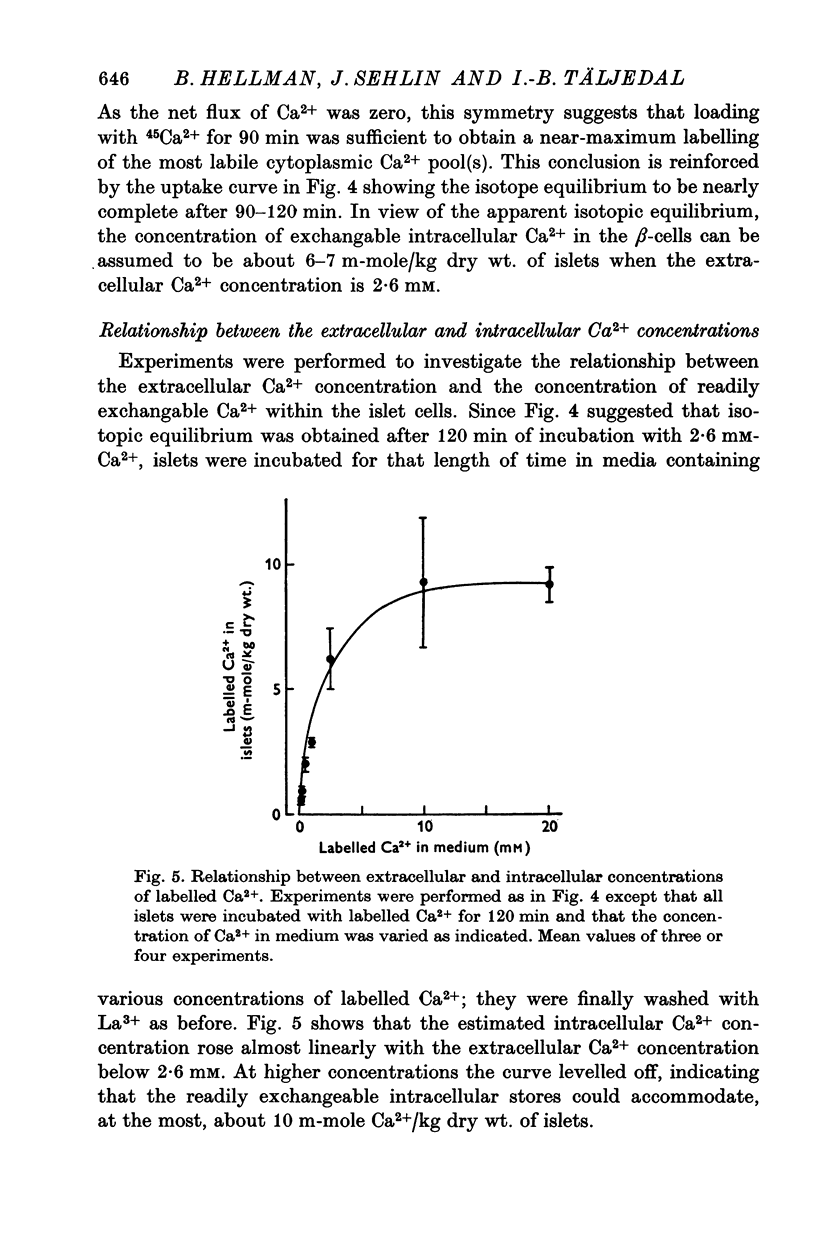
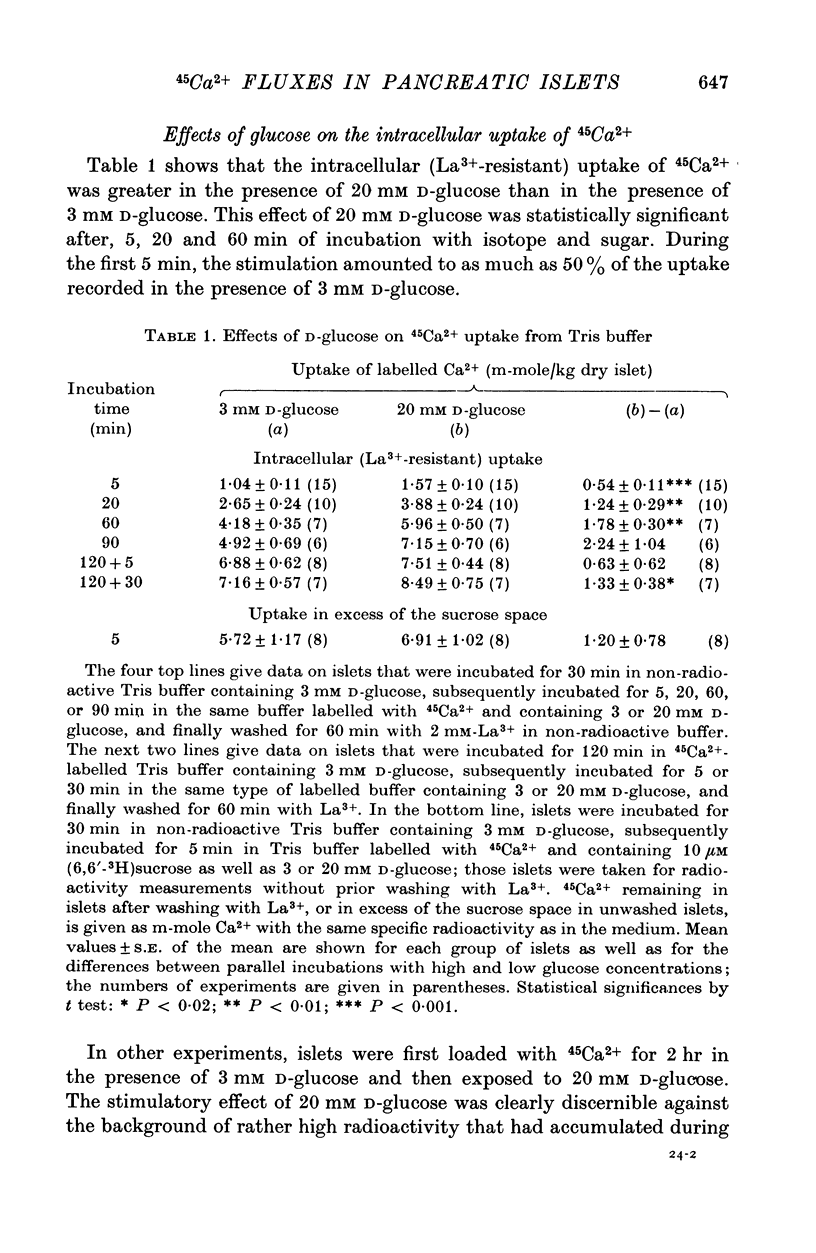
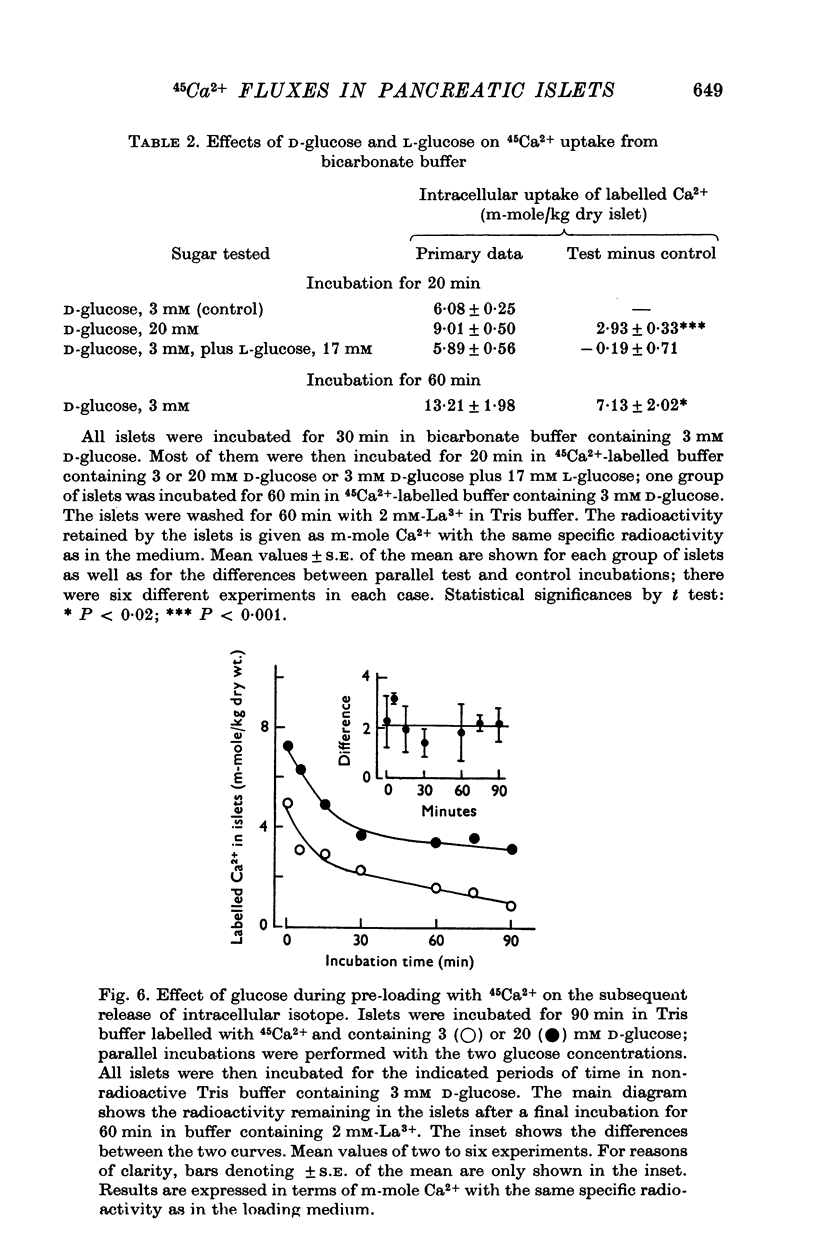
Abstract
1. Fluxes of 45Ca2+ were studied in pancreatic islets from non-inbred ob/ob-mice. Because La3+ blocked the transmembrane fluxes of 45Ca2+ in islet cells, incubations aimed at measuring glucose-induced changes of the intracellular Ca2+ were ended by washing the islets with 2 mM-La3+ for 60 min. 2. Uptake of 45Ca2+ progressed for 2 hr; the intracellular concentration of exchangable Ca2+ was about 7 m-mole/kg dry wt., as estimated from the isotope distribution at apparent equilibrium in islets exposed to 3 mM D-glucose. Raising the D-glucose concentration to 20 mM enhanced the 45 Ca2+ uptake whether or not the islets had first been equilibrated with the isotope. The stimulatory effect of D-glucose was observed in Tris buffer containing no anions but Cl- as well as in polyanionic bicarbonate buffer. The effect could not be reproduced with equimolar L-glucose. 3. The rate of 45Ca2+ release was the same whether the islets had been pre-loaded in the presence of 3 or 20 mM D-glucose. Thus the 45Ca2+ that had been taken up in response to 20 mM D-glucose appeared to be released much more slowly than the bulk of intracellular 45Ca2+. The release of 45Ca2+ was not significantly influenced by D-glucose during the release period. Incubation for 30 min was require for half of the radioactivity to be released. 4. The rates of insulin secretion were about the same in uni-anionic Tris buffer as in polyanionic bicarbonate buffer. A marked insulin secretory response to 20 mM D-glucose was observed in either buffer. 5. It is concluded that 20 mM D-glucose causes a net uptake of Ca2+ from the extracellular fluid into the interior of the beta-cells. This uptake is probably not regulated at the level of the plasma membrane but more likely reflects an increased affinity of some intracellular phase or compartment for the ion. Because the observed uptake and release of intracellular 45Ca2+ are slow processes in comparison with the rapid effects of extracellular Ca2+ on insulin secretion, insulin secretion may also depend on a more superficial and La3+-displacable Ca2+ pool.
Full text
PDF


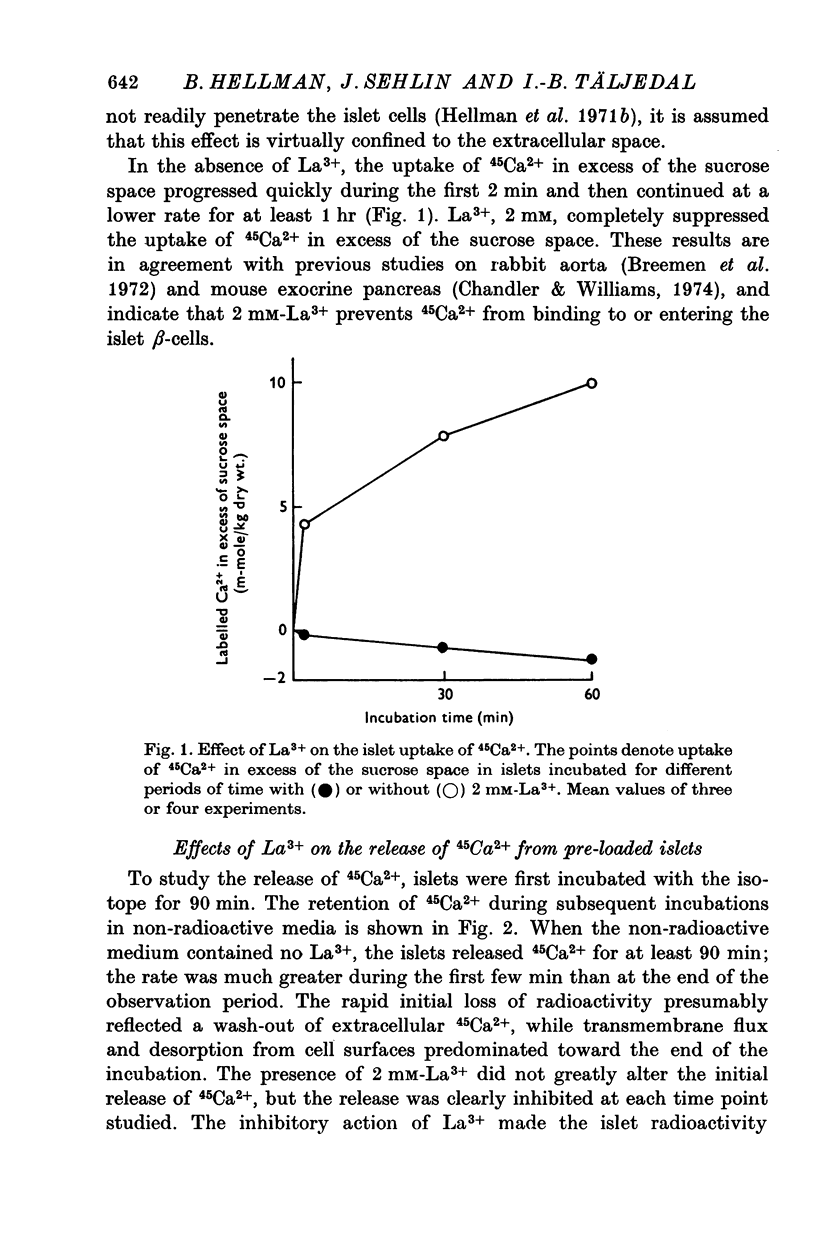
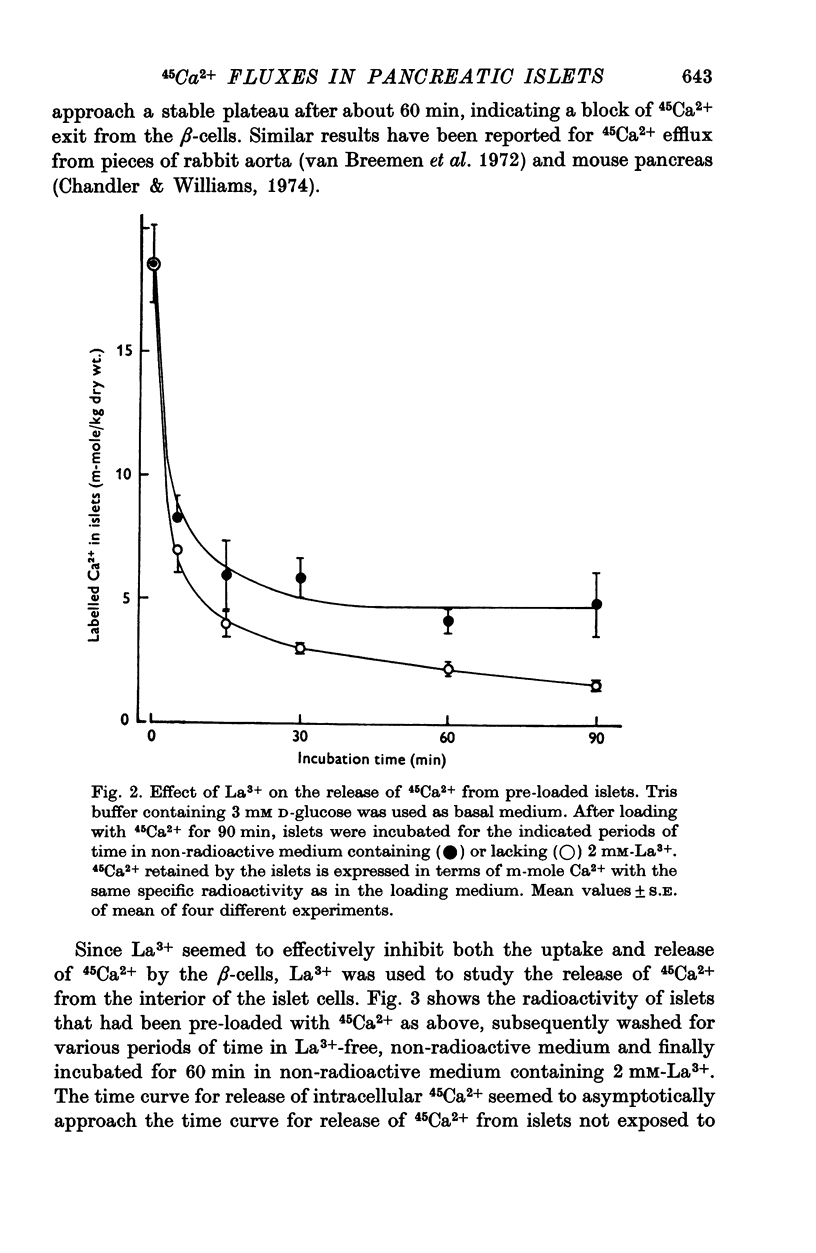
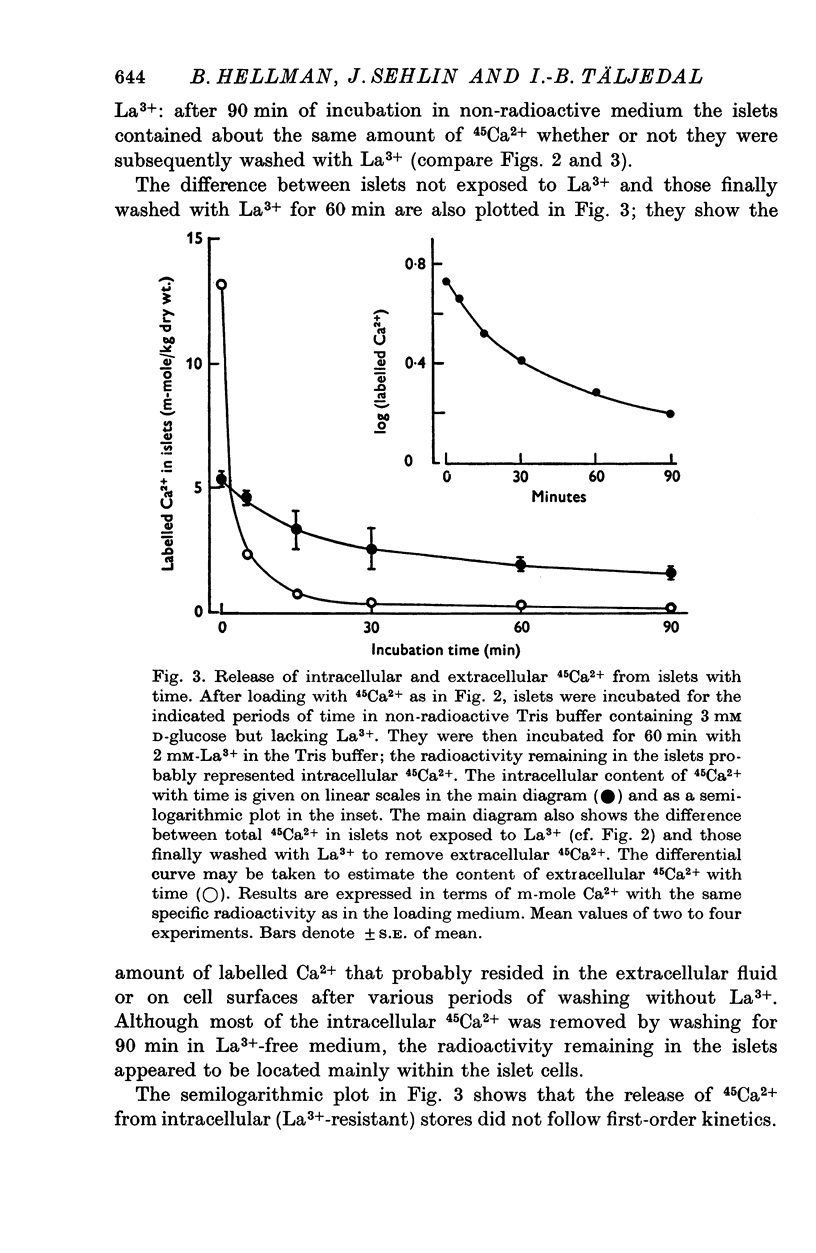
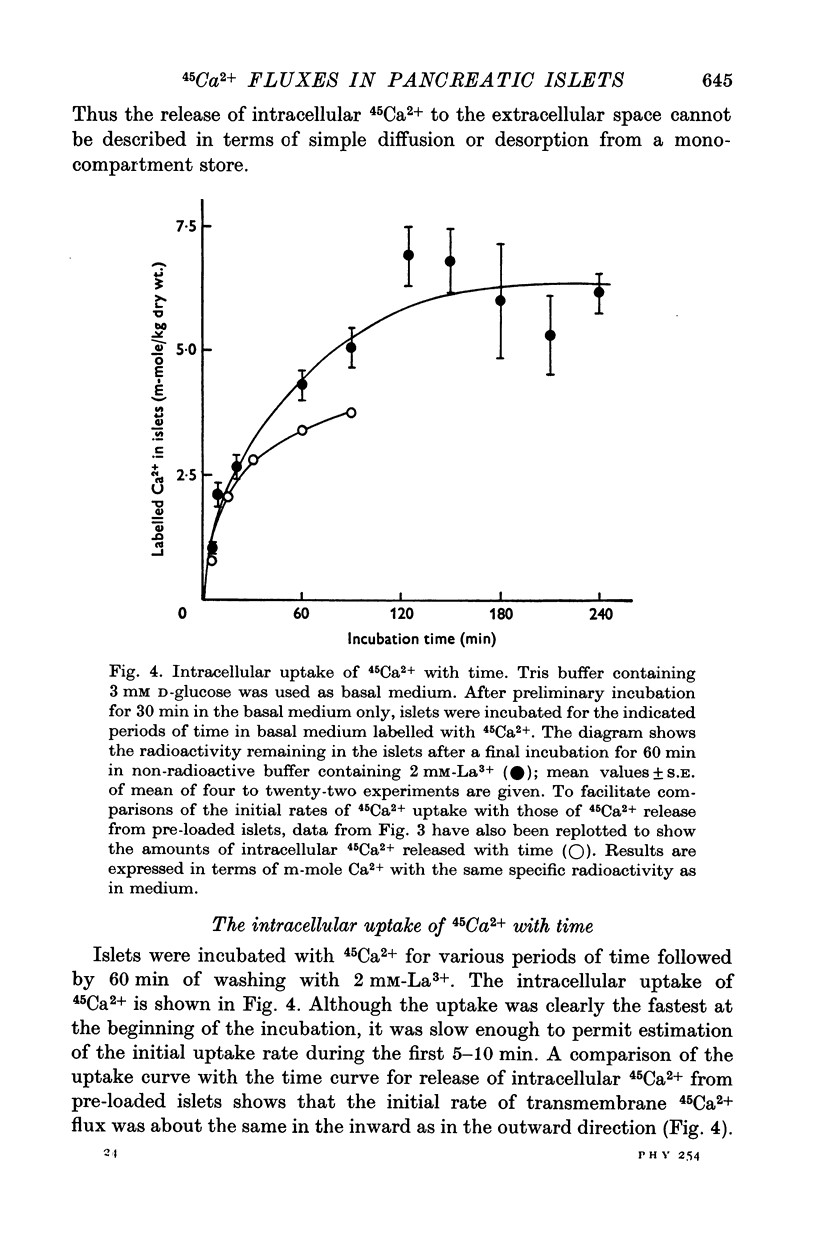








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler D. E., Williams J. A. Pancreatic acinar cells: effects of lanthanum ions on amylase release and calcium ion fluxes. J Physiol. 1974 Dec;243(3):831–846. doi: 10.1113/jphysiol.1974.sp010779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970 Sep;210(2):265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M. A threshold distribution hypothesis for packet storage of insulin. II. Effect of calcium. Diabetes. 1972;21(2 Suppl):584–593. doi: 10.2337/diab.21.2.s584. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Täljedal I. B. Iodoacetamide-induced sensitization of the pancreatic beta-cells to glucose stimulation. Biochem J. 1973 Apr;132(4):775–789. doi: 10.1042/bj1320775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Täljedal I. B. Role of thiol groups in insulin release: studies with poorly permeating disulphides. Mol Pharmacol. 1973 Nov;9(6):792–801. [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Calcium uptake by pancreatic -cells as measured with the aid of 45 Ca and mannitol- 3 H. Am J Physiol. 1971 Dec;221(6):1795–1801. doi: 10.1152/ajplegacy.1971.221.6.1795. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Transport of -aminoisobutyric acid in mammalian pancretic -cells. Diabetologia. 1971 Aug;7(4):256–265. doi: 10.1007/BF01211878. [DOI] [PubMed] [Google Scholar]
- Herman L., Sato T., Hales C. N. The electron microscopic localization of cations to pancreatic islets of Langerhans and their possible tole in insulin secretion. J Ultrastruct Res. 1973 Feb;42(3):298–311. doi: 10.1016/s0022-5320(73)90058-0. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. 3. Uptake of 45 calcium by isolated islets of Langerhans. Endocrinology. 1971 Jan;88(1):72–80. doi: 10.1210/endo-88-1-72. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Brisson G. R., Baird L. E. Stimulus-secretion coupling of glucose-induced insulin release. X. Effect of glucose on 45 Ca efflux from perifused islets. Am J Physiol. 1973 Feb;224(2):389–394. doi: 10.1152/ajplegacy.1973.224.2.389. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J. Insulin secretion: multifactorial regulation for a single process of release. The Minkowski award lecture delivered on September 7, 1972 before the European Association for the study of Diabetes at Madrid, Spain. Diabetologia. 1973 Jun;9(3):167–173. doi: 10.1007/BF01219778. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Sakamoto Y. Electrical characteristics of pancreatic islet cells. J Physiol. 1975 Mar;246(2):421–437. doi: 10.1113/jphysiol.1975.sp010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Schäfer H. J., Klöppel G. The significance of calcium in insulin secretion. Ultrastructural studies on identification and localization of calcium in activated and inactivated B cells of mice. Virchows Arch A Pathol Anat Histol. 1974;362(3):231–245. doi: 10.1007/BF00432197. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]


