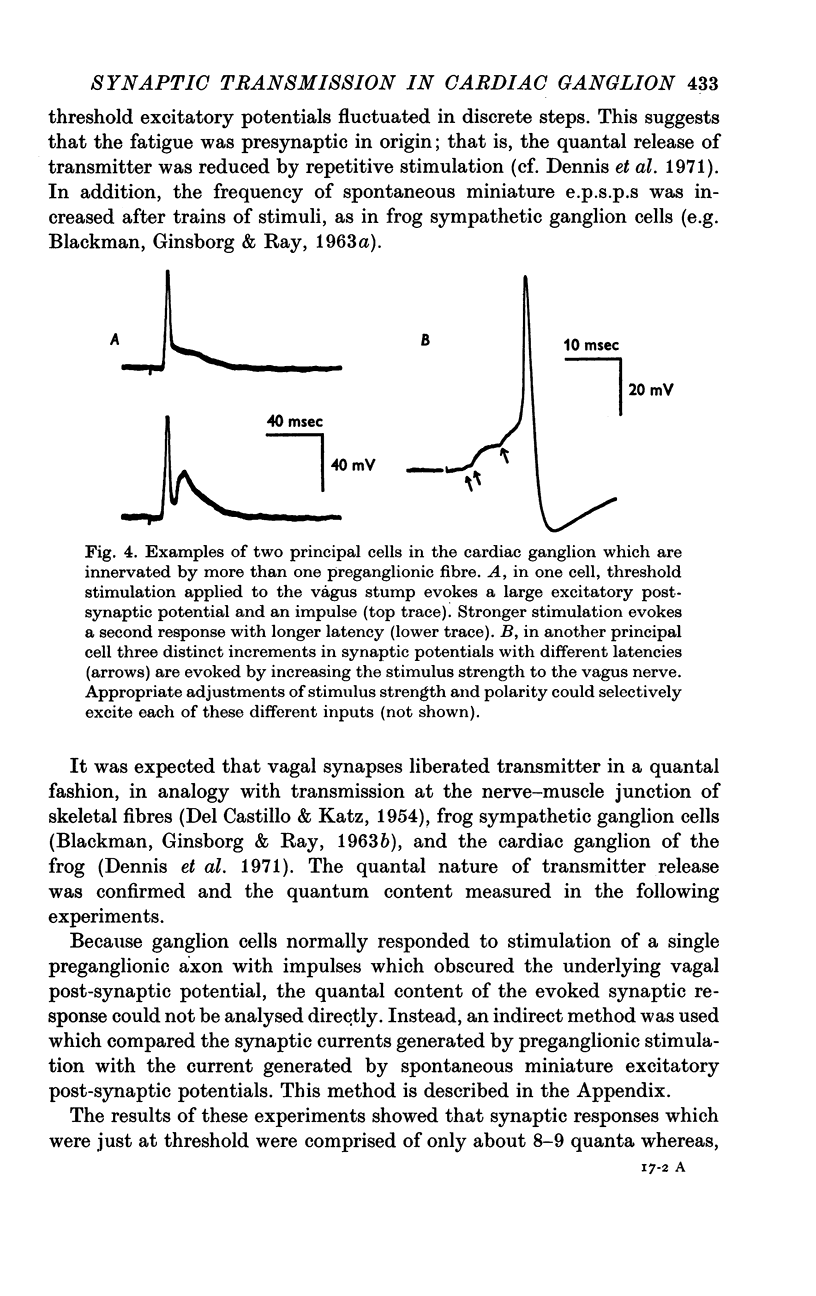
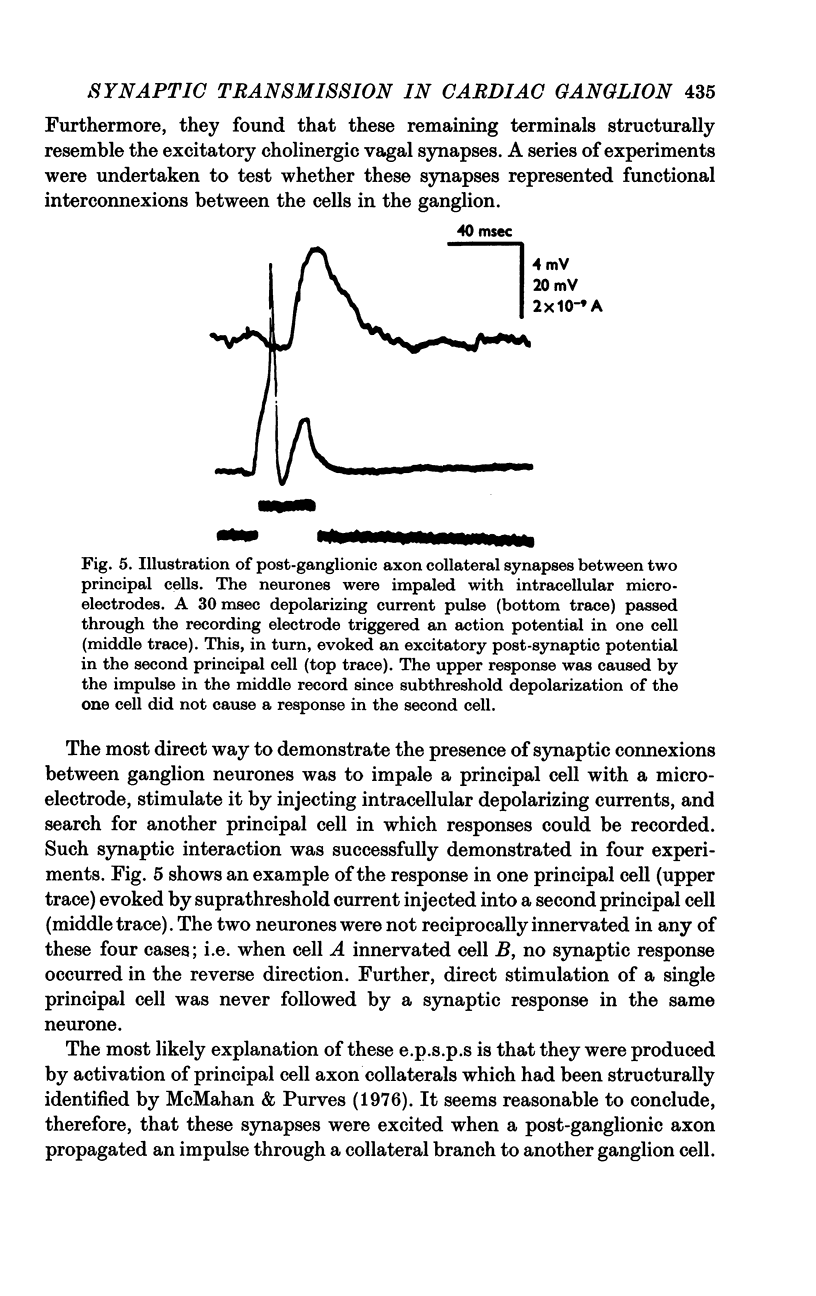
1. The cardiac ganglion of the mudpuppy is situated on a thin sheet of tissue. Two nerve cell types can be distinguished readily in the living preparation - principal cells and smaller interneurones which synapse with the principal cells. The purpose of this study was to investigate synaptic transmission and the functional organization of neuronal connections of ganglion cells with intracellular micro-electrodes. 2. Stimulation of the preganglionic, vagus, nerves evoked a large excitatory response in principal cells. About three quarters of these neurones were innervated by a single vagal axon. The remaining cells received two or more preganglionic nerve fibres. 3. The quantum content of vagal excitatory post-synaptic potentials (e.p.s.p.s) was measured. Normally, the e.p.s.p. was suprathreshold and consisted of about twenty-two quanta, whereas only about nine quanta were required to reach threshold and initiate an action potential. 4. Intracellular stimulation of principal cells evoked e.p.s.p.s in neighbouring principal cells. The responses were blocked by cholinergic antagonists. These potentials were caused by excitation of principal cell axon collateral synapses. 5. Principal cells also formed electrical junctions with each other. These electrical junctions were very weak. Although they transmitted slow potential changes, only a small response was recorded in one cell when an electrically coupled neighbouring cell fired an impulse. The resistance of the electrical junction between principal cells was calculated to be about 5-8 X 10(8) omega. 6. Stable penetrations of interneurones were only rarely achieved, making it difficult to study their functional relationship to principal cells. Action potentials were recorded from interneurones in a few instances. 7. These data demonstrate that parasympathetic ganglion cells in the heart of the mudpuppy receive innervation from more than one source involving both chemical and electrical synapses, and that some of the synapses are intrinsic to the ganglion.
Full text
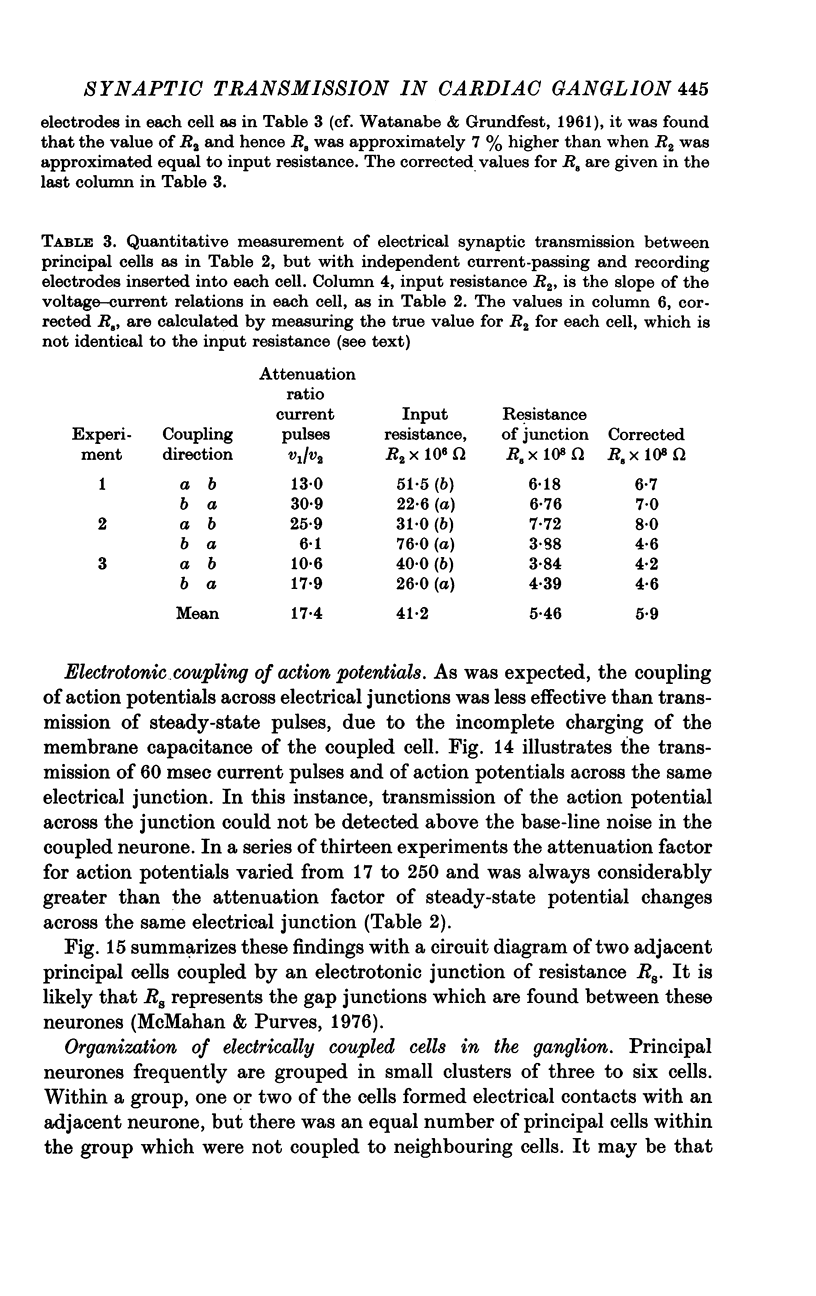
PDF




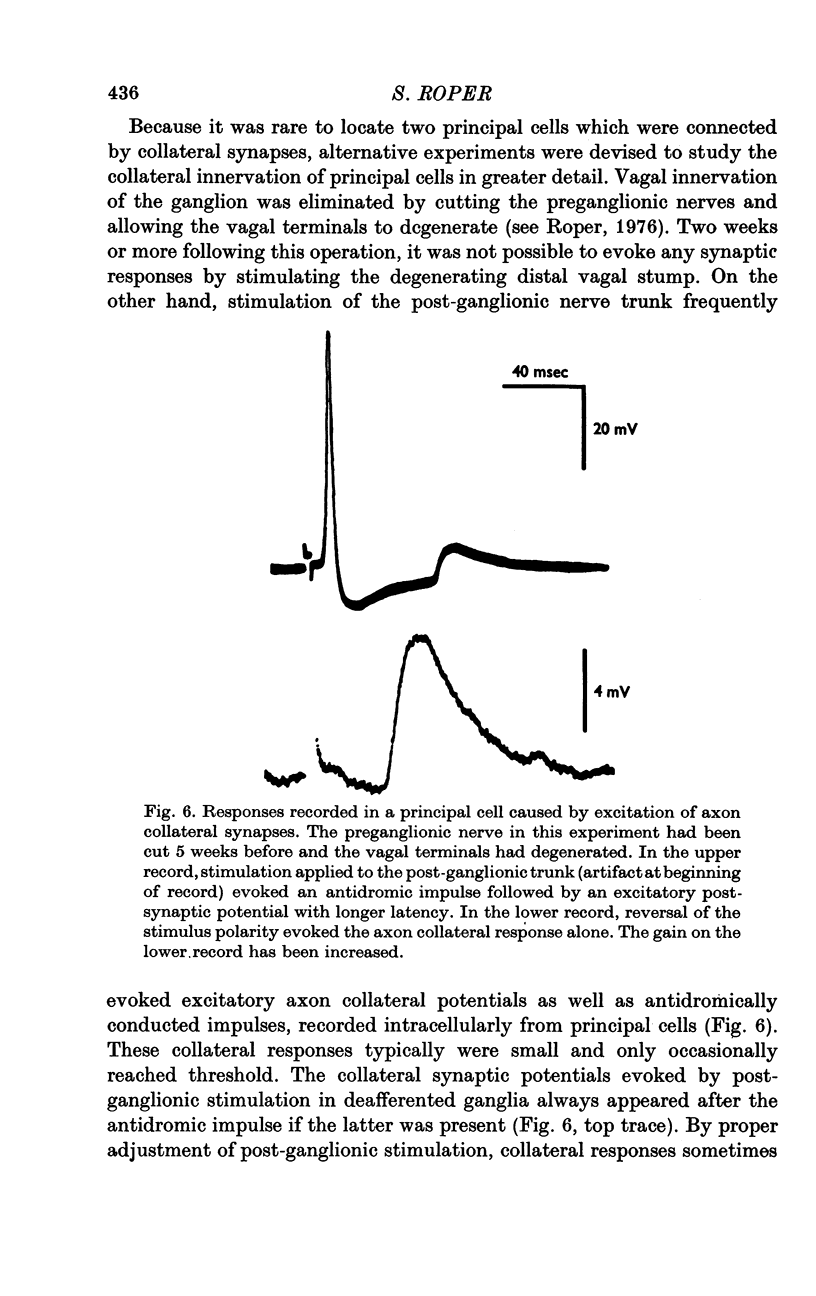

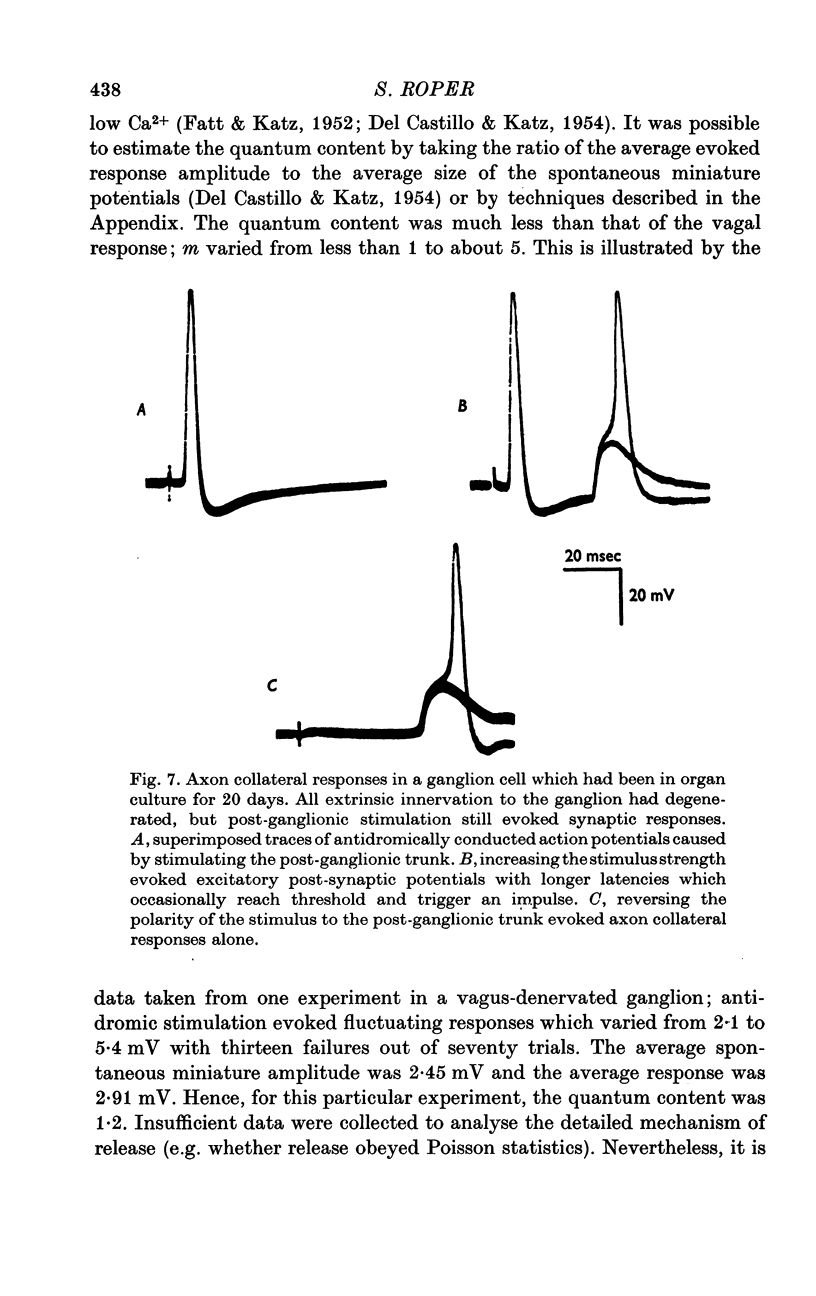
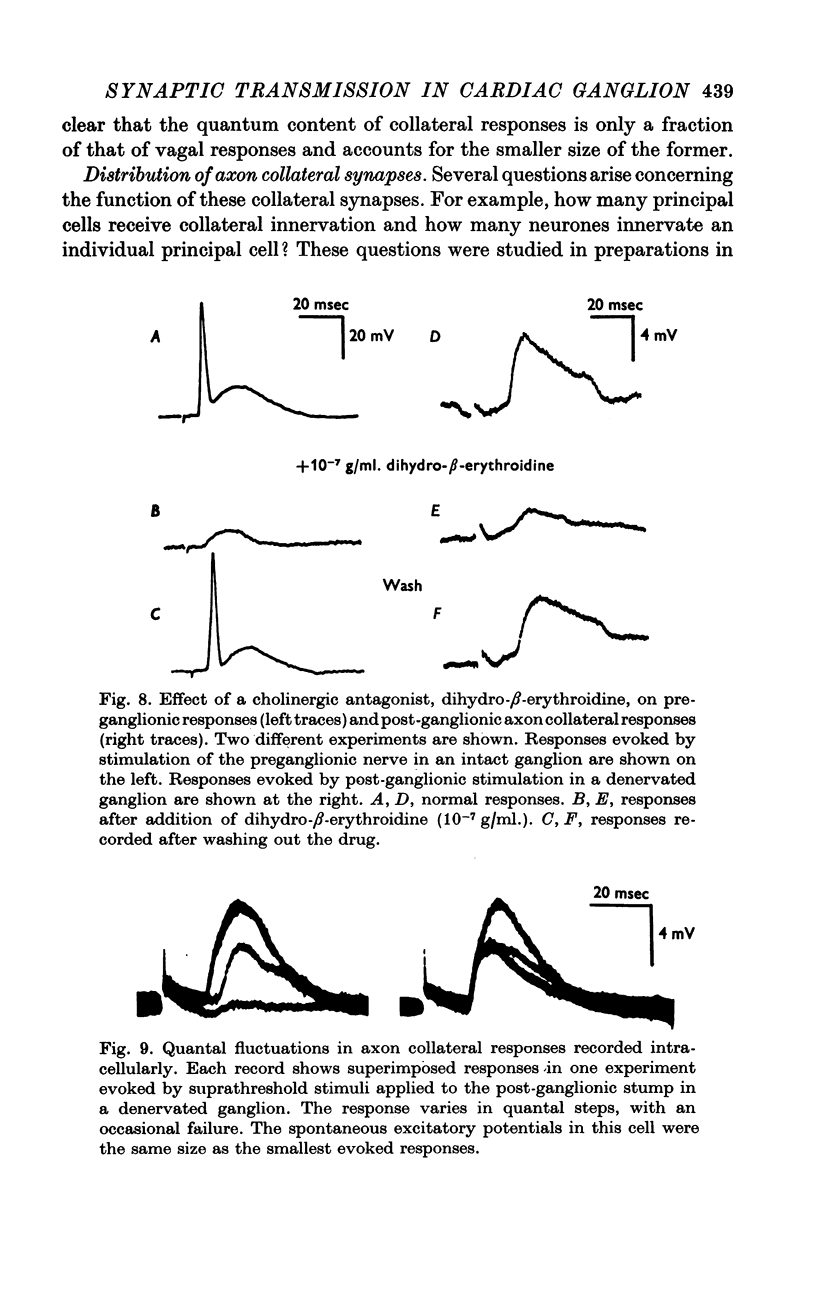
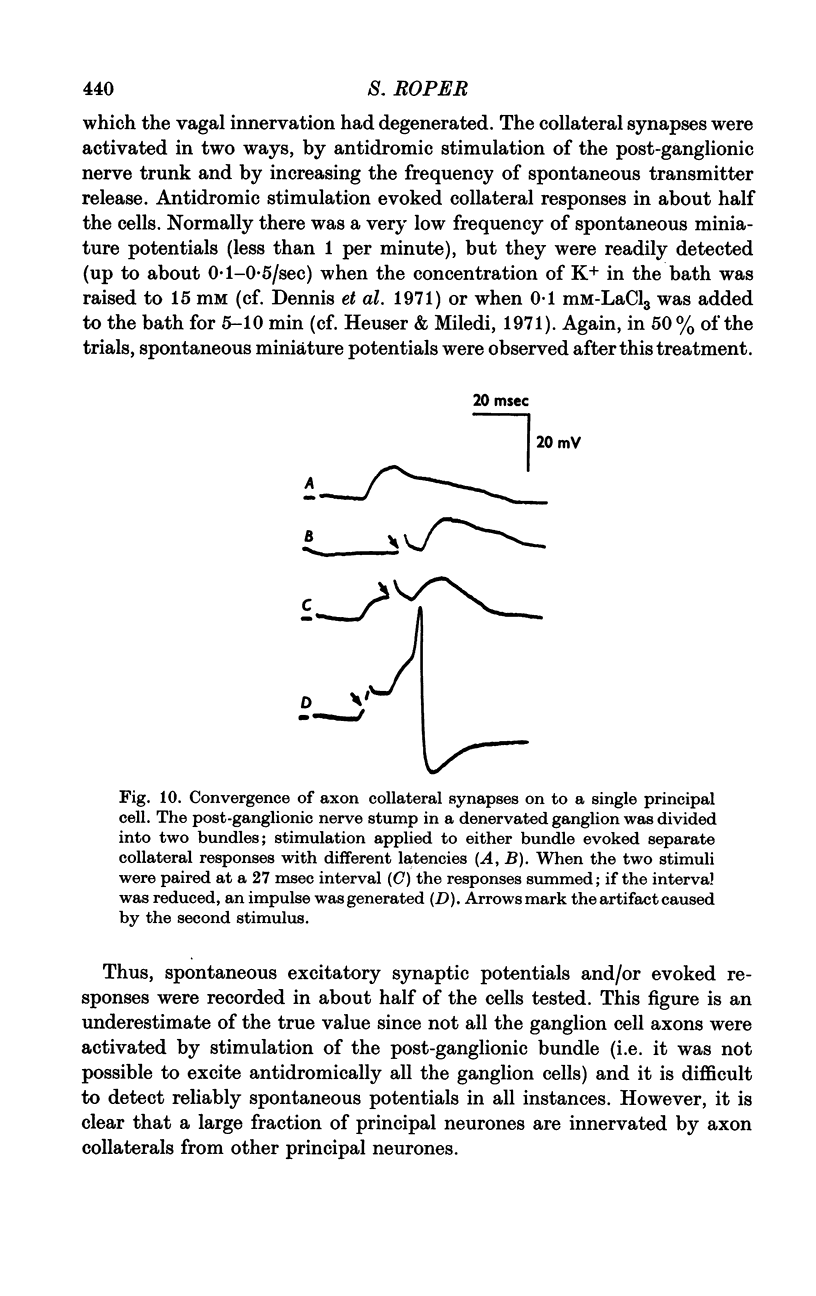
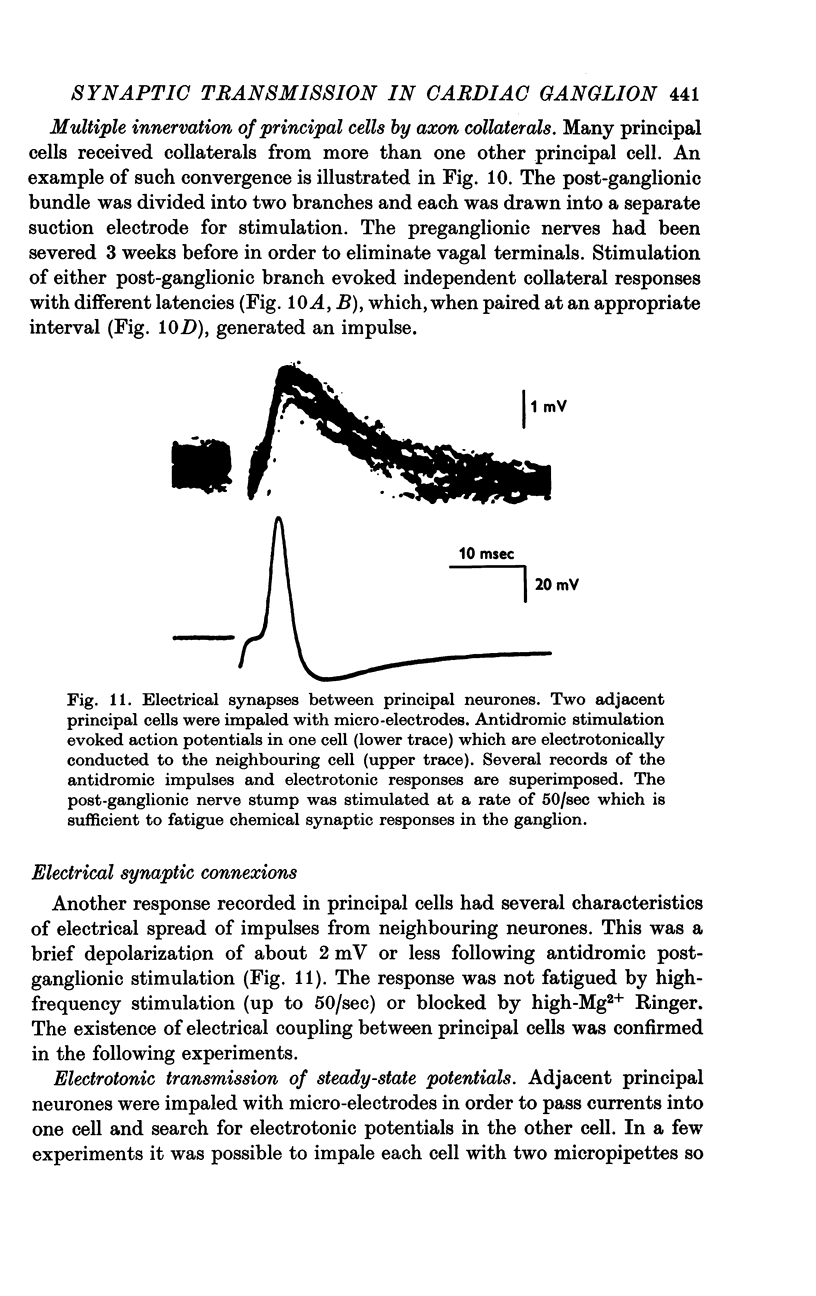
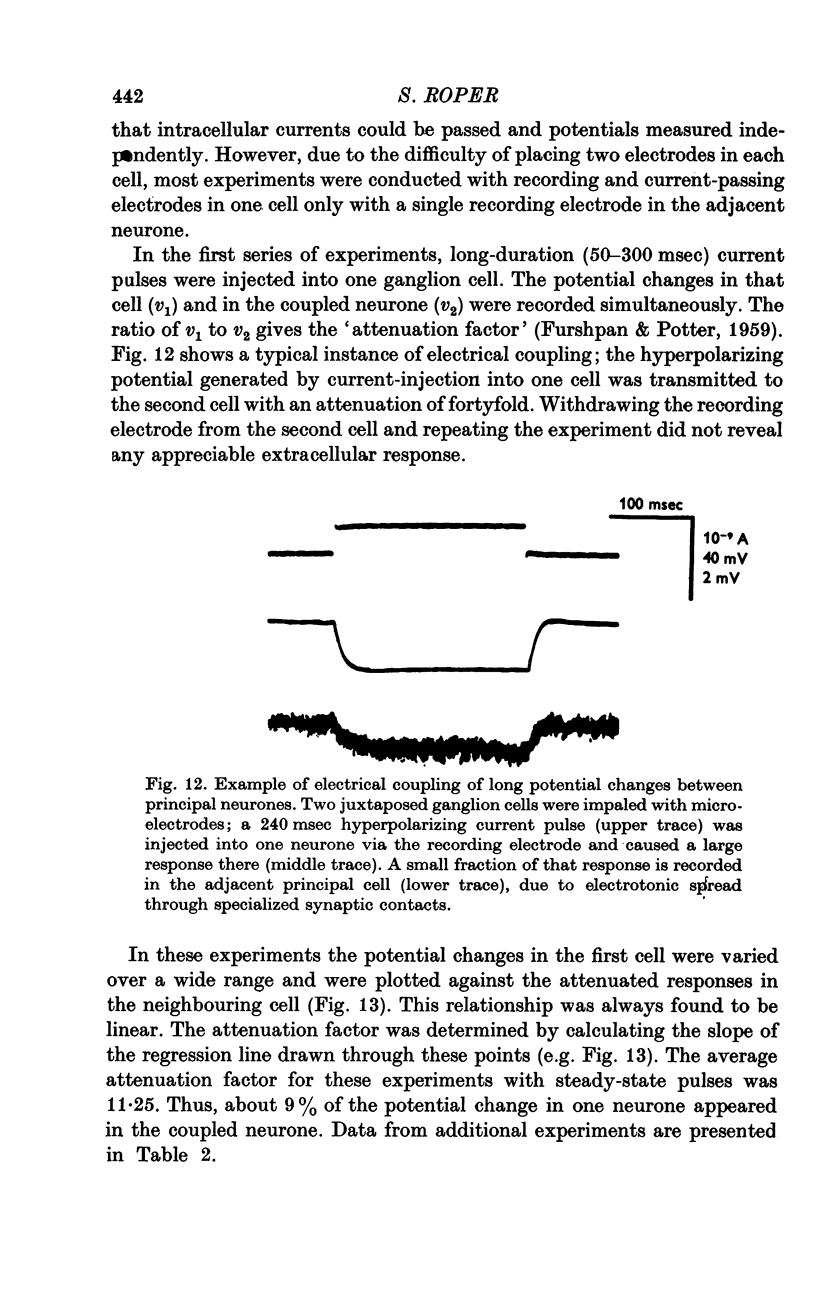
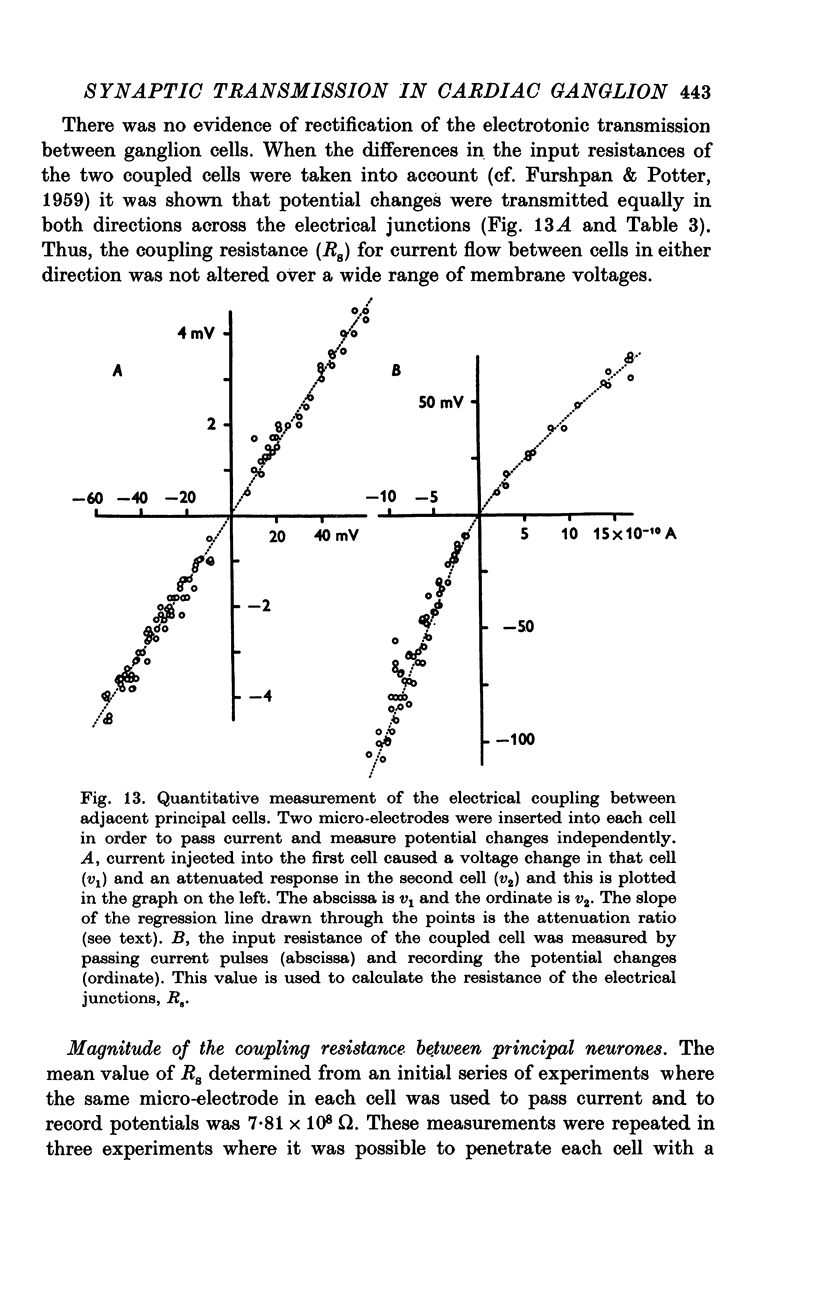
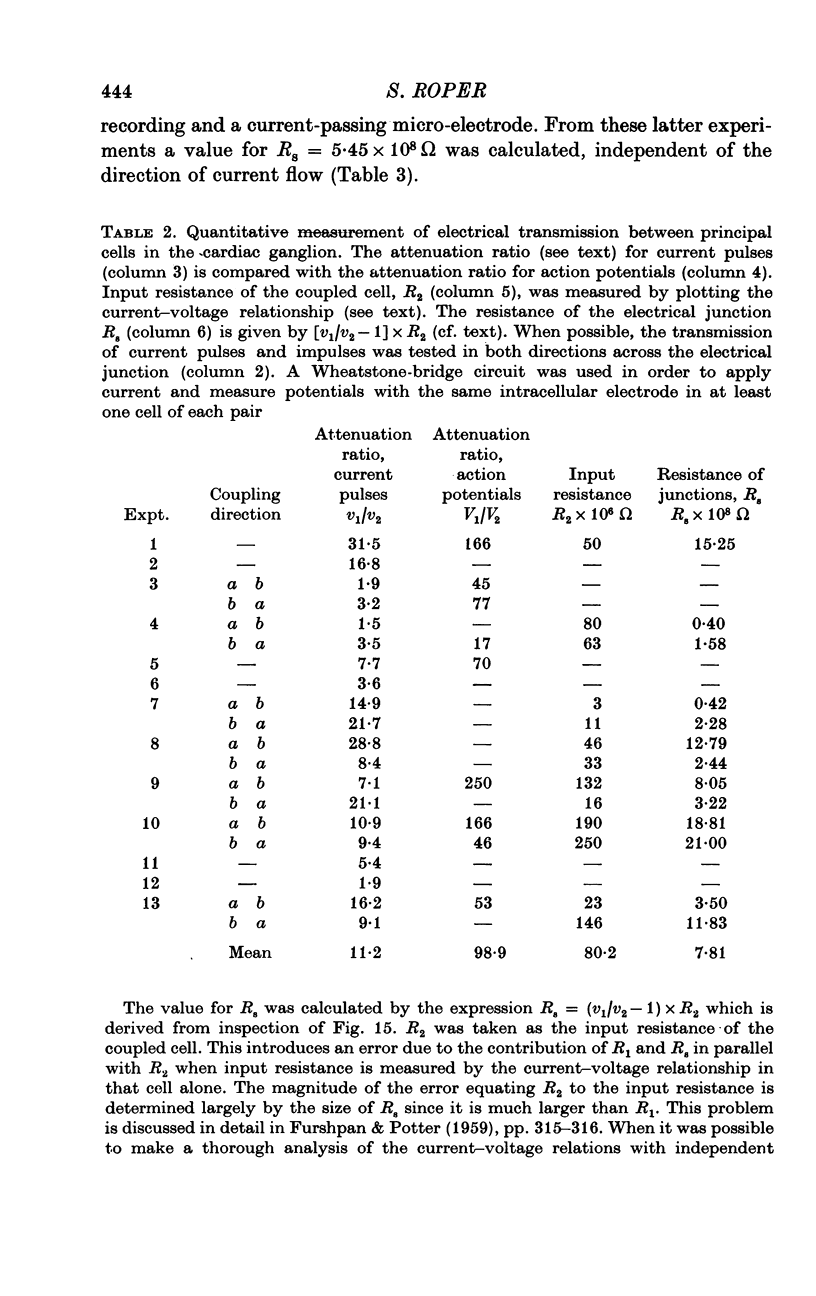





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKMAN J. G., GINSBORG B. L., RAY C. On the quantal release of the transmitter at a sympathetic synapse. J Physiol. 1963 Jul;167:402–415. doi: 10.1113/jphysiol.1963.sp007158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Spontaneous synaptic activity in sympathetic ganglion cells of the frog. J Physiol. 1963 Jul;167:389–401. doi: 10.1113/jphysiol.1963.sp007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., LIBET B. Origin and blockade of the synaptic responses of curarized sympathetic ganglia. J Physiol. 1961 Aug;157:484–503. doi: 10.1113/jphysiol.1961.sp006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Jacobowitz D. Catecholamine fluorescence studies of adrenergic neurons and chromaffin cells in sympathetic ganglia. Fed Proc. 1970 Nov-Dec;29(6):1929–1944. [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Coggeshall R. E. Opposite synaptic actions mediated by different branches of an identifiable interneuron in Aplysia. Science. 1967 Jan 20;155(3760):346–349. doi: 10.1126/science.155.3760.346. [DOI] [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. QUANTAL COMPONENTS OF THE SYNAPTIC POTENTIAL IN THE CILIARY GANGLION OF THE CHICK. J Physiol. 1964 Dec;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. R., Raisman G. The ultrastructure and somatic efferent synapses of small granule-containing cells in the superior cervical ganglion. J Anat. 1969 Sep;105(Pt 2):255–282. [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The formation of synapses in mammalian sympathetic ganglia reinnervated with preganglionic or somatic nerves. J Physiol. 1974 Feb;237(1):217–242. doi: 10.1113/jphysiol.1974.sp010479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- McMahan U. J., Purves D. Visual identification of two kinds of nerve cells and their synaptic contacts in a living autonomic ganglion of the mudpuppy (Necturus maculosus). J Physiol. 1976 Jan;254(2):405–425. doi: 10.1113/jphysiol.1976.sp011238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Lague P. H., Obata K., Claude P., Furshpan E. J., Potter D. D. Evidence for cholinergic synapses between dissociated rat sympathetic neurons in cell culture. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3602–3606. doi: 10.1073/pnas.71.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Casella C. Synaptically mediated potentials elicited by the stimulation of post-ganglionic trunks in the guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):55–67. doi: 10.1007/BF00587046. [DOI] [PubMed] [Google Scholar]
- Raisman G., Field P. M., Ostberg A. J., Iversen L. L., Zigmond R. E. A quantitative ultrastructural and biochemical analysis of the process of reinnervation of the superior cervical ganglion in the adult rat. Brain Res. 1974 May 10;71(1):1–16. doi: 10.1016/0006-8993(74)90187-5. [DOI] [PubMed] [Google Scholar]
- Roper S. The acetylcholine sensitivity of the surface membrane of multiply-innervated parasympathetic ganglion cells in the mudpuppy before and after partial denervation. J Physiol. 1976 Jan;254(2):455–473. doi: 10.1113/jphysiol.1976.sp011240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal release of acetylcholine from the nerve endings of the guinea-pig superior cervical ganglion. Pflugers Arch. 1971;329(3):207–219. doi: 10.1007/BF00586615. [DOI] [PubMed] [Google Scholar]
- WATANABE A., GRUNDFEST H. Impulse propagation at the septal and commissural junctions of crayfish lateral giant axons. J Gen Physiol. 1961 Nov;45:267–308. doi: 10.1085/jgp.45.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]


