Abstract
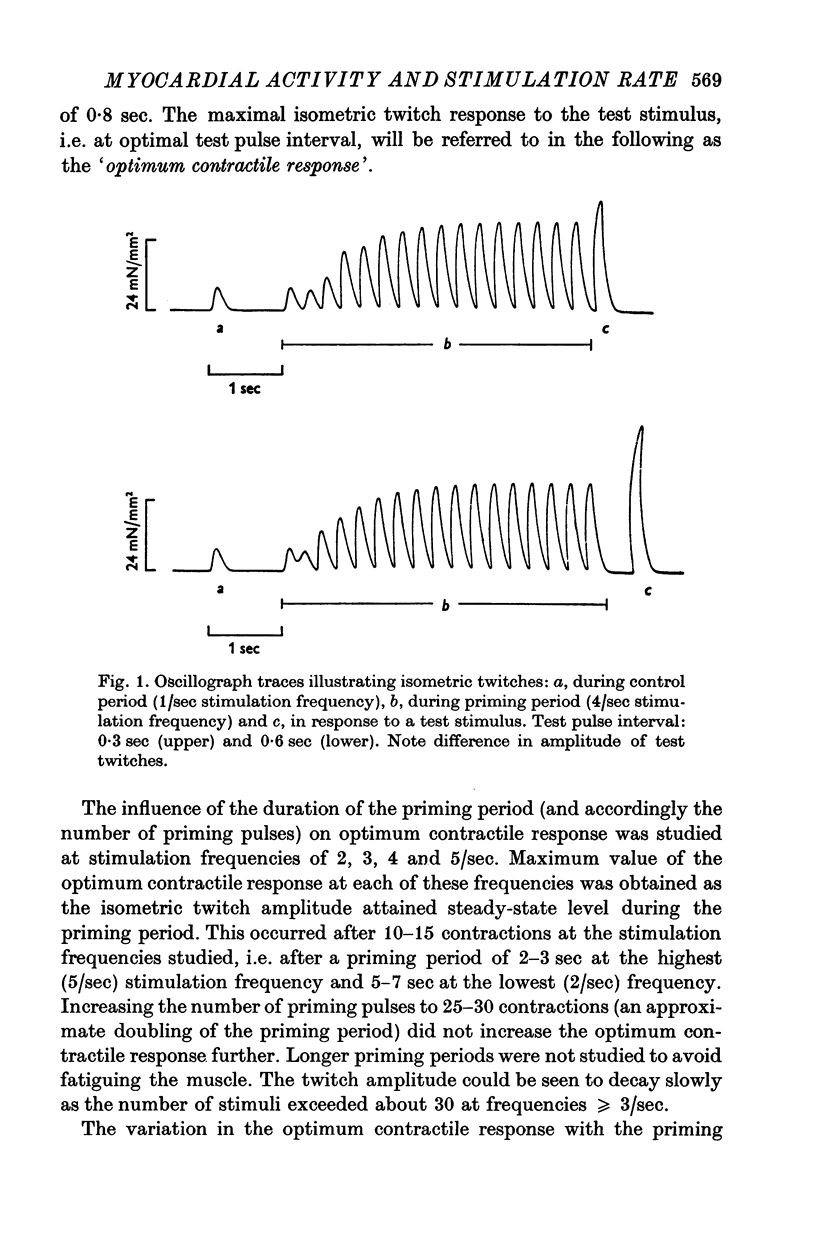
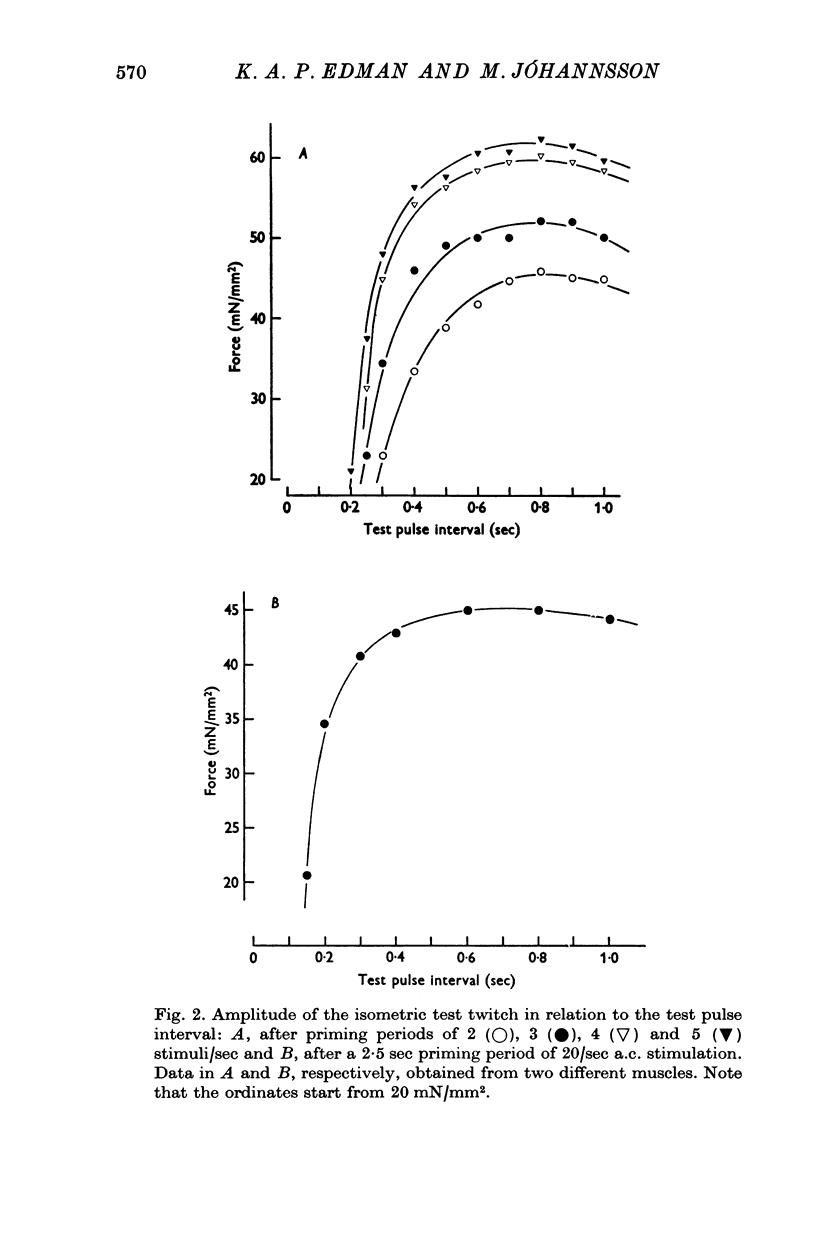
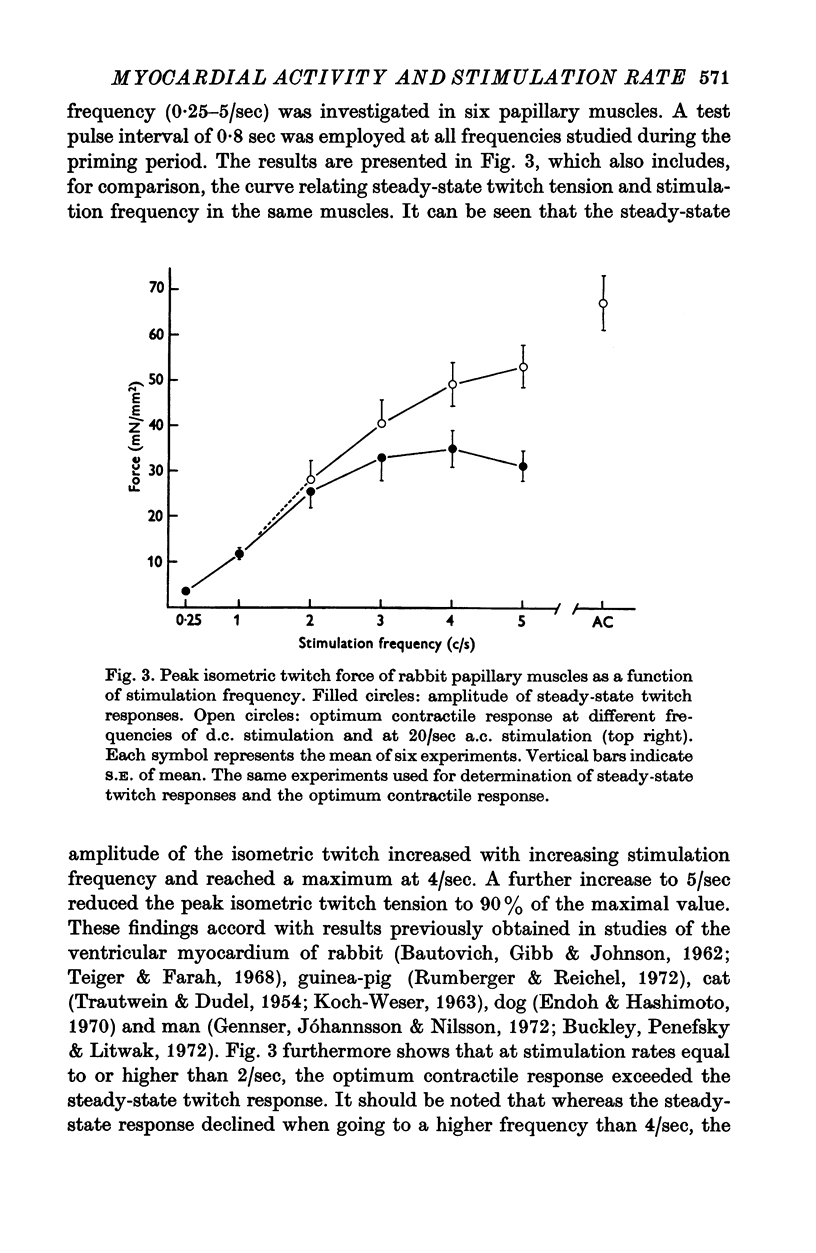
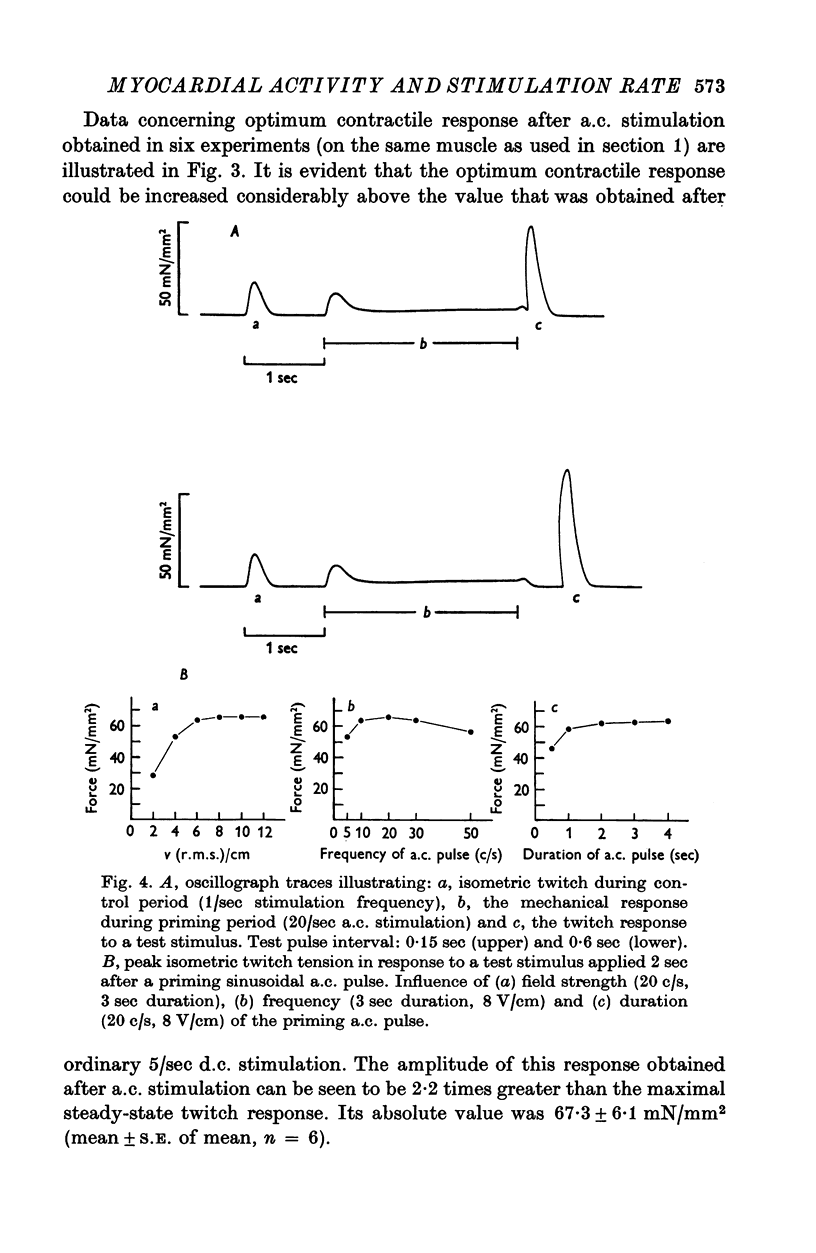
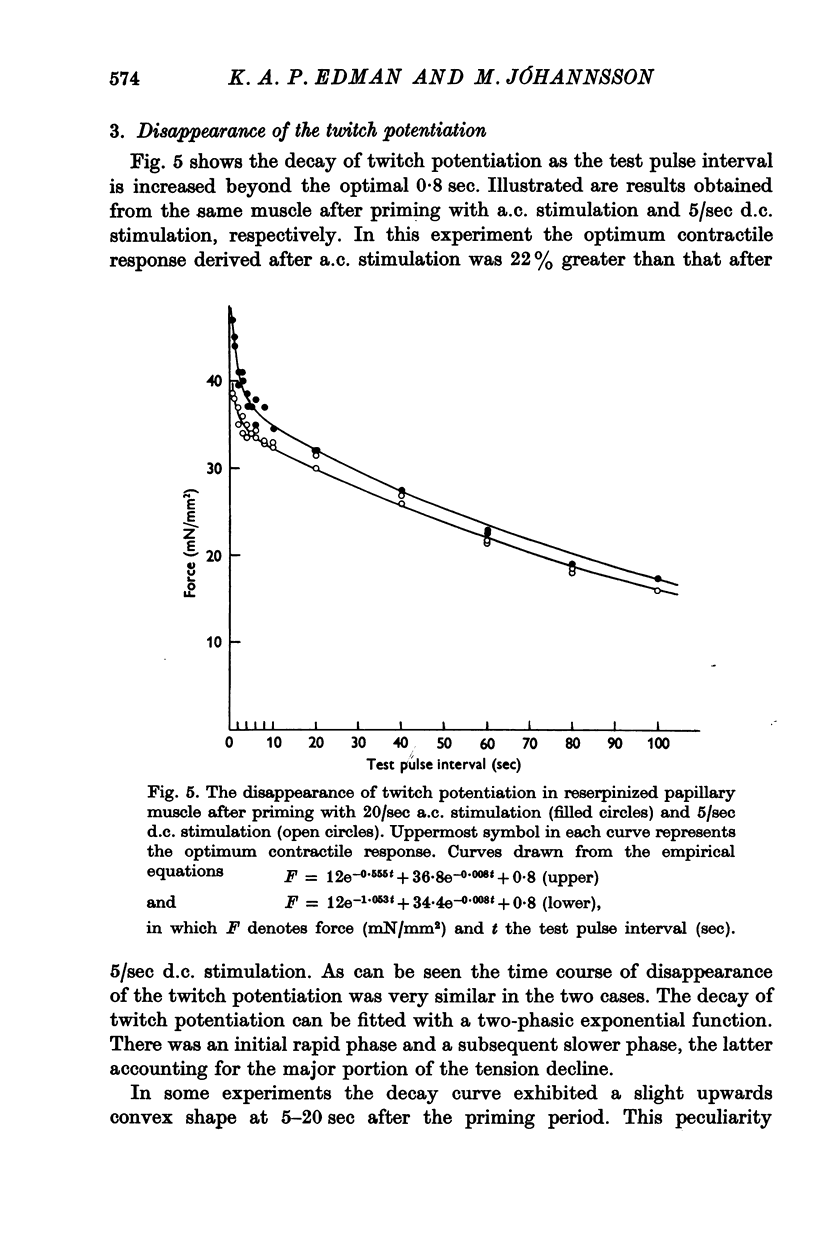
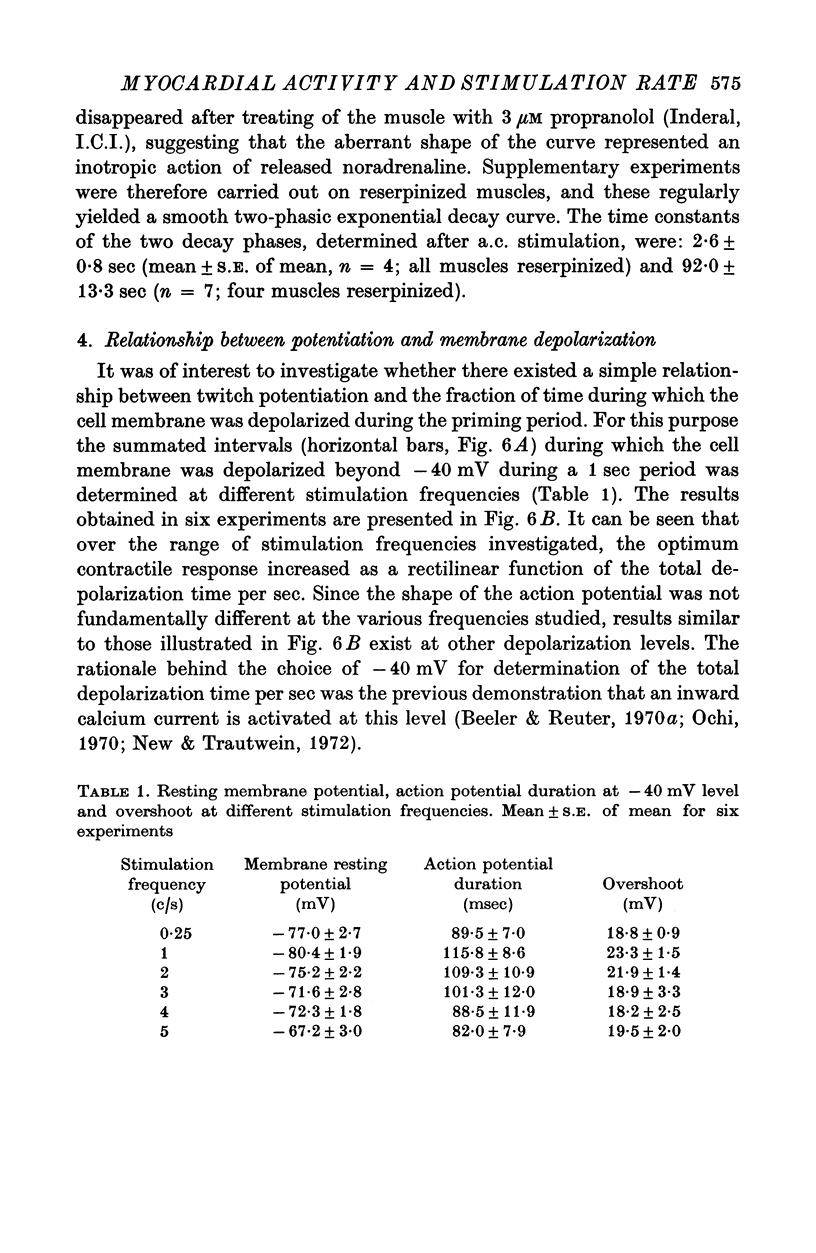
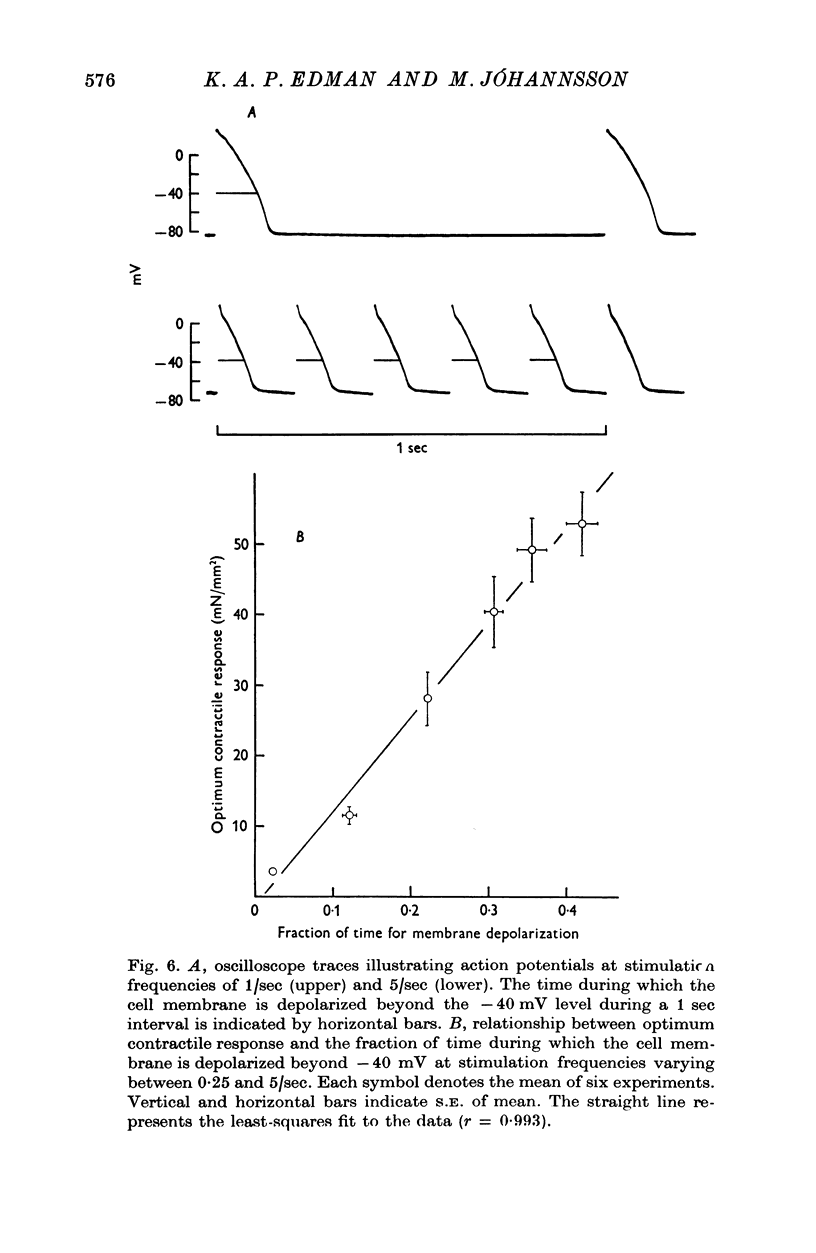
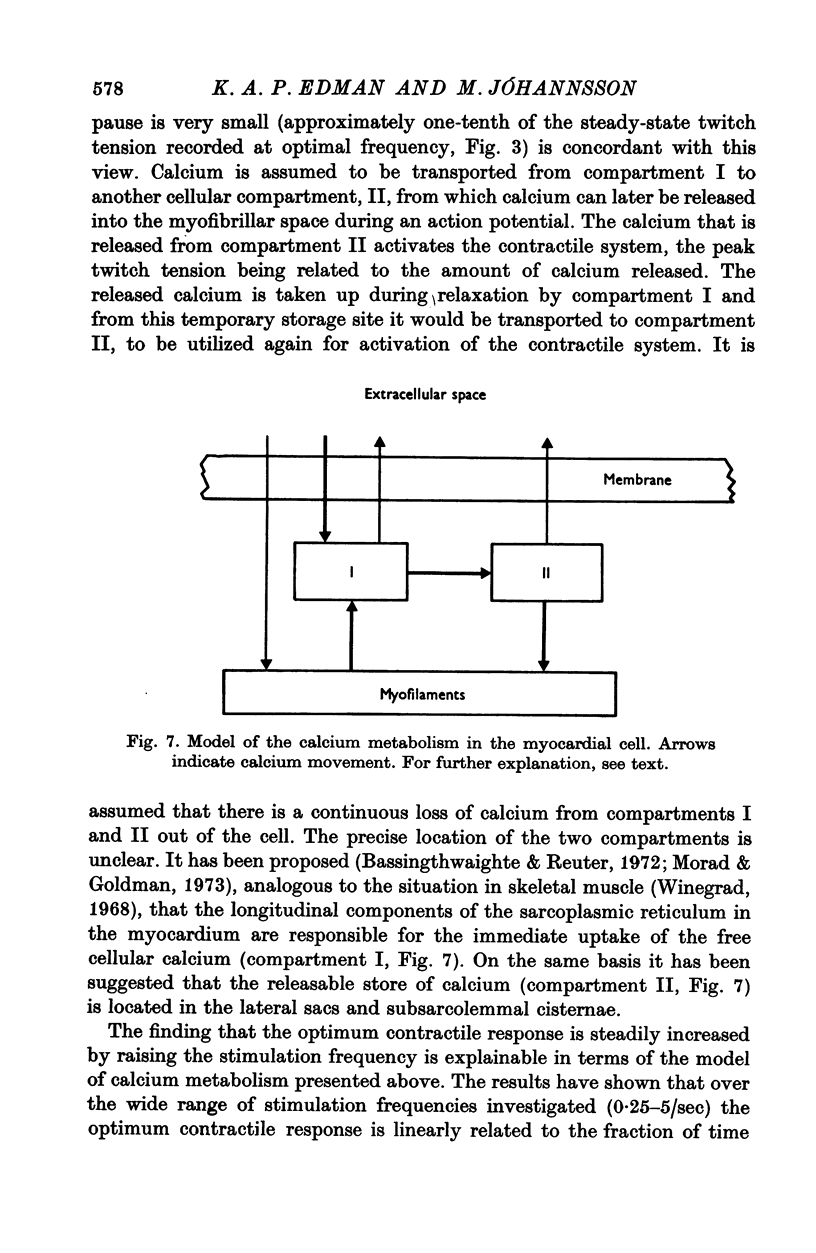
1. The relationship between active force and stimulation frequency (0-25-5/sec) was studied at 36-37 degrees C in isolated papillary muscles of the rabbit. 2. The muscle's force producing capability at a given frequency was determined as the isometric twitch response to a test stimulus that was applied at various times after a priming period. The optimum contractile response was obtained at an interval of 0-8 sec between the test pulse and the last stimulus of the priming period. 3. The optimum contractile response exceeded the steady-state twitch amplitude at all stimulation frequencies higher than 1/sec. While the steady-state twitch resonse declined at frequencies higher than 4/sec, the optimum contractile response was steadily increased as the stimulation frequency was raised. 4. The optimum contractile response was also determined after priming the muscle with a sinusoidal a.c. pulse (field strength, 10 V (r.m.s.)/cm; frequency, 20 c/s; duration, 2-5 sec). The optimum contractile response obtained after a.c. stimulation was 2-2 times greater than the maximal steady-state response. Its absolute value was 67-3+/-6-1 mN/mm2 (mean +/-S.E. of mean, n = 6). 5. The twitch potentiation produced by priming the muscle at a given frequency decayed exponentially in two phases after optimum contractile response had been attained. The time constants of the two phases, determined after a.c. stimulation, were 2-6+/-0-8 (n = 4) and 92-0+/-13-3 sec (n = 7), respectively. 6. The optimum contractile response determined at various stimulation frequencies was linearly related to the fraction of time during which the cell membrane was depolarized (beyond -40 mV) by the action potentials. 7. The results are interpreted in terms of a two-component model of the metabolism of activator calcium in the excitation-contraction coupling.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUTOVICH G., GIBB D. B., JOHNSON E. A. The force of contraction of the rabbit papillary muscle preparation as a function of the frequency and pattern of stimulation. Aust J Exp Biol Med Sci. 1962 Dec;40:455–472. doi: 10.1038/icb.1962.50. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. The relation between membrane potential, membrane currents and activation of contraction in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):211–229. doi: 10.1113/jphysiol.1970.sp009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley N. M., Penefsky Z. J., Litwak R. S. Comparative force-frequency relationships in human and other mammalian ventricular myocardium. Pflugers Arch. 1972;332(4):259–270. doi: 10.1007/BF00588574. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Nilsson E. The mechanical parameters of myocardial contraction studied at a constant length of the contractile element. Acta Physiol Scand. 1968 Jan-Feb;72(1):205–219. doi: 10.1111/j.1748-1716.1968.tb03843.x. [DOI] [PubMed] [Google Scholar]
- Endo M., Hashimoto K. Frequency-force relationship in the blood-perfused canine papillary muscle preparation. Jpn J Physiol. 1970 Jun 15;20(3):320–331. doi: 10.2170/jjphysiol.20.320. [DOI] [PubMed] [Google Scholar]
- Gennser G., Jóhannsson M., Nilsson E. The influence of contraction frequency and of a local anesthetic (Mepivacaine) on human fetal myocardium. Acta Physiol Scand. 1972 Aug;85(4):559–568. doi: 10.1111/j.1748-1716.1971.tb05294.x. [DOI] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. Voltage dependence and time dependence of contraction in sheep cardiac Purkinje fibers. Circ Res. 1971 Apr;28(4):446–460. doi: 10.1161/01.res.28.4.446. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmer N. G., Lindström K. An electrometer amplifier with low input capacitance and large input dynamic range. IEEE Trans Biomed Eng. 1972 Mar;19(2):162–164. doi: 10.1109/TBME.1972.324061. [DOI] [PubMed] [Google Scholar]
- Jewell B. R., Rovell J. M. Influence of previous mechanical events on the contractility of isolated cat papillary muscle. J Physiol. 1973 Dec;235(3):715–740. doi: 10.1113/jphysiol.1973.sp010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH-WESER J., BLINKS J. R. THE INFLUENCE OF THE INTERVAL BETWEEN BEATS ON MYOCARDIAL CONTRACTILITY. Pharmacol Rev. 1963 Sep;15:601–652. [PubMed] [Google Scholar]
- KOCH-WESER J. Effect of rate changes on strength and time course of contraction of papillary muscle. Am J Physiol. 1963 Mar;204:451–457. doi: 10.1152/ajplegacy.1963.204.3.451. [DOI] [PubMed] [Google Scholar]
- LANGER G. A. CALCIUM EXCHANGE IN DOG VENTRICULAR MUSCLE: RELATION TO FREQUENCY OF CONTRACTION AND MAINTENANCE OF CONTRACTILITY. Circ Res. 1965 Jul;17:78–89. doi: 10.1161/01.res.17.1.78. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Ion fluxes in cardiac excitation and contraction and their relation to myocardial contractility. Physiol Rev. 1968 Oct;48(4):708–757. doi: 10.1152/physrev.1968.48.4.708. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Mainwood G. W., Korecky B. The electrical and mechanical response of rat papillary muscle to paired pulse stimulation. Can J Physiol Pharmacol. 1970 Apr;48(4):216–225. doi: 10.1139/y70-039. [DOI] [PubMed] [Google Scholar]
- New W., Trautwein W. The ionic nature of slow inward current and its relation to contraction. Pflugers Arch. 1972;334(1):24–38. doi: 10.1007/BF00585998. [DOI] [PubMed] [Google Scholar]
- Ochi R. The slow inward current and the action of manganese ions in guinea-pig's myocardium. Pflugers Arch. 1970;316(1):81–94. doi: 10.1007/BF00587898. [DOI] [PubMed] [Google Scholar]
- Rumberger E., Reichel H. The force-frequency relationship: a comparative study between warm- and cold-blooded animals. Pflugers Arch. 1972;332(3):206–217. [PubMed] [Google Scholar]
- STEN-KNUDSEN O. Is muscle contraction initiated by internal current flow? J Physiol. 1960 May;151:363–384. doi: 10.1113/jphysiol.1960.sp006444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands S. D., Winegrad S. Treppe and total calcium content of the frog ventricle. Am J Physiol. 1970 Mar;218(3):908–910. doi: 10.1152/ajplegacy.1970.218.3.908. [DOI] [PubMed] [Google Scholar]
- Spilker B., Cervoni P. Electrical membrane activity and frequency-force relations in normal, denervated and reserpine-pretreated cat heart muscle. J Pharmacol Exp Ther. 1969 Jul;168(1):60–69. [PubMed] [Google Scholar]
- TRAUTWEIN W., DUDEL J. Aktionspotential und Mechanogramm des Warmblüterherzmuskels als Funktion der Schlagfrequenz. Pflugers Arch. 1954;260(1):24–39. doi: 10.1007/BF00363777. [DOI] [PubMed] [Google Scholar]
- Teiger D. G., Farah A. The effect of calcium and sodium ion on the frequency-force relationship in cardiac muscle. J Pharmacol Exp Ther. 1968 Nov;164(1):1–9. [PubMed] [Google Scholar]
- Tritthart H., Kaufmann R., Volkmer H. P., Bayer R., Krause H. Ca-movement controlling myocardial contractility. I. Voltage-, current- and time-dependence of mechanical activity under voltage clamp conditions (cat papillary muscles and trabeculae). Pflugers Arch. 1973 Feb 6;338(3):207–231. doi: 10.1007/BF00587388. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Intracellular calcium movements of frog skeletal muscle during recovery from tetanus. J Gen Physiol. 1968 Jan;51(1):65–83. doi: 10.1085/jgp.51.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. H., Heppner R. L., Weidmann S. Inotropic effects of electric currents. I. Positive and negative effects of constant electric currents or current pulses applied during cardiac action potentials. II. Hypotheses: calcium movements, excitation-contraction coupling and inotropic effects. Circ Res. 1969 Mar;24(3):409–445. doi: 10.1161/01.res.24.3.409. [DOI] [PubMed] [Google Scholar]


