Abstract
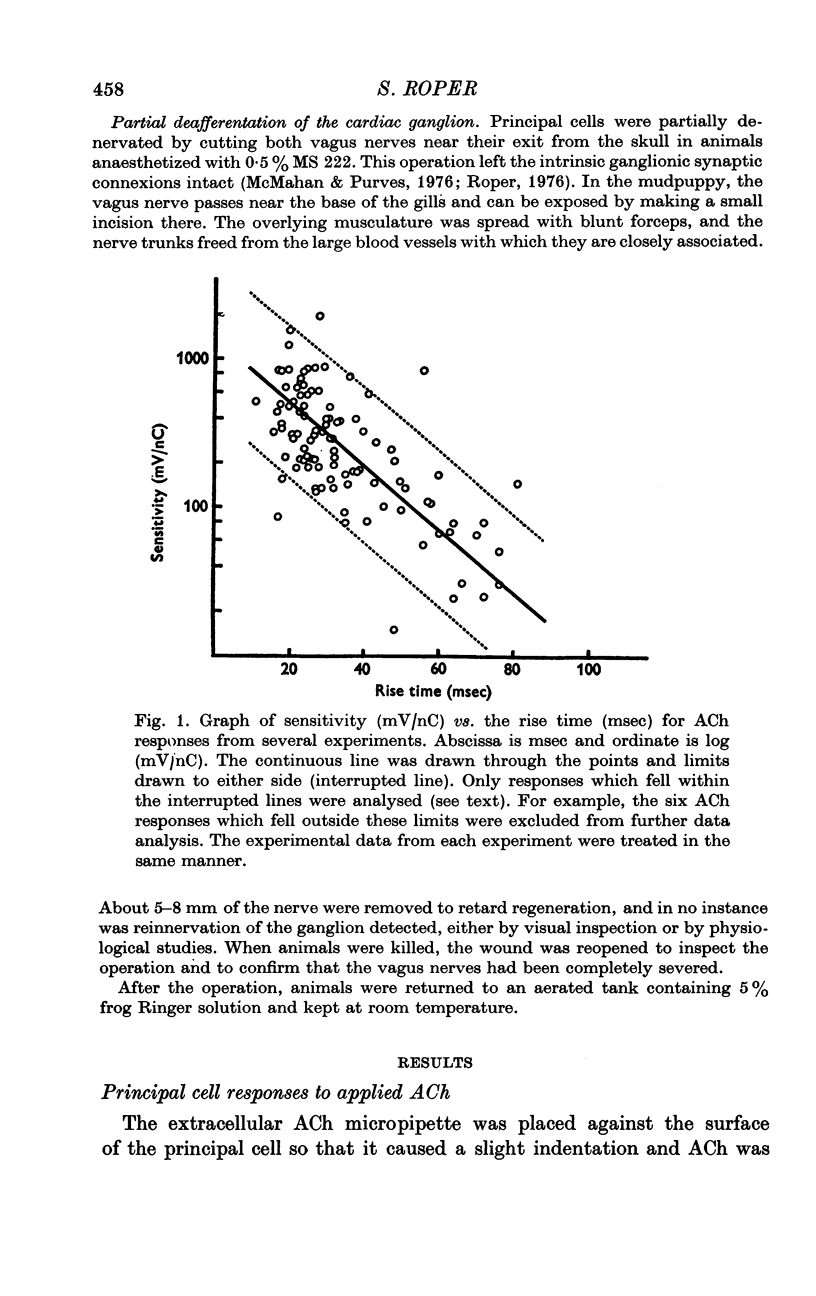
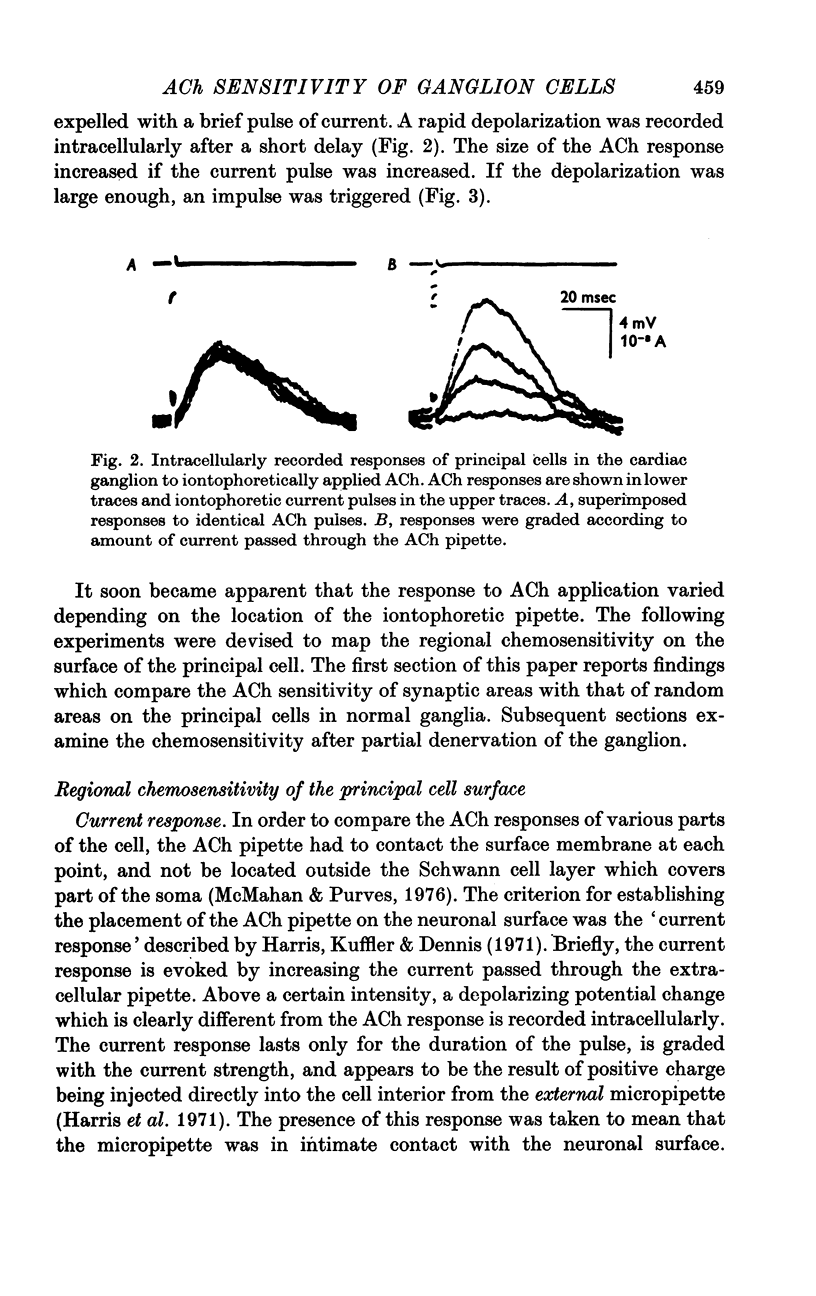
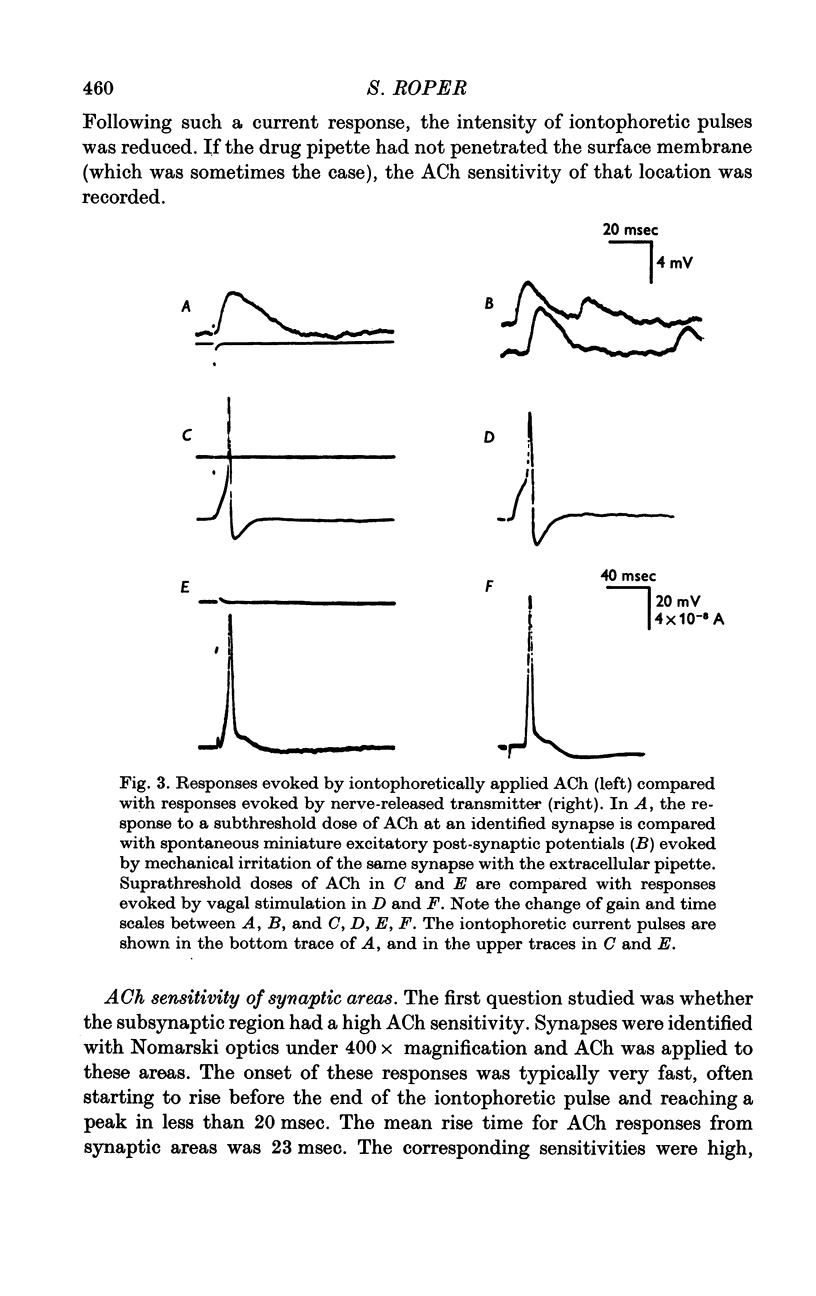
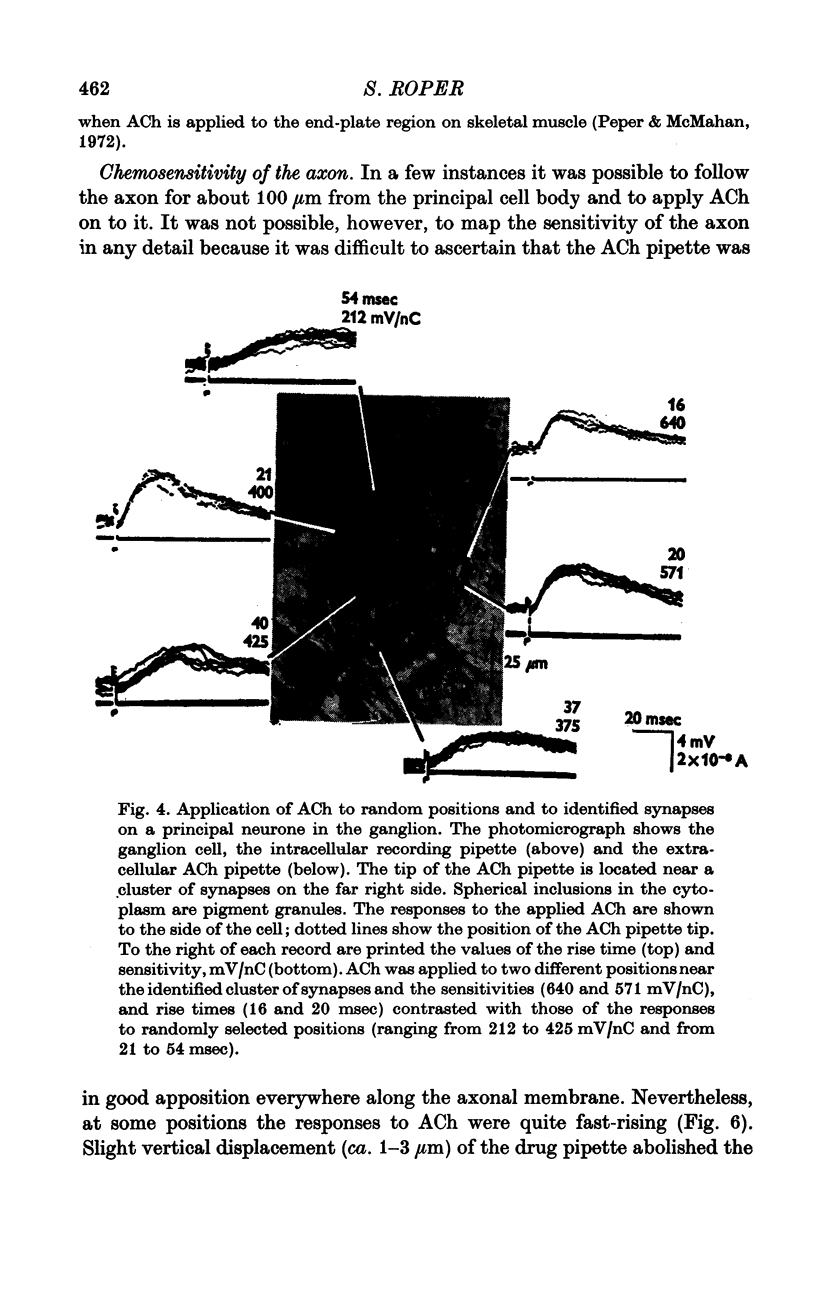
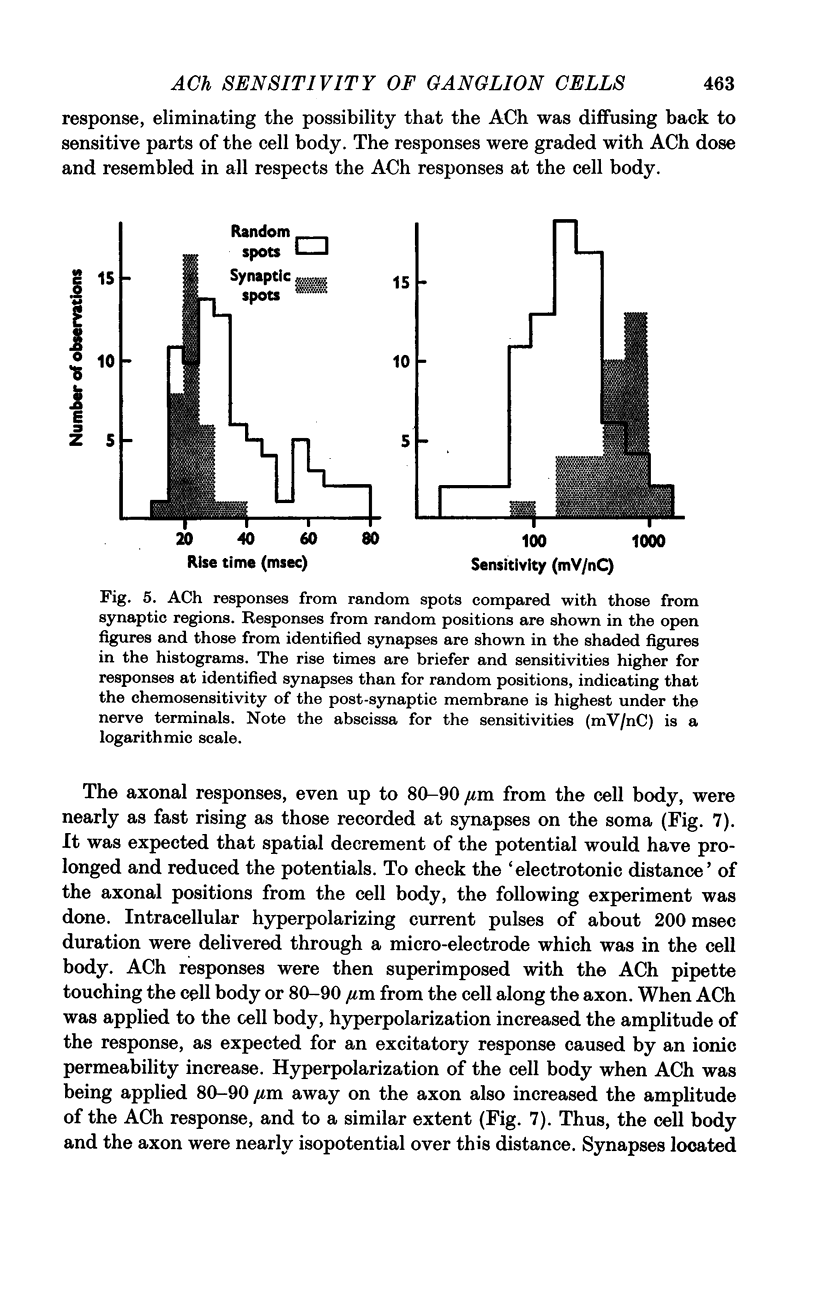
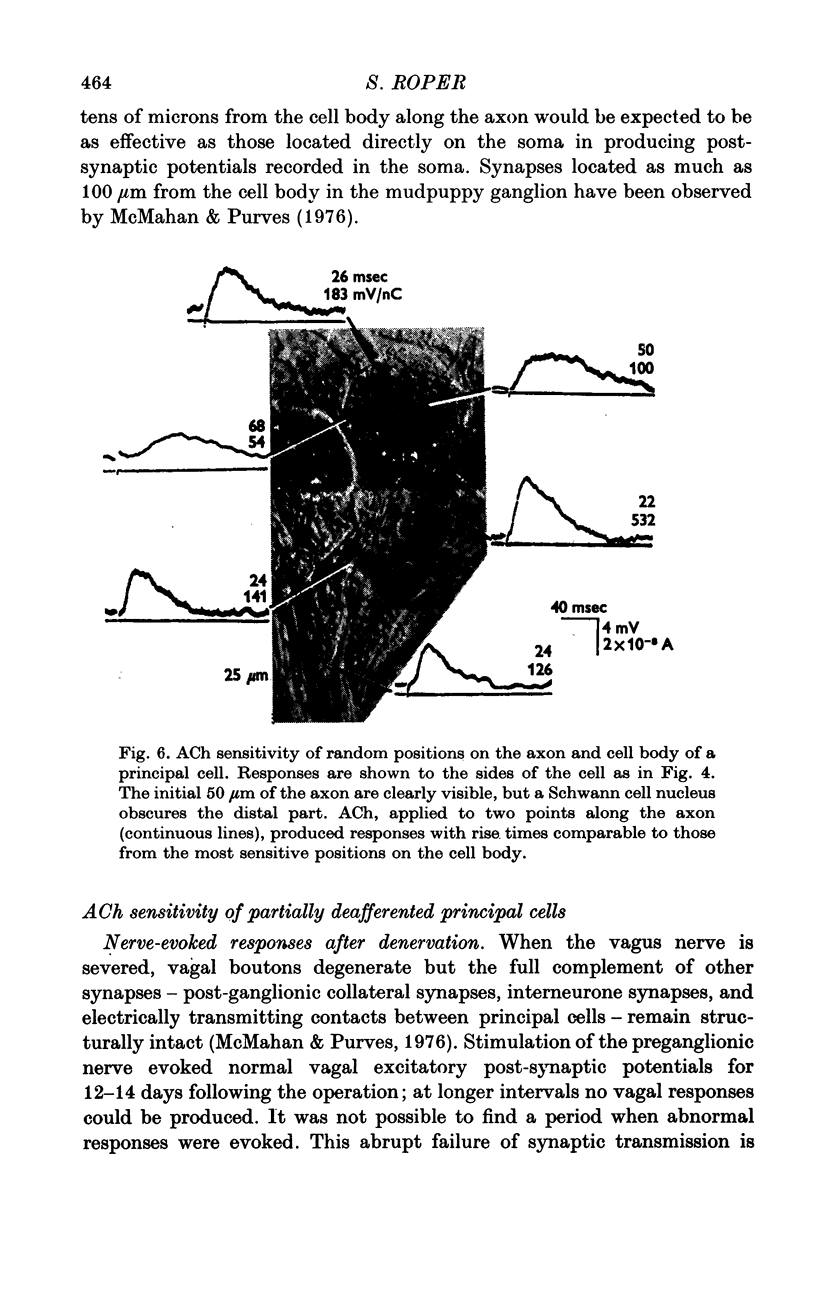
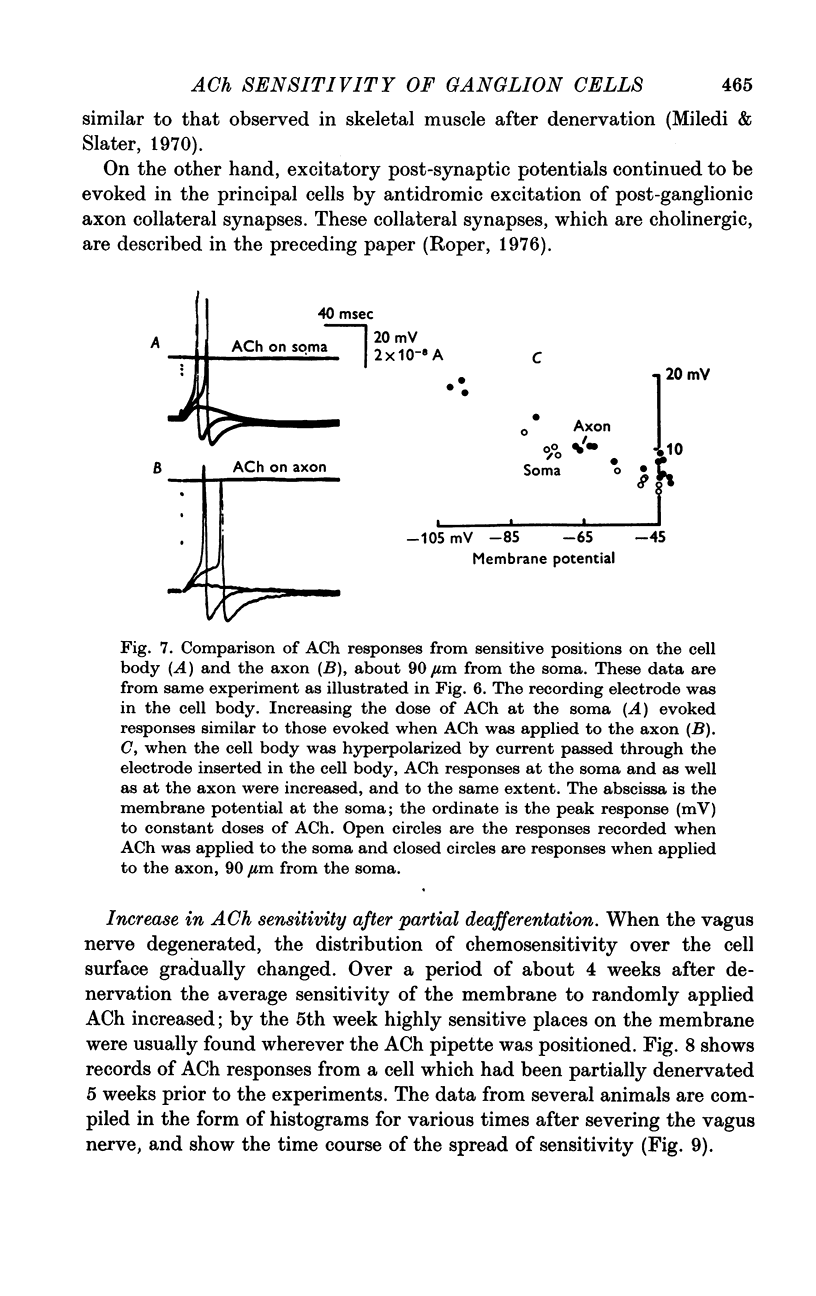
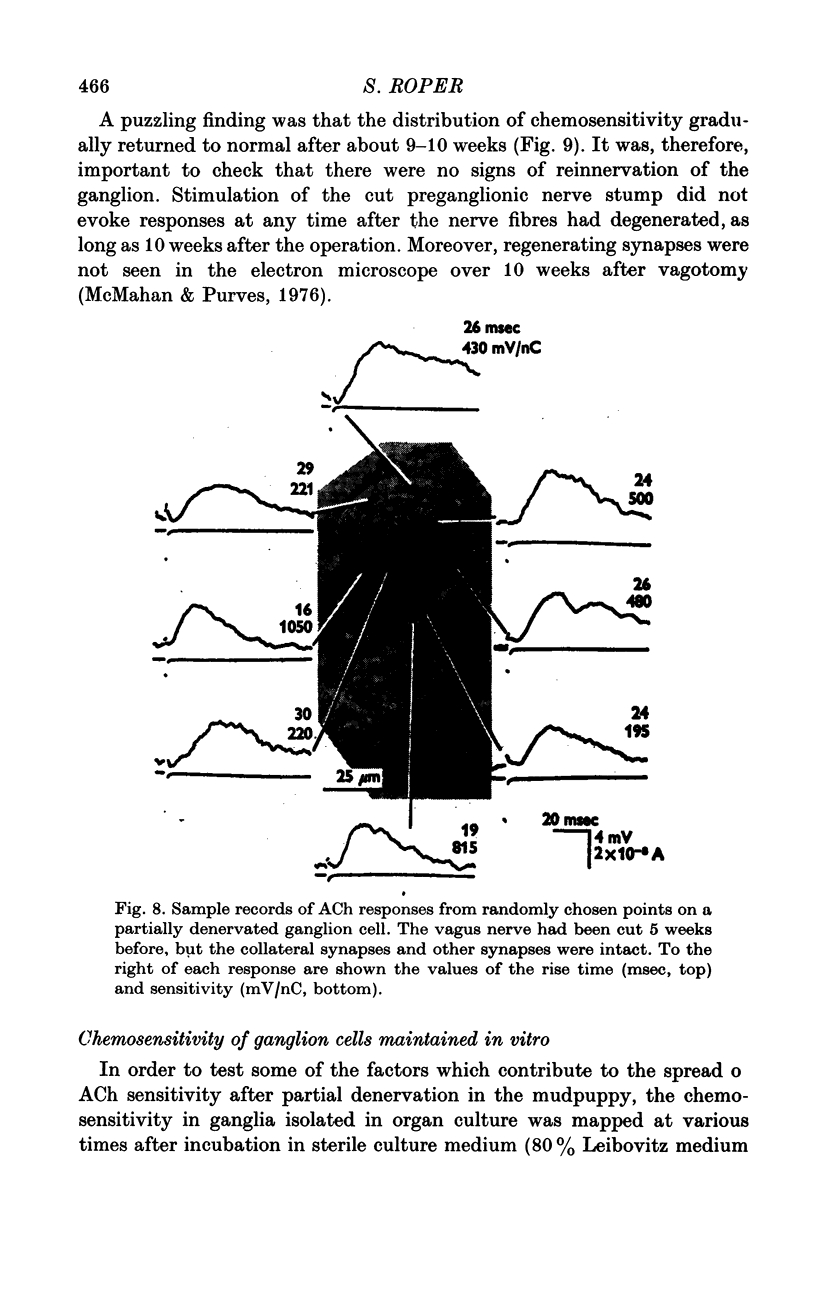
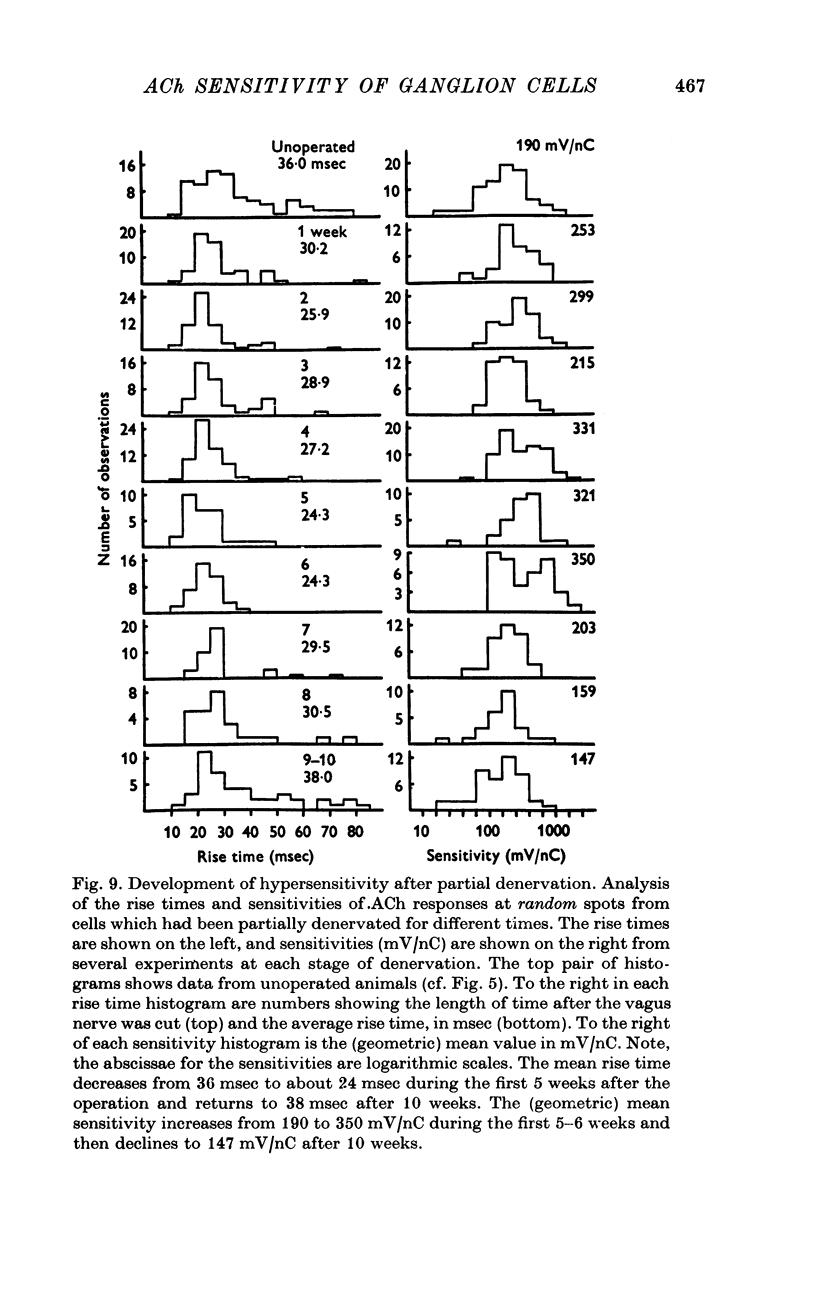
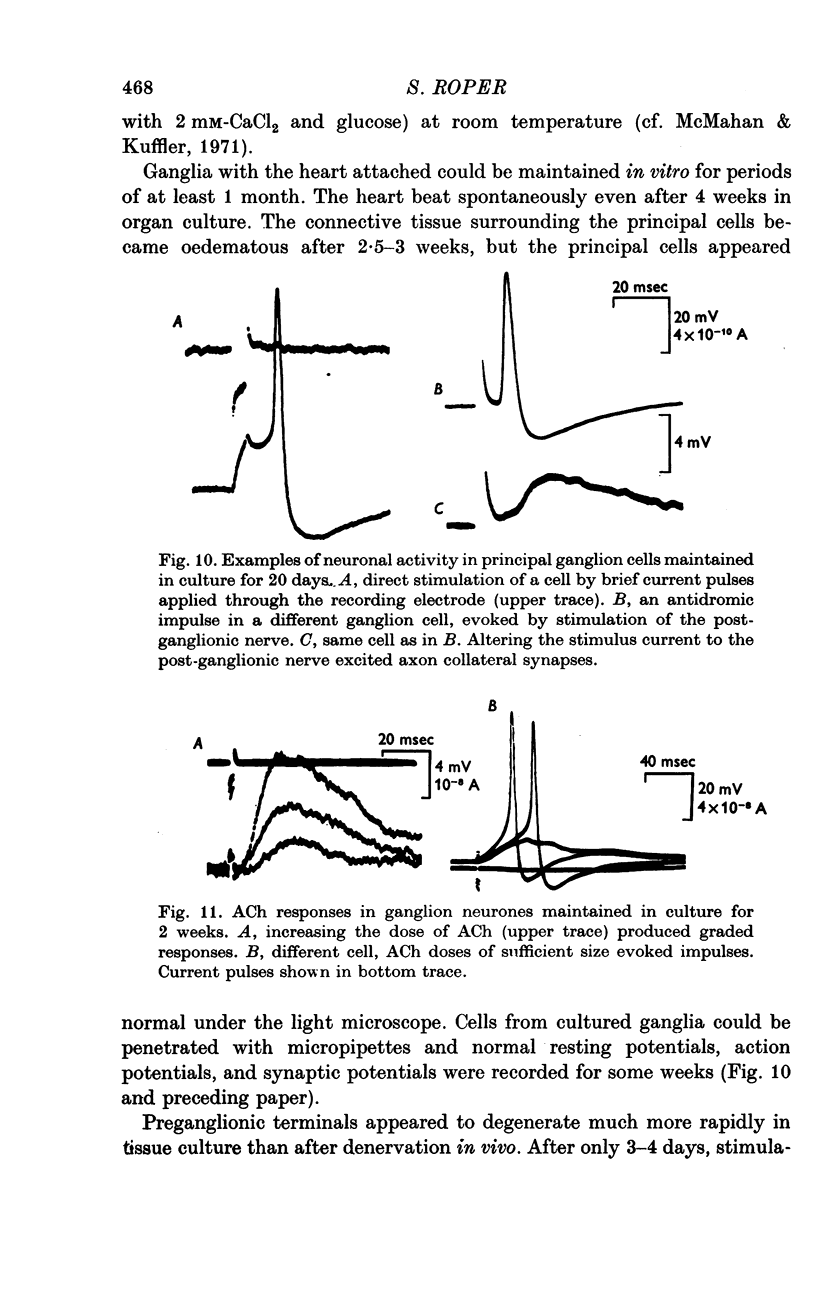
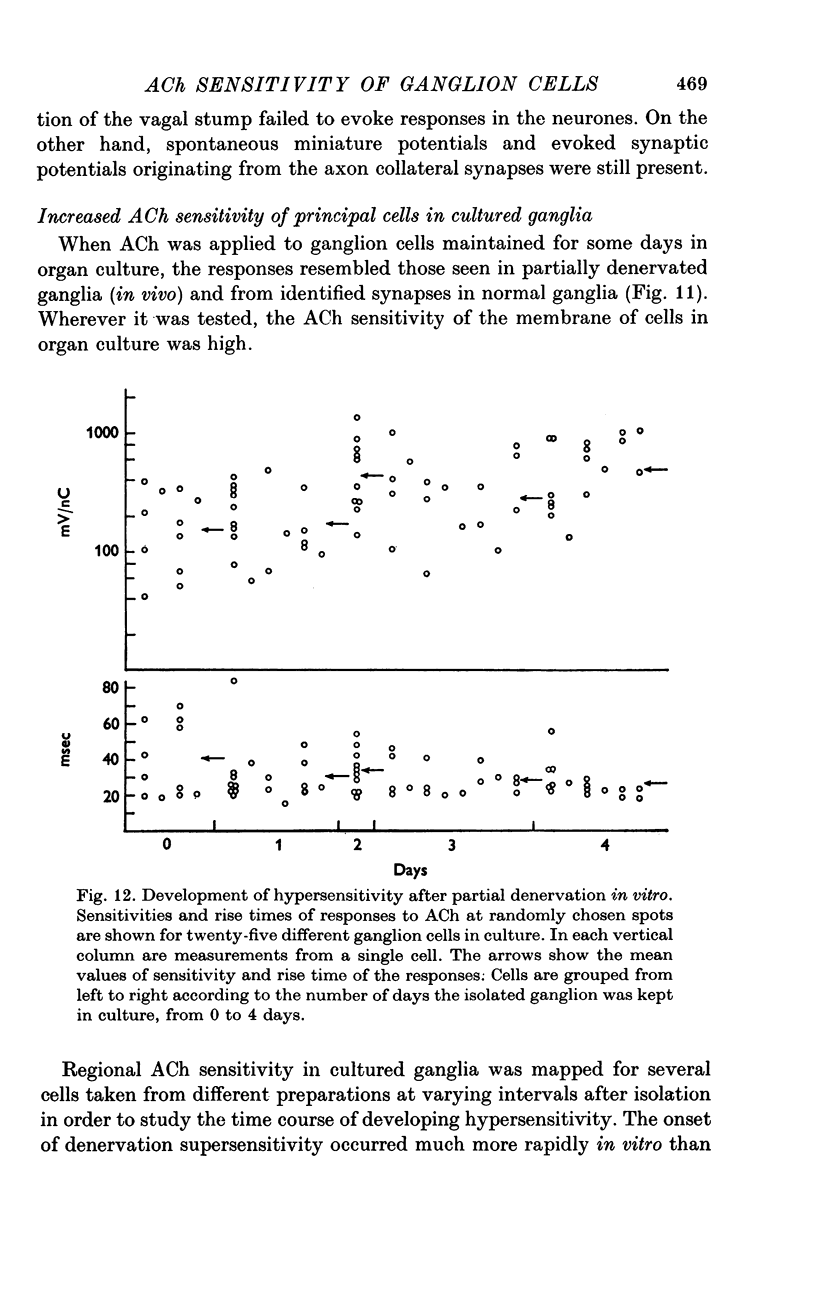
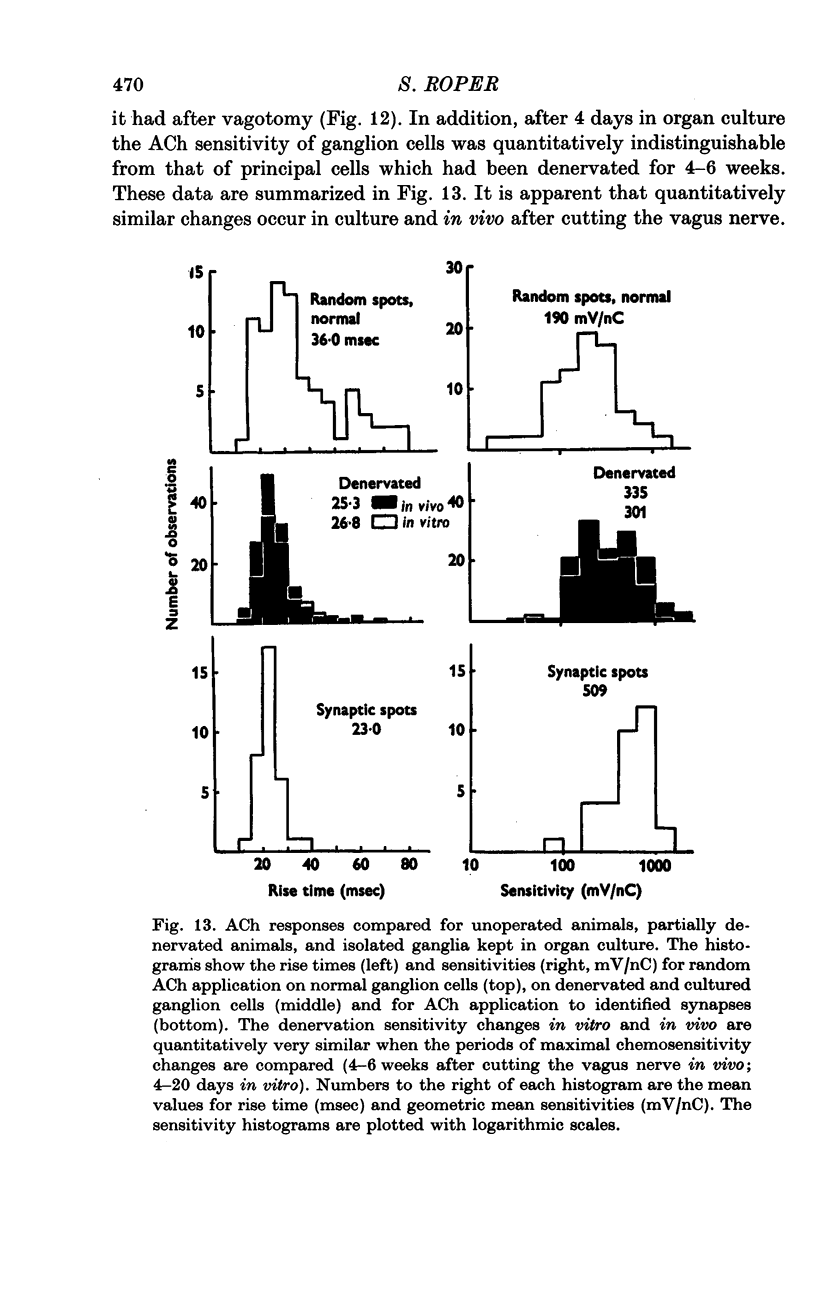
1. The surface chemosensitivity to iontophoretically applied acetylcholine (ACh) of single nerve cells in the cardiac ganglion of the mudpuppy was examined. 2. Some synapses on the neurones can be recognized in the living preparation with differential interference contrast optics. Identified synaptic regions of the ganglion cells were more sensitive to ACh than were other areas. The mean sensitivity of synaptic areas was 509 mV/nC, but that of random spots on the cell surface (which were mainly non-synaptic) was only 190 mV/nC. The mean rise time of ACh responses at synapses was 23 msec and at random spots was 36 msec. These data suggest that the density of ACh receptors is highest under the synapses on the post-synaptic membrane. 3. When some, but not all, of the presynaptic terminals on the ganglion cells are destroyed by cutting the vagus nerve, the sensitivity of the entire surface membrane to applied ACh increases. This increase in sensitivity reaches a maximum about 4-6 weeks after the operation. 4. Synaptic transmission at excitatory collateral synapses which remain after vagal degeneration is not altered by this hypersensitivity. 5. Neurones from ganglia which have been isolated and maintained in organ culture also become hypersensitive to applied ACh. this heightened chemosensitivity deveoops much faster in vitro; hypersensitivity in cultured ganglia becomes manifest within 4-5 days, in contrast with 4-6 weeks after vagus degeneration in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Dennis M. J., Harris A. J. The development of chemosensitivity in extrasynaptic areas of the neuronal surface after denervation of parasympathetic ganglion cells in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):555–563. doi: 10.1098/rspb.1971.0047. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Purves D. Visual identification of two kinds of nerve cells and their synaptic contacts in a living autonomic ganglion of the mudpuppy (Necturus maculosus). J Physiol. 1976 Jan;254(2):405–425. doi: 10.1113/jphysiol.1976.sp011238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Sakmann B. The effect of contractile activity on fibrillation and extrajunctional acetylcholine-sensitivity in rat muscle maintained in organ culture. J Physiol. 1974 Feb;237(1):157–182. doi: 10.1113/jphysiol.1974.sp010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper S. An electrophysiological study of chemical and electrical synapses on neurones in the parasympathetic cardiac ganglion of the mudpuppy, Necturus maculosus: evidence for intrinsic ganglionic innervation. J Physiol. 1976 Jan;254(2):427–454. doi: 10.1113/jphysiol.1976.sp011239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRENDELENBURG U. Supersensitivity and subsensitivity to sympathomimetic amines. Pharmacol Rev. 1963 Jun;15:225–276. [PubMed] [Google Scholar]





