Abstract
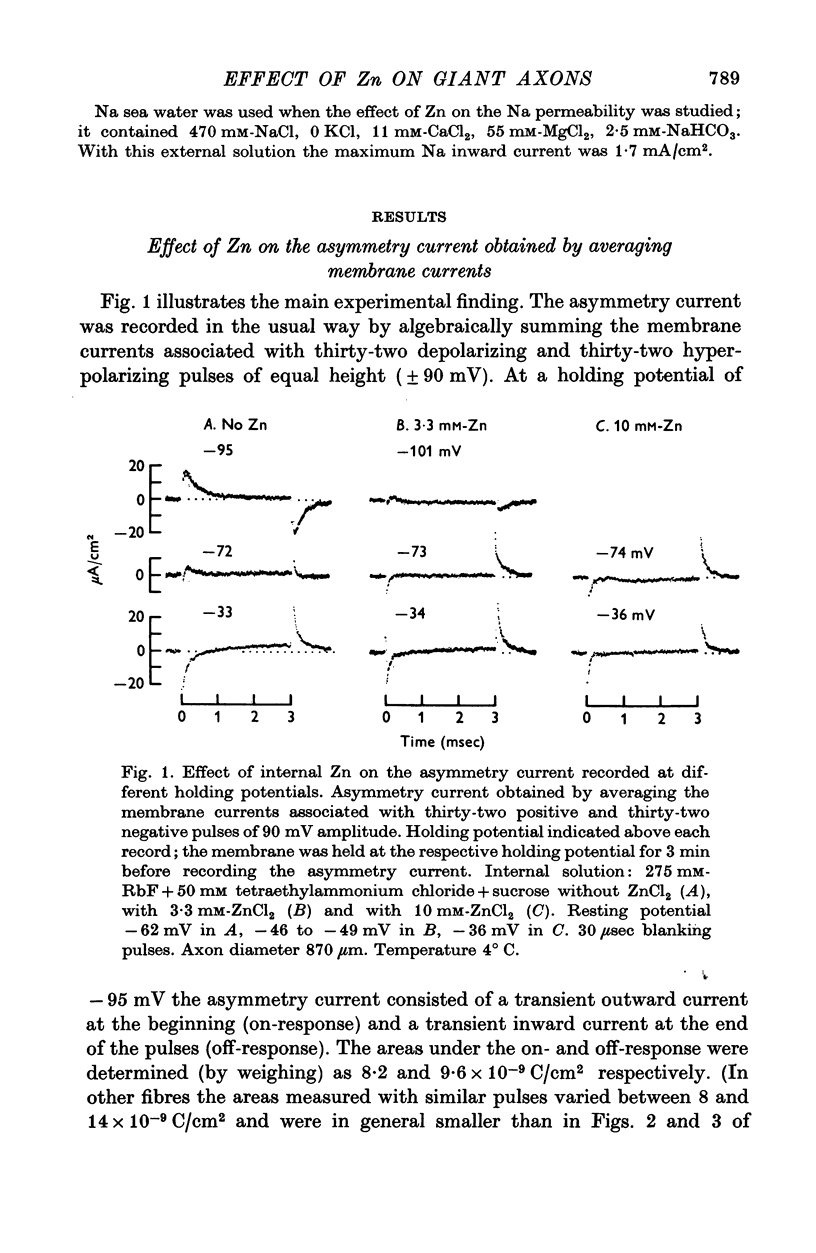
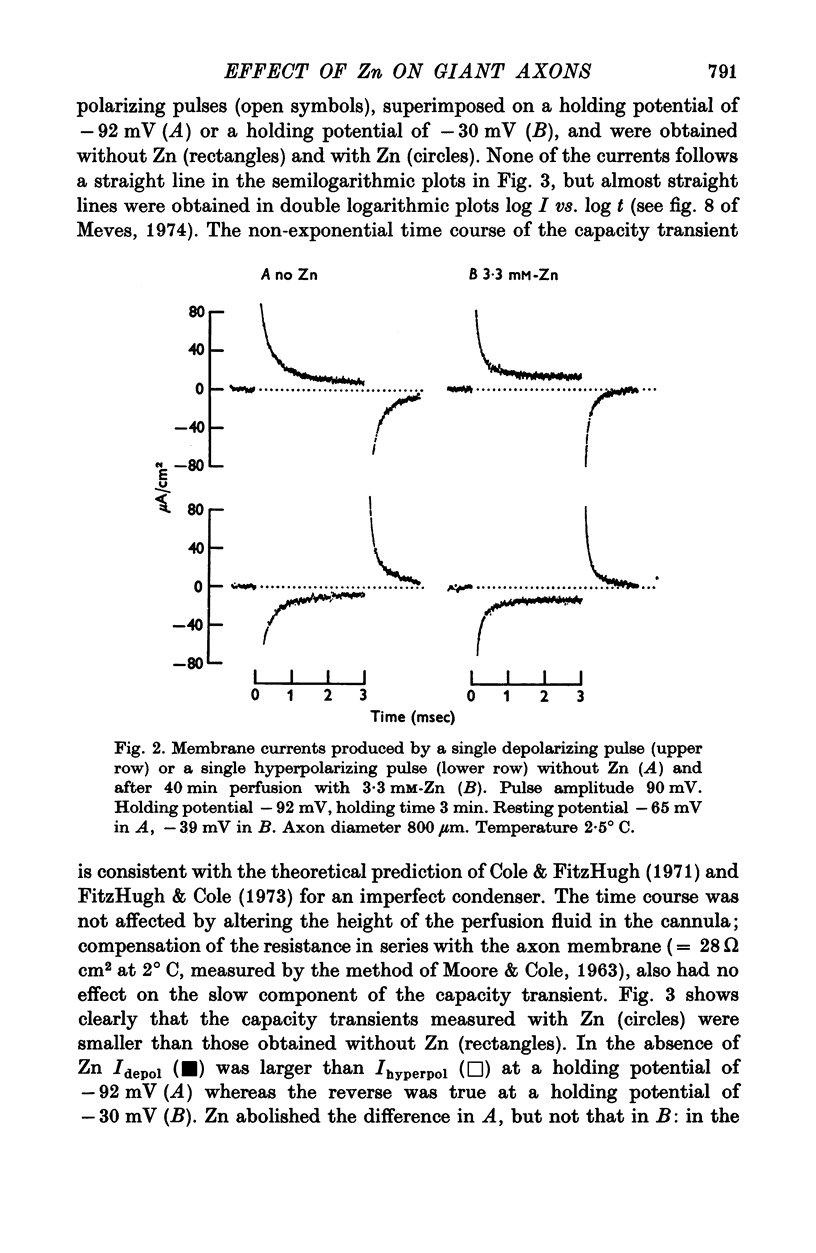
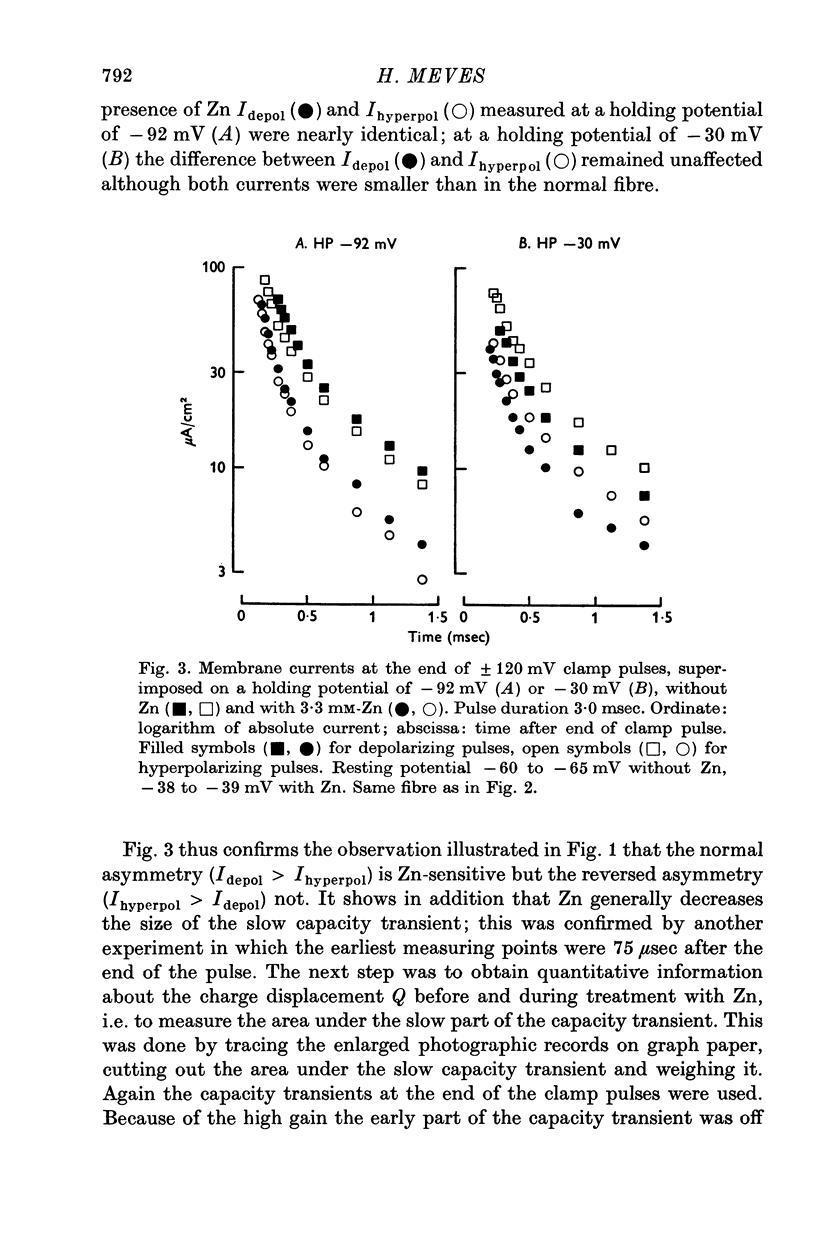
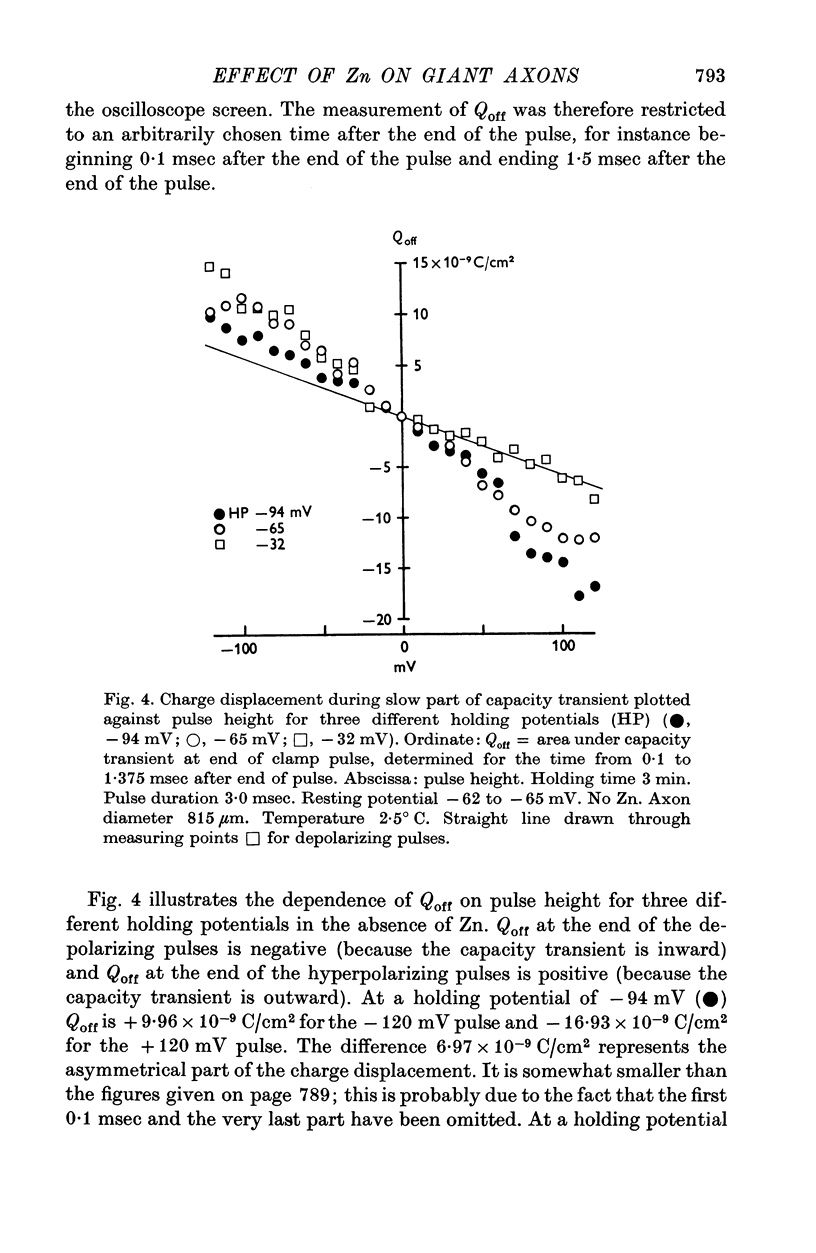
1. Displacement currents produced by single depolarizing or hyperpolarizing voltage-clamp pulses (Idepol and Ihyperpol) were recorded from intracellularly perfused squid giant axons treated with tetrodotoxin and tetraethylammonium chloride. The effect of internal Zn on the slow part of the displacement current was studied at different holding potentials. 2. Internal Zn in a concentration of 3-3 mM markedly reduced the slow charge displacement associated with depolarizing and hyperpolarizing pulses. 3. At a holding potential more negative than -60 mV Idepol is normally larger than Ihyperpol if measured with pulses of equal height. The asymmetry Idepol greater than Ihyperpol (which possibly reflects the movement of gating charges) was abolished by Zn. 4. The reversed asymmetry Ihyperpol greater than Idepol which is normally seen at holding potentials less negative than -60 mV was not blocked by Zn. This suggests that the underlying mechanism is different from that of the asymmetry Idepol greater than Ihyperpol. 5. The Zn-sensitive slow charge displacement during single depolarizing pulses was strongly reduced by lowering the holding potential from about -90 to about -30 mV. 6. The observations with single clamp pulses were confirmed by averaging and summing the currents associated with an equal number of depolarizing and hyperpolarizing pulses. 7. The effect of internal Zn on the charge displacement is thought to be due to a reaction with mobile charges in the membrane dielectric. Internal Zn in a concetration of 0-5-1 mM did not significantly shift the Na inactivation curve, indicating that it does not react with surface charges at the inner side of the membrane.
Full text
PDF


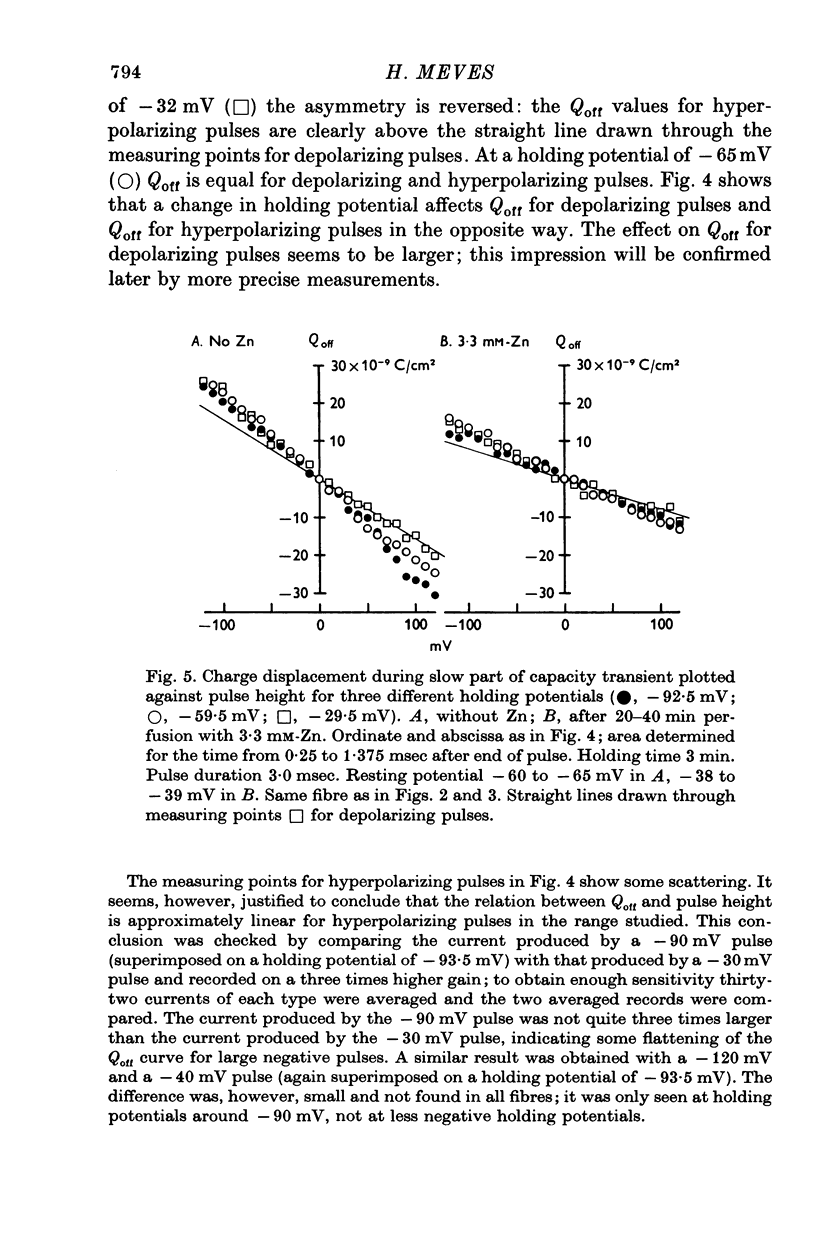

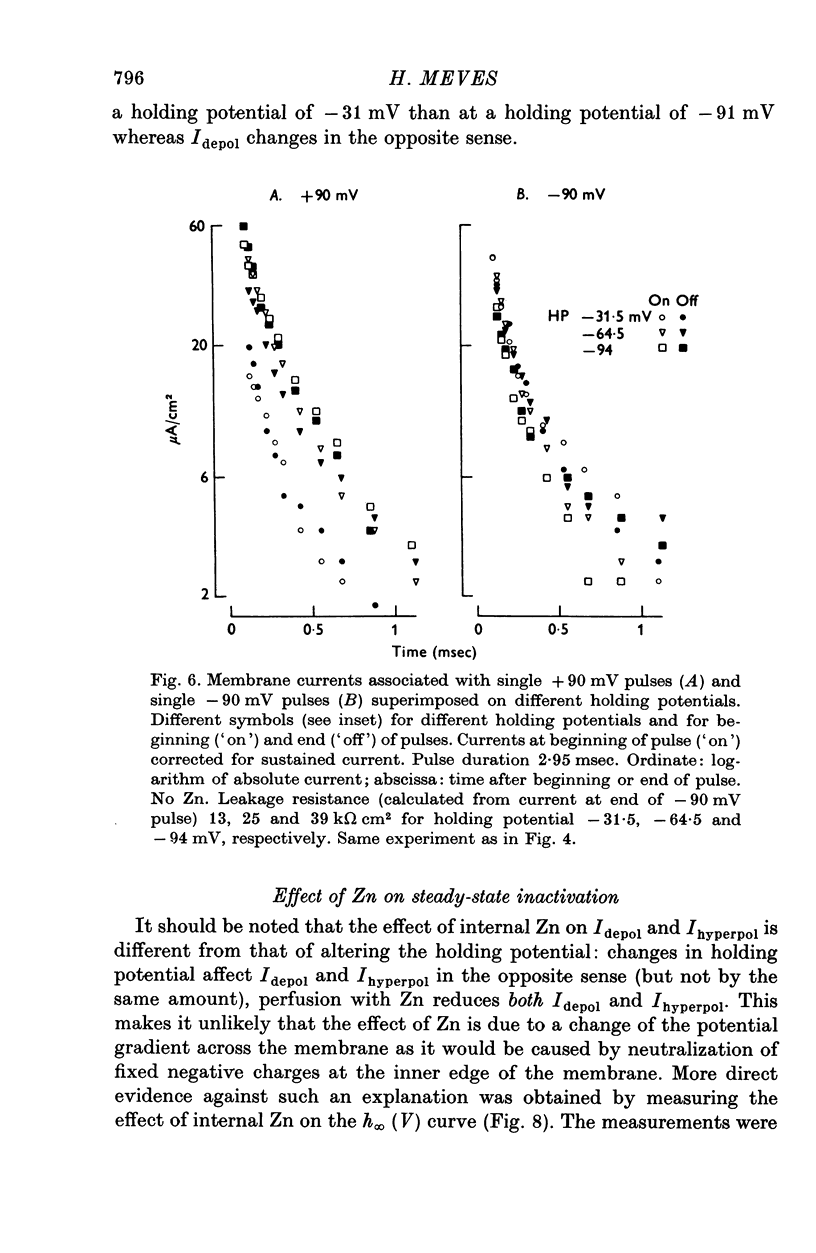
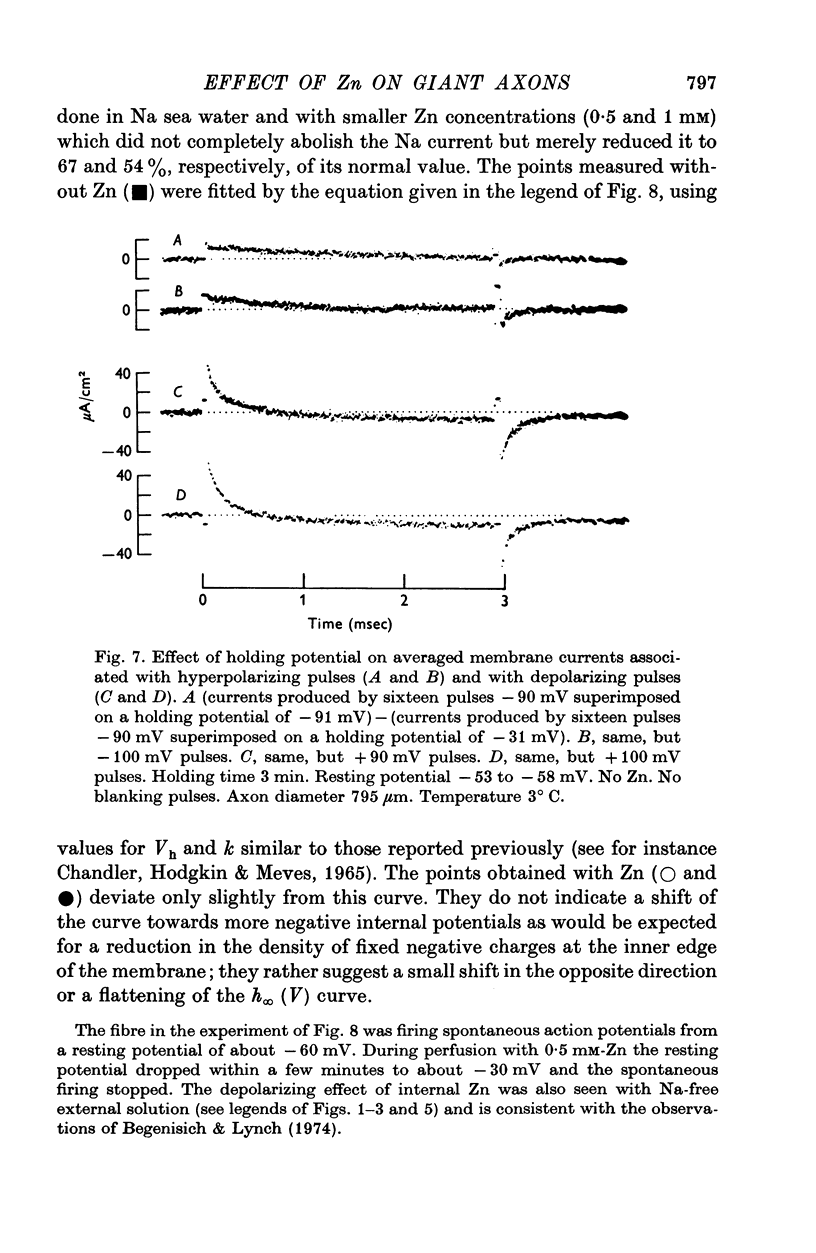
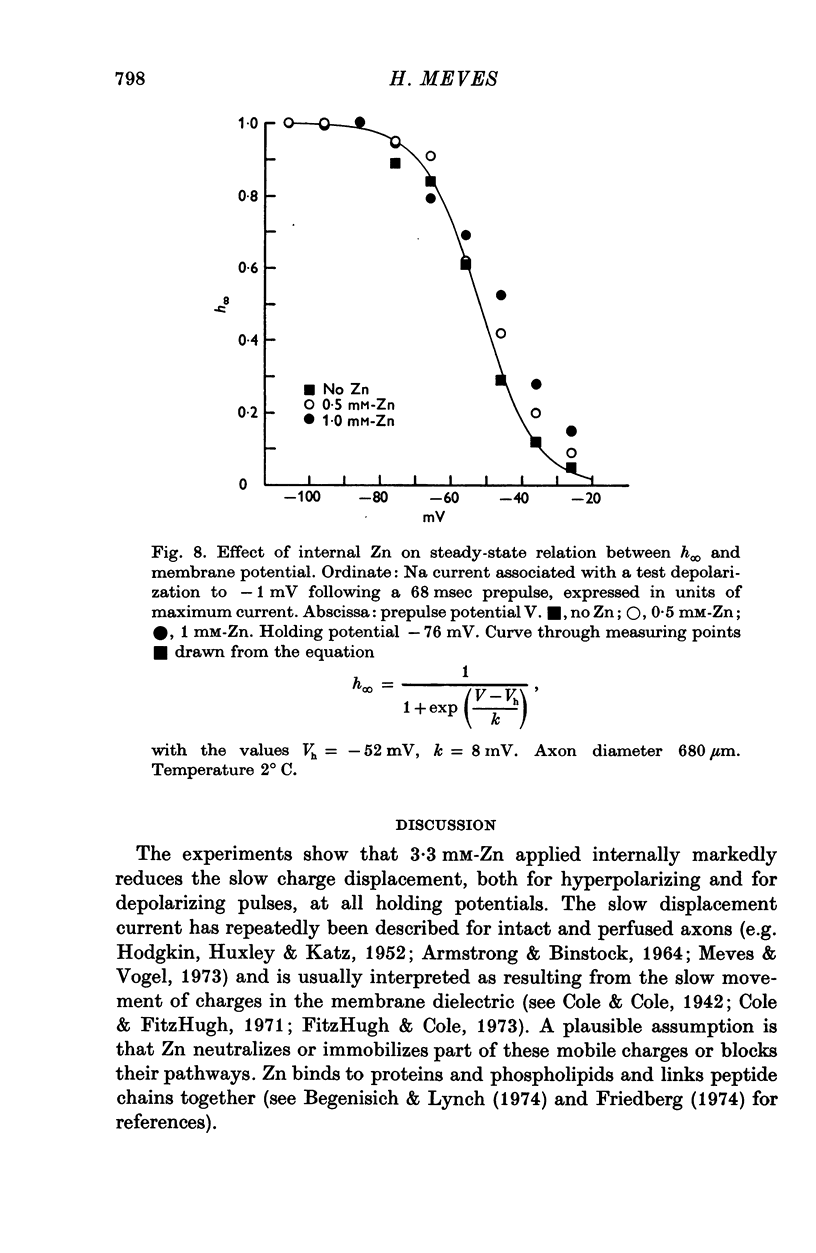



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. THE EFFECTS OF SEVERAL ALCOHOLS ON THE PROPERTIES OF THE SQUID GIANT AXON. J Gen Physiol. 1964 Nov;48:265–277. doi: 10.1085/jgp.48.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T., Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974 Jun;63(6):675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Gating currents of the sodium channels: three ways to block them. Science. 1974 Feb 22;183(4126):753–754. doi: 10.1126/science.183.4126.753. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. R., Strickholm A. Evidence for a conformational change in nerve membrane with depolarization. Nature. 1971 Dec 24;234(5330):470–471. doi: 10.1038/234470a0. [DOI] [PubMed] [Google Scholar]
- Fitzhugh R., Cole K. S. Voltage and current clamp transients with membrane dielectric loss. Biophys J. 1973 Nov;13(11):1125–1140. doi: 10.1016/S0006-3495(73)86050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg F. Effects of metal binding on protein structure. Q Rev Biophys. 1974 Feb;7(1):1–33. doi: 10.1017/s0033583500001335. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J Physiol. 1974 Jun;239(2):393–434. doi: 10.1113/jphysiol.1974.sp010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Rudy B. Proceedings: Demonstration of a first-order voltage-dependent transition of the sodium activation gates. J Physiol. 1974 Jun;239(2):100P–101P. [PubMed] [Google Scholar]
- Meves H. The effect of holding potential on the asymmetry currents in squid gaint axons. J Physiol. 1974 Dec;243(3):847–867. doi: 10.1113/jphysiol.1974.sp010780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H. A study of lipid bilayer membrane stability using precise measurements of specific capacitance. Biophys J. 1970 Dec;10(12):1127–1148. doi: 10.1016/S0006-3495(70)86360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


