Abstract
1. An electrophysiological investigation of efferent synapses in the retina of the turtle was conducted by recording intracellularly from amacrine cells. These cells have been selected because in birds they have been shown to have direct anatomical connexions with centrifugal fibre terminals. 2. Amacrine cells could be easily distinguished from most other retinal cells, except ganglion cells, by their different photo-responses. Because both amacrine and ganglion cells may generate action potentials they were distinguished by their responses to optic nerve stimulation. 3. The response of ganglion cells to single shock stimulation of the optic nerve consists of an antidromic action potential followed by a late synaptic potential. 4. Cells which did not show antidromic responses but were electrically excitable, by passing direct current through the recording electrode, were considered to be amacrine cells. 5. Amacrine cells generate an e.p.s.p. in response to optic nerve stimulation. An analysis of the e.p.s.p. suggests that it may be due to a single afferent fibre terminating in the proximity of the cell soma. By analogy to the bird, it is concluded that the amacrine cells e.p.s.p.s result from the activation of centrifugal fibres.
Full text
PDF


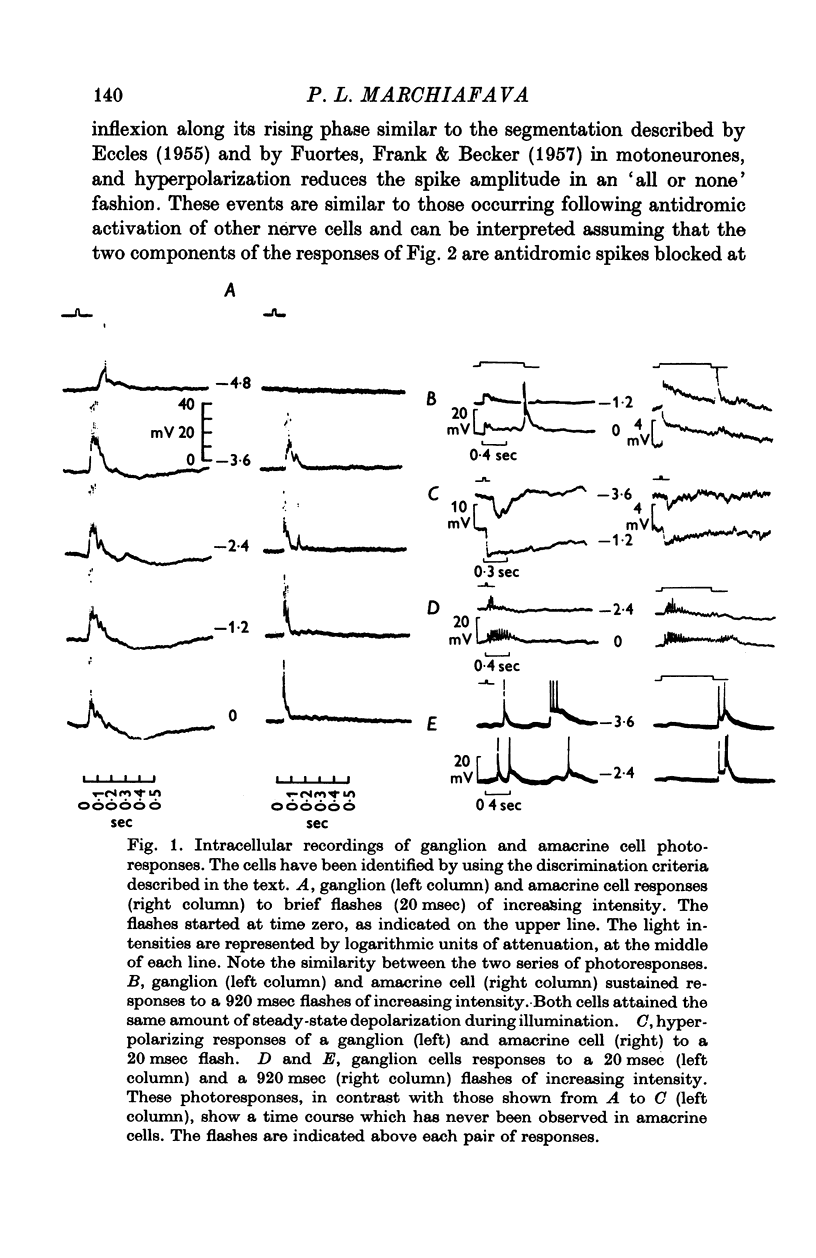
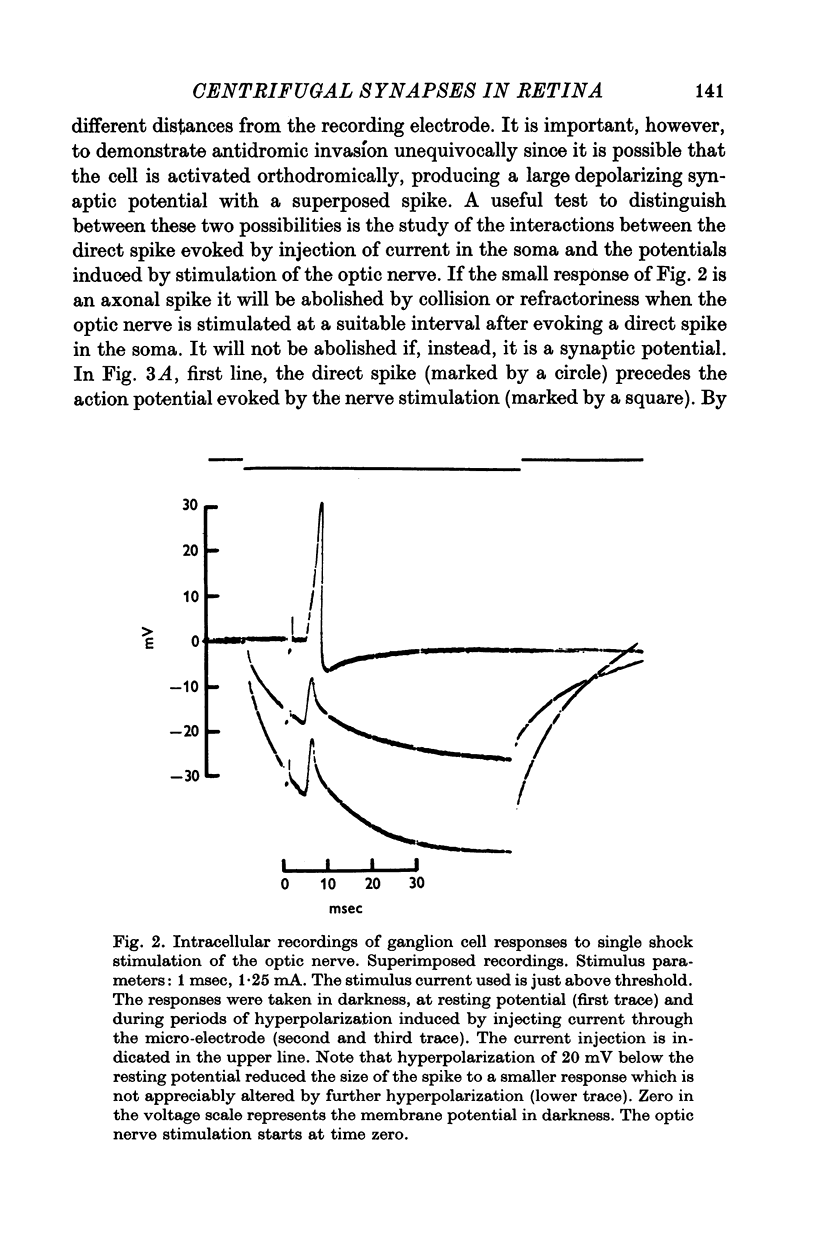
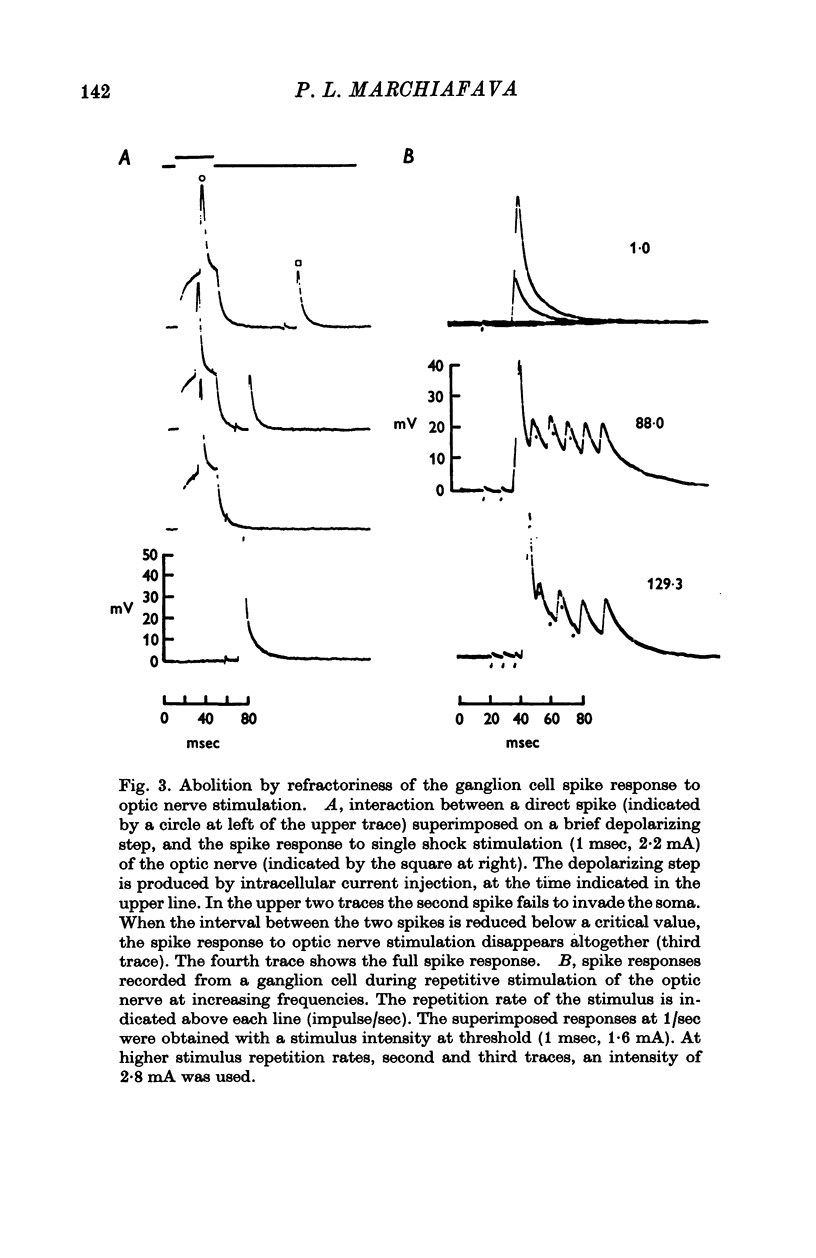

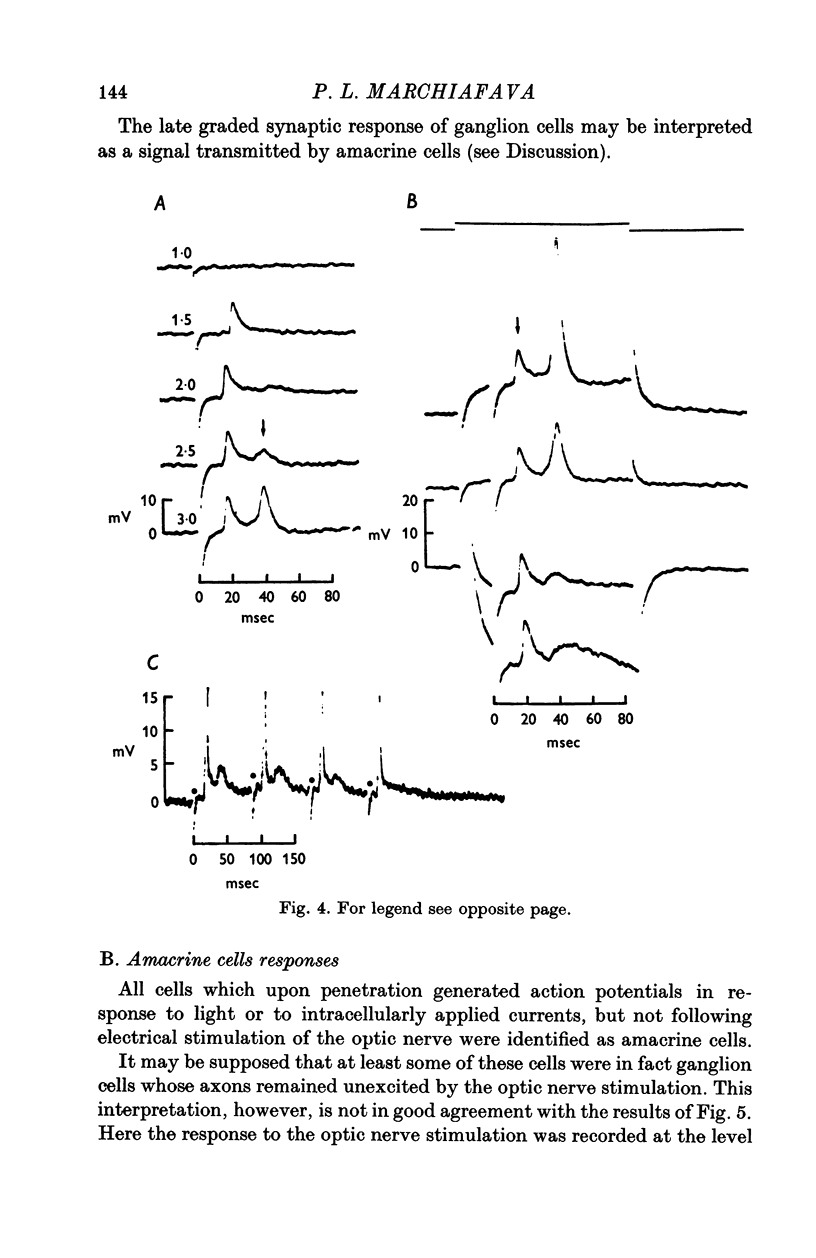






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branston N. M., Fleming D. G. Efferent fibers in the frog optic nerve. Exp Neurol. 1968 Apr;20(4):611–623. doi: 10.1016/0014-4886(68)90112-x. [DOI] [PubMed] [Google Scholar]
- Byzov A. L., Utina I. A. Tsentrobezhnye vliianiia na amakrinovye kletki setchatki liagushki. Neirofiziologiia. 1971 May-Jun;3(3):293–300. [PubMed] [Google Scholar]
- COWAN W. M., ADAMSON L., POWELL T. P. An experimental study of the avian visual system. J Anat. 1961 Oct;95:545–563. [PMC free article] [PubMed] [Google Scholar]
- COWAN W. M., POWELL T. P. CENTRIFUGAL FIBRES IN THE AVIAN VISUAL SYSTEM. Proc R Soc Lond B Biol Sci. 1963 Sep 17;158:232–252. doi: 10.1098/rspb.1963.0045. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Martin A. R. Estimates of probability of transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Nov;210(4):933–945. doi: 10.1113/jphysiol.1970.sp009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Cowan W. M. An electron microscope study of normal and degenerating centrifugal fiber terminals in the pigeon retina. Z Zellforsch Mikrosk Anat. 1966;71(1):14–28. doi: 10.1007/BF00339827. [DOI] [PubMed] [Google Scholar]
- Dubin M. W. The inner plexiform layer of the vertebrate retina: a quantitative and comparative electron microscopic analysis. J Comp Neurol. 1970 Dec;140(4):479–505. doi: 10.1002/cne.901400406. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C. The central action of antidromic impulses in motor nerve fibres. Pflugers Arch. 1955;260(5):385–415. doi: 10.1007/BF00363548. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., FRANK K., BECKER M. C. Steps in the production of motoneuron spikes. J Gen Physiol. 1957 May 20;40(5):735–752. doi: 10.1085/jgp.40.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuortes M. G., Schwartz E. A., Simon E. J. Colour-dependence of cone responses in the turtle retina. J Physiol. 1973 Oct;234(1):199–216. doi: 10.1113/jphysiol.1973.sp010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuortes M. G., Simon E. J. Interactions leading to horizontal cell responses in the turtle retina. J Physiol. 1974 Jul;240(1):177–198. doi: 10.1113/jphysiol.1974.sp010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SMITH R. S. DELAYED DEPOLARIZATION AND THE REPETITIVE RESPONSE TO INTRACELLULAR STIMULATION OF MAMMALIAN MOTONEURONES. J Physiol. 1963 Oct;168:890–910. doi: 10.1113/jphysiol.1963.sp007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego A., Cruz J. Mammalian Retina: Associational Nerve Cells in Ganglion Cell Layer. Science. 1965 Dec 3;150(3701):1313–1314. doi: 10.1126/science.150.3701.1313. [DOI] [PubMed] [Google Scholar]
- Gliozzi A. Effect of electrical stimulation of the optic nerve on retinal potentials. Arch Ital Biol. 1966 Dec;104(4):511–515. [PubMed] [Google Scholar]
- Holden A. L. Antidromic activation of the isthmo-optic nucleus. J Physiol. 1968 Jul;197(1):183–198. doi: 10.1113/jphysiol.1968.sp008554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden A. L., Powell T. P. The functional organization of the isthmo-optic nucleus in the pigeon. J Physiol. 1972 Jun;223(2):419–447. doi: 10.1113/jphysiol.1972.sp009856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden A. L. The centrifugal system running to the pigeon retina. J Physiol. 1968 Jul;197(1):199–219. doi: 10.1113/jphysiol.1968.sp008555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERNELL D. THE DELAYED DEPOLARIZATION IN CAT AND RAT MOTONEURONES. Prog Brain Res. 1964;12:42–55. doi: 10.1016/s0079-6123(08)60616-0. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Electrophysiological study of single neurons in the inner nuclear layer of the carp retina. Vision Res. 1969 Jan;9(1):37–55. doi: 10.1016/0042-6989(69)90030-3. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol. 1973 Nov;235(1):133–153. doi: 10.1113/jphysiol.1973.sp010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATURANA H. R. Efferent fibres in the optic nerve of the toad (Bufo bufo). J Anat. 1958 Jan;92(1):21–27. [PMC free article] [PubMed] [Google Scholar]
- MCGILL J. I. ORGANIZATION WITHIN THE CENTRAL AND CENTRIFUGAL FIBRE PATHWAYS IN THE AVIAN VISUAL SYSTEM. Nature. 1964 Oct 24;204:395–396. doi: 10.1038/204395a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Naka K. I. Identification of intracellular responses in the frog retina. Brain Res. 1972 Jul 13;42(1):59–71. doi: 10.1016/0006-8993(72)90042-x. [DOI] [PubMed] [Google Scholar]
- Matsumoto N. Responses of the amacrine cell to optic nerve stimulation in the frog retina. Vision Res. 1975 Apr;15(4):509–514. doi: 10.1016/0042-6989(75)90028-0. [DOI] [PubMed] [Google Scholar]
- Maturana H. R., Frenk S. Synaptic connections of the centrifugal fibers in the pigeon retina. Science. 1965 Oct 15;150(3694):359–361. doi: 10.1126/science.150.3694.359. [DOI] [PubMed] [Google Scholar]
- McGill J. I., Powell T. P., Cowan W. M. The organization of the projection of the centrifugal fibres to the retina in the pigeon. J Anat. 1966 Jan;100(Pt 1):35–49. [PMC free article] [PubMed] [Google Scholar]
- McGill J. I., Powell T. P., Cowan W. M. The retinal representation upon the optic tectum and isthmo-optic nucleus in the pigeon. J Anat. 1966 Jan;100(Pt 1):5–33. [PMC free article] [PubMed] [Google Scholar]
- Miles F. A. Centrifugal effects in the avian retina. Science. 1970 Nov 27;170(3961):992–995. doi: 10.1126/science.170.3961.992. [DOI] [PubMed] [Google Scholar]
- Naka K., Otsuka T. Morphological and functional identifications of catfish retinal neurons. II. Morphological identification. J Neurophysiol. 1975 Jan;38(1):72–91. doi: 10.1152/jn.1975.38.1.72. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Burke R. E. Delayed depolarization in cat spinal motoneurons. Exp Neurol. 1967 Jan;17(1):16–26. doi: 10.1016/0014-4886(67)90118-5. [DOI] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Smith T. G., Nelson P. G., Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967 Sep;30(5):1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Organization of on-off cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):1–14. doi: 10.1113/jphysiol.1973.sp010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of bipolar cells in the retina of the turtle. J Physiol. 1974 Jan;236(1):211–224. doi: 10.1113/jphysiol.1974.sp010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J. Feedback loop between cones and horizontal cells in the turtle retina. Fed Proc. 1974 Apr;33(4):1078–1082. [PubMed] [Google Scholar]
- Simon E. J. Two types of luminosity horizontal cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):199–211. doi: 10.1113/jphysiol.1973.sp010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Otsu K. Bipolar-amacrine transmission in the carp retina. Vision Res. 1973 Feb;13(2):295–307. doi: 10.1016/0042-6989(73)90108-9. [DOI] [PubMed] [Google Scholar]
- Toyoda J. Membrane resistance changes underlying the bipolar cell response in the carp retina. Vision Res. 1973 Feb;13(2):283–294. doi: 10.1016/0042-6989(73)90107-7. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. T. Synaptic orgnization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol. 1974 Mar;3(1):1–33. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]


