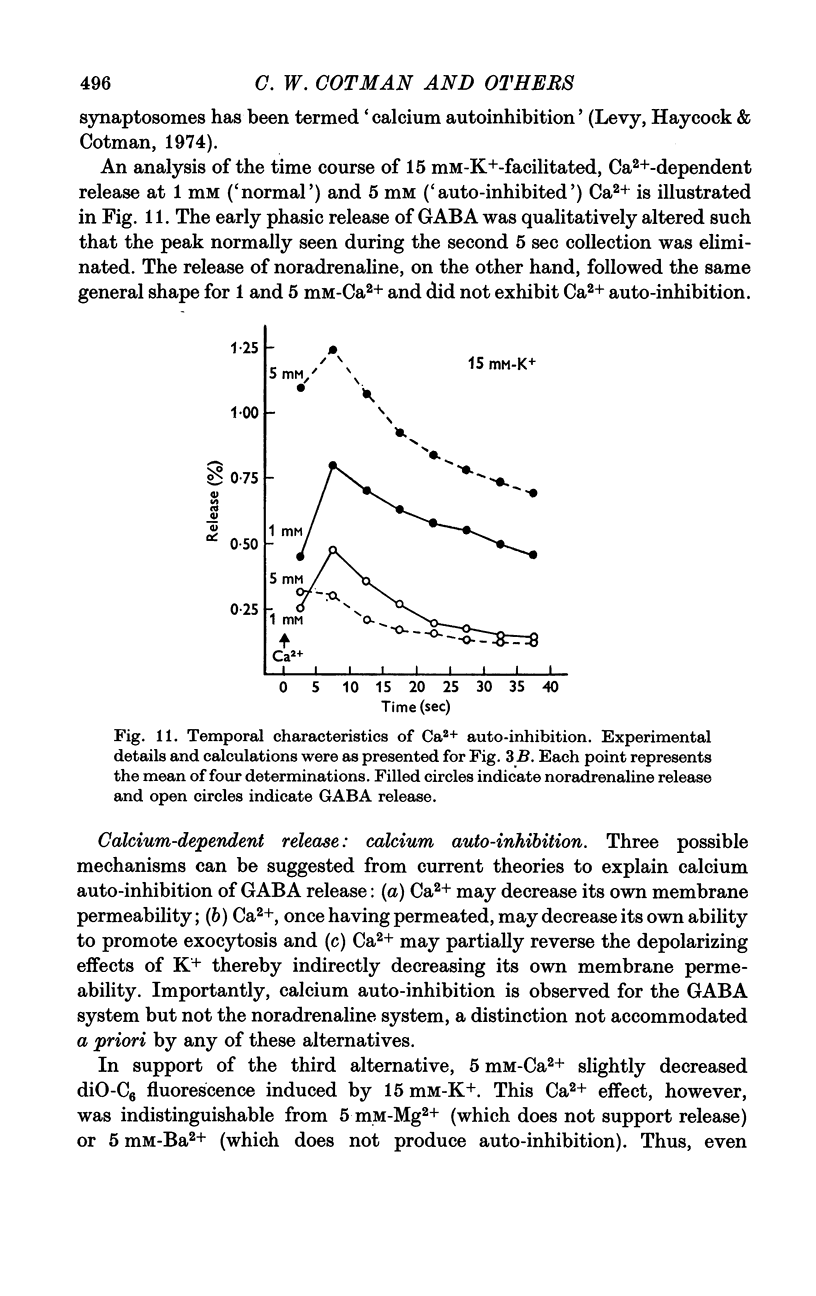
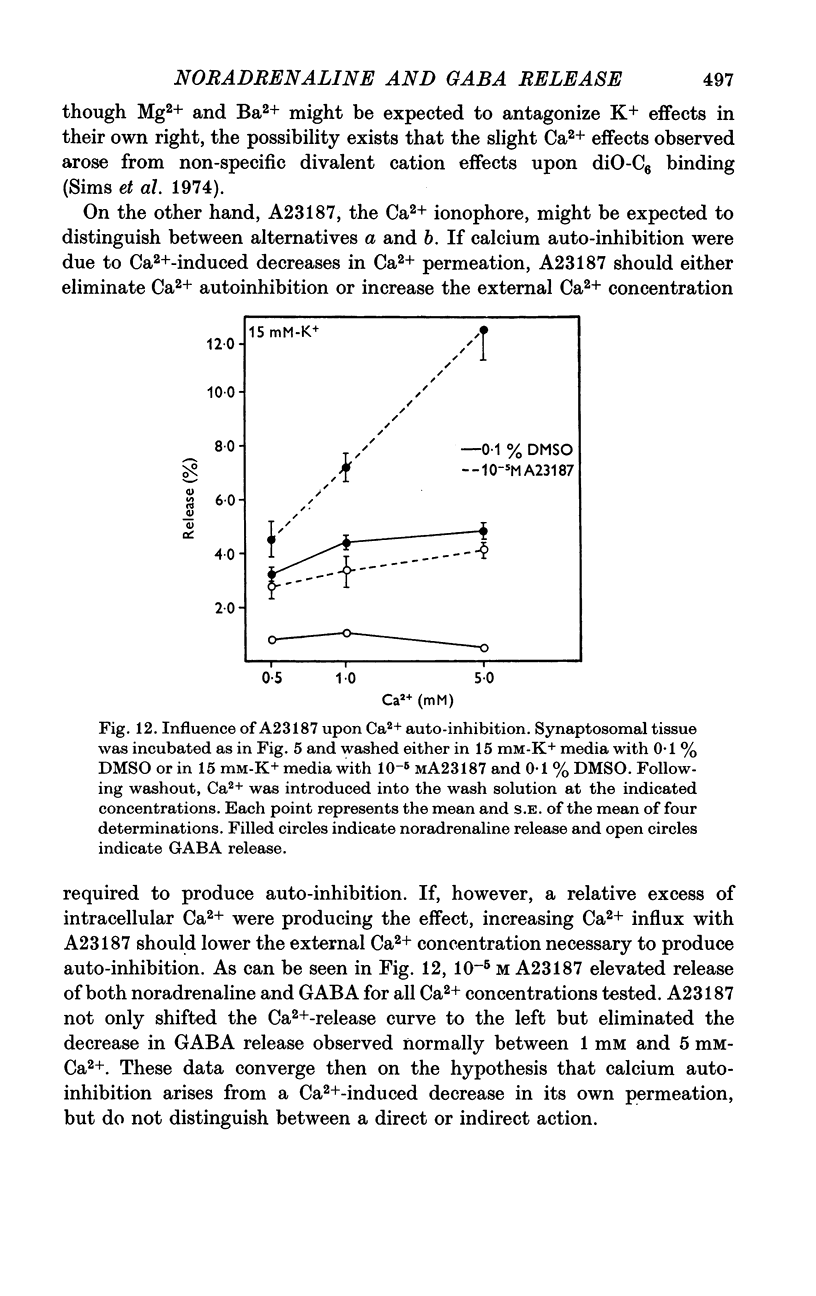
Abstract
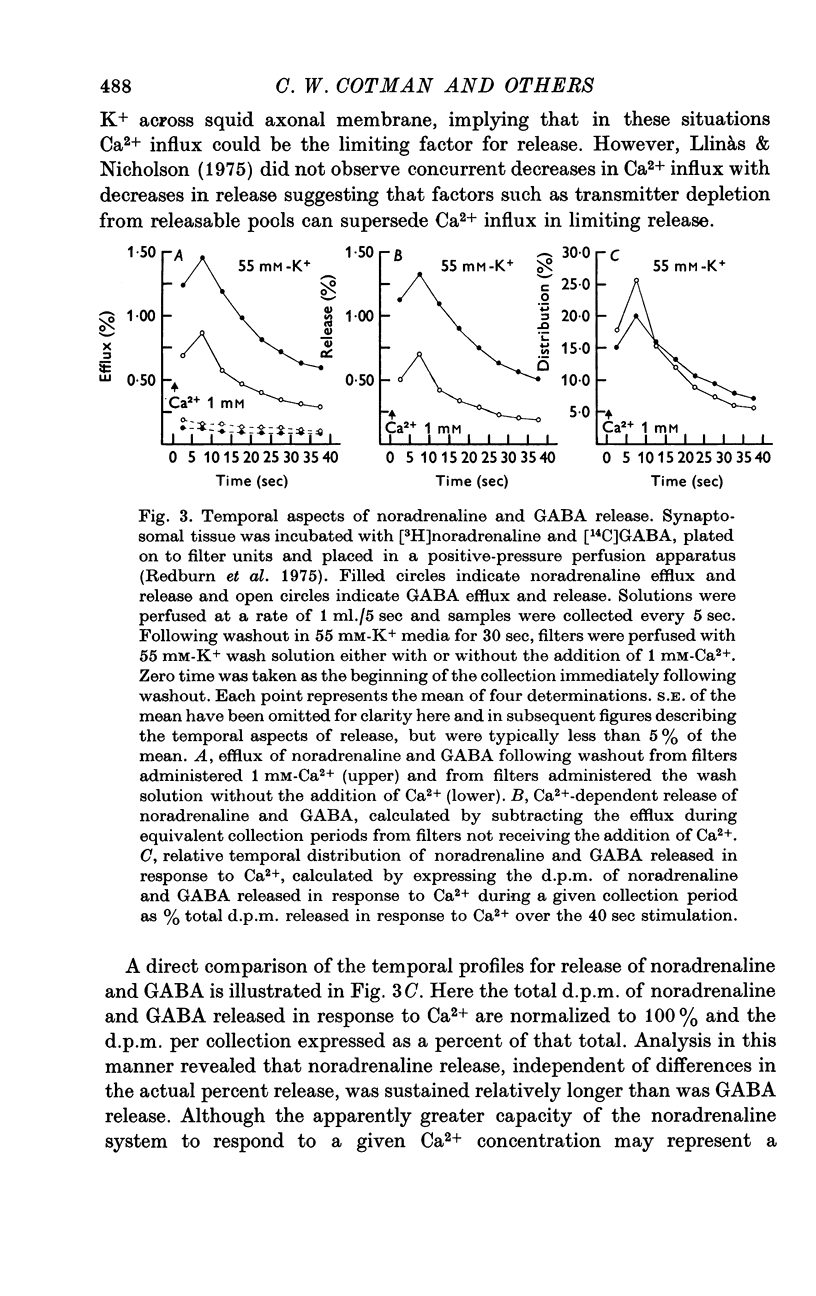
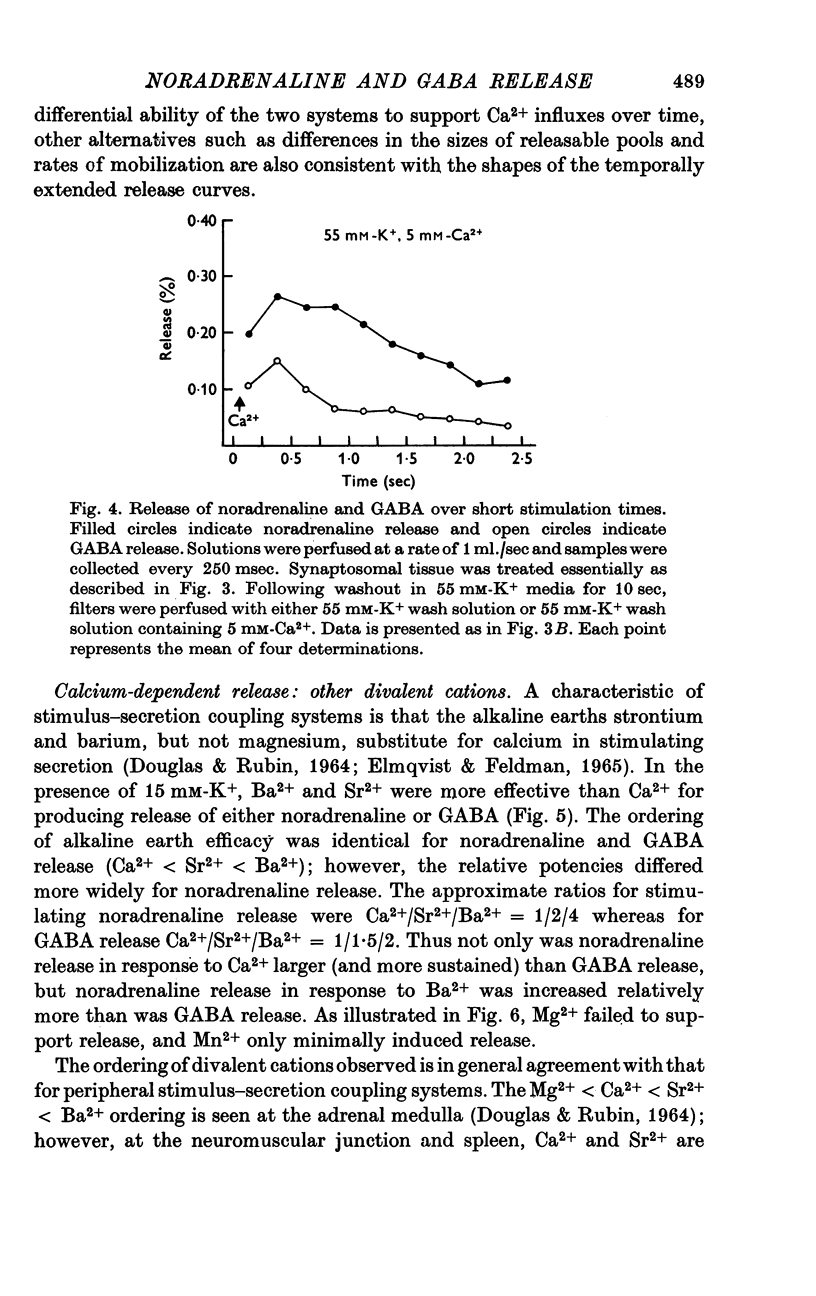
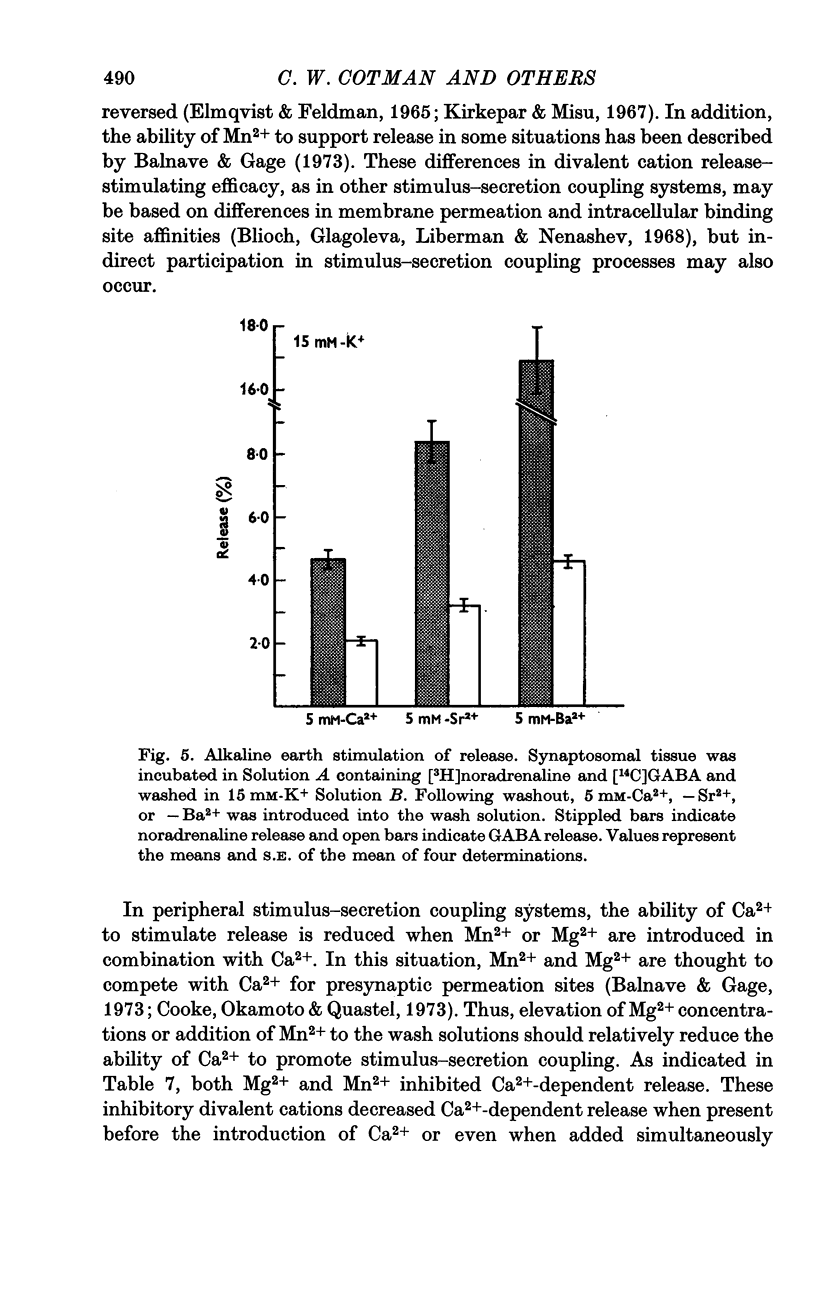
1. Brain synaptosomal fractions released both endogenous and exogenously loaded noradrenaline and gamma-aminobutyric acid (GABA) in response to calcium. Elevation of magnesium concentrations in the release media decreased the calcium-dependent release. 2. The release of noradrenaline and GABA occurred within 250 msec following the application of calcium. Following the initial response to calcium, release progressively decreased with continued application of calcium. GABA release declined more rapidly than noradrenaline release, consistent with a noradrenaline distribution having greater accessibility to the release process. 3. Sodium was required for the loading of noradrenaline and GABA into pools released by calcium. On the other hand, the presence of sodoium was not required for release from previously loaded pools. 4. Microsomal fractions did not exhibit calcium-dependent release of noradrenaline or GABA. Furthermore, exogenously loaded lysine was not released from synaptosomal fractions in response to calcium. 5. Barium and strontium, but not magnesium, stimulated noradrenaline and GABA release in the absence of calcium. The ordering of alkaline earth efficacies was barium greater than strontium greater than calcium. 6. Manganese inhibited calcium-dependent release of noradrenaline and GABA to a greater extent than magnesium. 7. Release, in response to 1 mM calcium, increased linearly with the log. [K+]0, suggesting that a voltage-dependent calcium inophore limits release. The slope of release vs. log. [K+]0 was greater for noradrenaline than for GABA. 8. For a given [K+]0 less than 55 mM, increases in external calcium concentration above 1 mM increased noradrenaline release but decreased GABA release. These data suggest that calcium can decrease its own permeation and that differences in the release process may exist for different neurotransmitters. 9. In the presence of the artificial calcium ionophore, A23187, both noradrenaline and GABA release increased linearly with the log. [Ca2+]0. The slope for noradrenaline release was greater than that for GABA release. 10. Stimulus-secretion coupling in brain is suggested to be regulated at the level of a voltage dependent calcium permeation mechanism. However, basic differences in the interaction of calcium with the release process may exist between the noradrenaline and GABA systems.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave R. J., Gage P. W. The inhibitory effect of manganese on transmitter release at the neuromuscular junction of the toad. Br J Pharmacol. 1973 Feb;47(2):339–352. doi: 10.1111/j.1476-5381.1973.tb08332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley A. G., Powis G., Summers R. J. The effects of pargyline on overflow of transmitter and uptake of noradrenaline in the cat spleen. Br J Pharmacol. 1973 Apr;47(4):719–728. doi: 10.1111/j.1476-5381.1973.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Johnson E. M., Jr, Needleman P. Calcium-dependent norepinephrine release from presynaptic nerve endings in vitro. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2237–2240. doi: 10.1073/pnas.69.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn R. W., Goodwin F. K., Murphy D. L., Bunney W. E., Jr, Davis J. M. Quantitative studies of norepinephrine uptake by synaptosomes. Biochem Pharmacol. 1968 Jun;17(6):957–964. doi: 10.1016/0006-2952(68)90354-7. [DOI] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. The specific effect of potassium on transmitter release by motor nerve terminals and its inhibition by calcium. J Physiol. 1973 Jan;228(2):435–458. doi: 10.1113/jphysiol.1973.sp010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Matthews D. A. Synaptic plasma membranes from rat brain synaptosomes: isolation and partial characterization. Biochim Biophys Acta. 1971 Dec 3;249(2):380–394. doi: 10.1016/0005-2736(71)90117-9. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Henry D. Catecholamines in fetal and newborn rat brain. J Neurochem. 1973 Jul;21(1):61–67. doi: 10.1111/j.1471-4159.1973.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Cuello A. C., Hiley R., Iversen L. L. Use of catechol O-methyltransferase for the enzyme radiochemical assay of dopamine. J Neurochem. 1973 Nov;21(5):1337–1340. doi: 10.1111/j.1471-4159.1973.tb07587.x. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Belleroche J. S., Bradford H. F. Metabolism of beds of mammalian cortical synaptosomes: response to depolarizing influences. J Neurochem. 1972 Mar;19(3):585–602. doi: 10.1111/j.1471-4159.1972.tb01376.x. [DOI] [PubMed] [Google Scholar]
- De Belleroche J. S., Bradford H. F. The stimulus-induced release of acetylcholine from synaptosome beds and its calcium dependence. J Neurochem. 1972 Jul;19(7):1817–1819. doi: 10.1111/j.1471-4159.1972.tb06229.x. [DOI] [PubMed] [Google Scholar]
- Descarries L., Droz B. Intraneural distribution of exogenous norepinephrine in the central nervous system of the rat. J Cell Biol. 1970 Feb;44(2):385–399. doi: 10.1083/jcb.44.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S., Krnjević K. Cortical inhibition and gamma-aminobutyric acid. Exp Brain Res. 1969;9(2):137–154. doi: 10.1007/BF00238327. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Influence of ionic environment on acetylcholine release from the motor nerve terminals. Acta Physiol Scand. 1966 May;67(1):34–42. doi: 10.1111/j.1748-1716.1966.tb03284.x. [DOI] [PubMed] [Google Scholar]
- Graham L. T., Jr, Aprison M. H. Fluorometric determination of aspartate, glutamate, and gamma-aminobutyrate in nerve tissue using enzymic methods. Anal Biochem. 1966 Jun;15(3):487–497. doi: 10.1016/0003-2697(66)90110-2. [DOI] [PubMed] [Google Scholar]
- Grynszpan-Winograd O. Morphological aspects of exocytosin in the adrenal medulla. Philos Trans R Soc Lond B Biol Sci. 1971 Jun 17;261(839):291–292. doi: 10.1098/rstb.1971.0058. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harreveld A. V., Trubatch J. Synaptic changes in frog brain after stimulation with potassium chloride. J Neurocytol. 1975 Feb;4(1):33–46. doi: 10.1007/BF01099093. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz R. W. The release of dopamine from synaptosomes from rat striatum by the ionophores X 537A and A 23187. Biochim Biophys Acta. 1975 Jan 14;375(1):138–152. doi: 10.1016/0005-2736(75)90079-6. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I. Mechanism of transmitter release. Prog Biophys Mol Biol. 1970;21:33–124. [PubMed] [Google Scholar]
- Hughes J., Roth R. H. Variation in noradrenaline output with changes in stimulus frequency and train length: role of different noradrenaline pools. Br J Pharmacol. 1974 Jul;51(3):373–381. doi: 10.1111/j.1476-5381.1974.tb10672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Bloom F. E. Studies of the uptake of 3 H-gaba and ( 3 H)glycine in slices and homogenates of rat brain and spinal cord by electron microscopic autoradiography. Brain Res. 1972 Jun 8;41(1):131–143. doi: 10.1016/0006-8993(72)90621-x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L. Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol. 1971 Apr;41(4):571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagayama M., Douglas W. W. Electron microscope evidence of calcium-induced exocytosis in mast cells treated with 48-80 or the ionophores A-23187 and X-537A. J Cell Biol. 1974 Aug;62(2):519–526. doi: 10.1083/jcb.62.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974 Jul 1;23(13):1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- Levi G., Raiteri M. Exchange of neurotransmitter amino acid at nerve endings can simulate high affinity uptake. Nature. 1974 Aug 30;250(5469):735–737. doi: 10.1038/250735a0. [DOI] [PubMed] [Google Scholar]
- Levi G., Raiteri M. Proceedings: Synaptosomal exchange of gamma-aminobutyric acid (GABA) can simulate high affinity uptake. Br J Pharmacol. 1974 Nov;52(3):435P–436P. [PMC free article] [PubMed] [Google Scholar]
- Levy W. B., Haycock J. W., Cotman C. W. Effects of polyvalent cations on stimulus-coupled secretion of (14C)-gamma-aminobutyric acid from isolated brain synaptosomes. Mol Pharmacol. 1974 May;10(3):438–449. [PubMed] [Google Scholar]
- Levy W. B., Redburn D. A., Cotman C. W. Stimulus-coupled secretion of gamma-aminobutyric acid from rat brain synaptosomes. Science. 1973 Aug 17;181(4100):676–678. doi: 10.1126/science.181.4100.676. [DOI] [PubMed] [Google Scholar]
- Llinás R., Nicholson C. Calcium role in depolarization-secretion coupling: an aequorin study in squid giant synapse. Proc Natl Acad Sci U S A. 1975 Jan;72(1):187–190. doi: 10.1073/pnas.72.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar P. C., Polak R. L. Stimulation by atropine of acetylcholine release and synthesis in cortical slices from rat brain. Br J Pharmacol. 1970 Nov;40(3):406–417. doi: 10.1111/j.1476-5381.1970.tb10622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder A. H., Snyder S. H. Potassium-induced release of amino acids from cerebral cortex and spinal cord slices of the rat. Brain Res. 1974 Aug 16;76(2):297–308. doi: 10.1016/0006-8993(74)90461-2. [DOI] [PubMed] [Google Scholar]
- Mulder A. H., Yamamura H. I., Kuhar M. J., Snyder S. H. Release of acetylcholine from hippocampal slices by potassium depolarization: dependence on high affinity choline uptake. Brain Res. 1974 Apr 19;70(2):372–376. doi: 10.1016/0006-8993(74)90329-1. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D. R., Reed P. W., Lardy H. A. Ultraviolet and fluorescent spectral properties of the divalent cation ionophore A23187 and its metal ion complexes. Biochemistry. 1974 Sep 10;13(19):4007–4014. doi: 10.1021/bi00716a029. [DOI] [PubMed] [Google Scholar]
- Redburn D. A., Biela J., Shelton D. L., Cotman C. W. Stimulus secretion coupling in vitro: a rapid perfusion apparatus for monitoring efflux of transmitter substances from tissue samples. Anal Biochem. 1975 Jul;67(1):268–278. doi: 10.1016/0003-2697(75)90294-8. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Siggins G. R., Oliver A. P., Hoffer B. J., Bloom F. E. Cyclic adenosine monophosphate and norepinephrine: effects on transmembrane properties of cerebellar Purkinje cells. Science. 1971 Jan 15;171(3967):192–194. doi: 10.1126/science.171.3967.192. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Michaelis-Menten kinetics of secretion of sympathetic neurotransmitter as a function of external calcium: effect of graded alpha adrenoceptor blockade. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(3):323–327. doi: 10.1007/BF00500294. [DOI] [PubMed] [Google Scholar]
- Varon S., Weinstein H., Baxter C. F., Roberts E. Uptake and metabolism of exogenous gamma-aminobutyric acid by subcellular particles in a sodium-containing medium. Biochem Pharmacol. 1965 Dec;14(12):1755–1764. doi: 10.1016/0006-2952(65)90265-0. [DOI] [PubMed] [Google Scholar]
- Wolfe L. S., Morgan I. G., Gombos G. Isolation of plasma membranes from rat brain. Biochim Biophys Acta. 1971 Sep 14;241(3):737–751. doi: 10.1016/0005-2736(71)90002-2. [DOI] [PubMed] [Google Scholar]


