Abstract
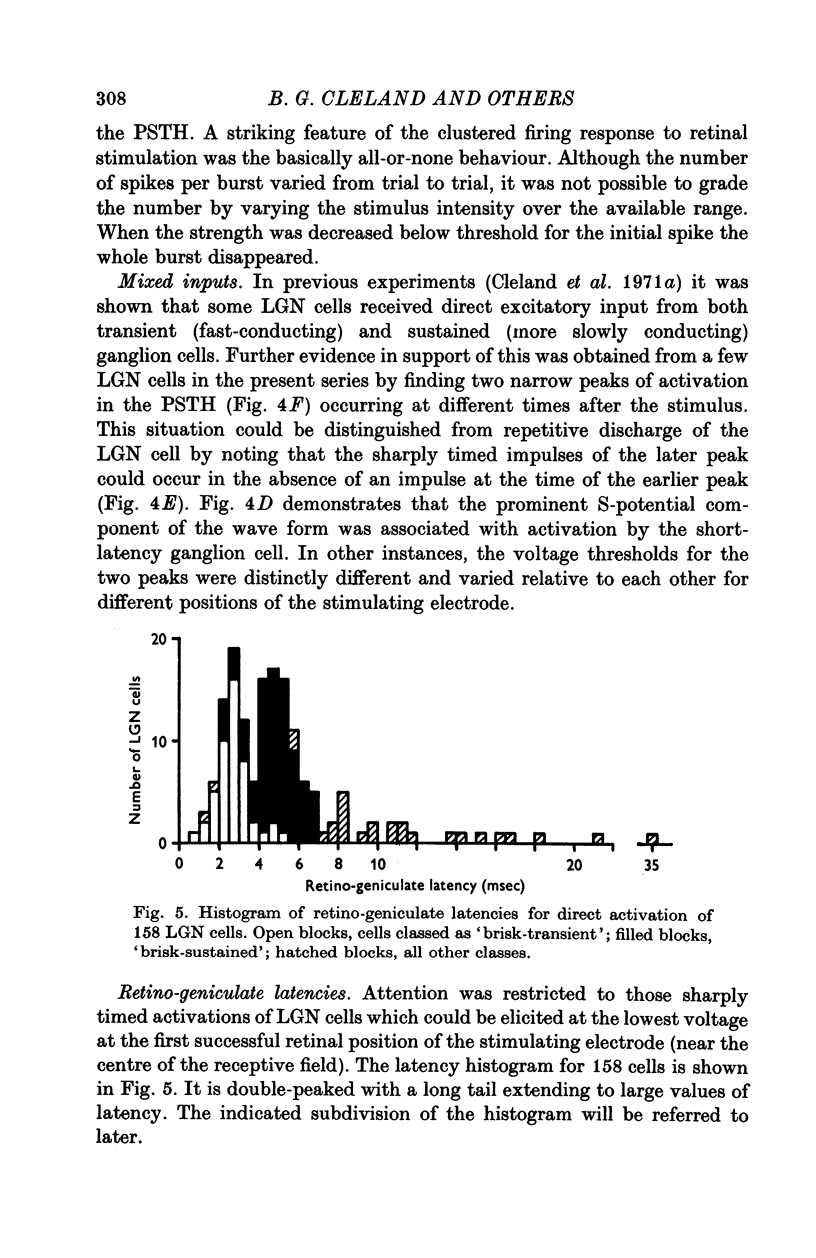
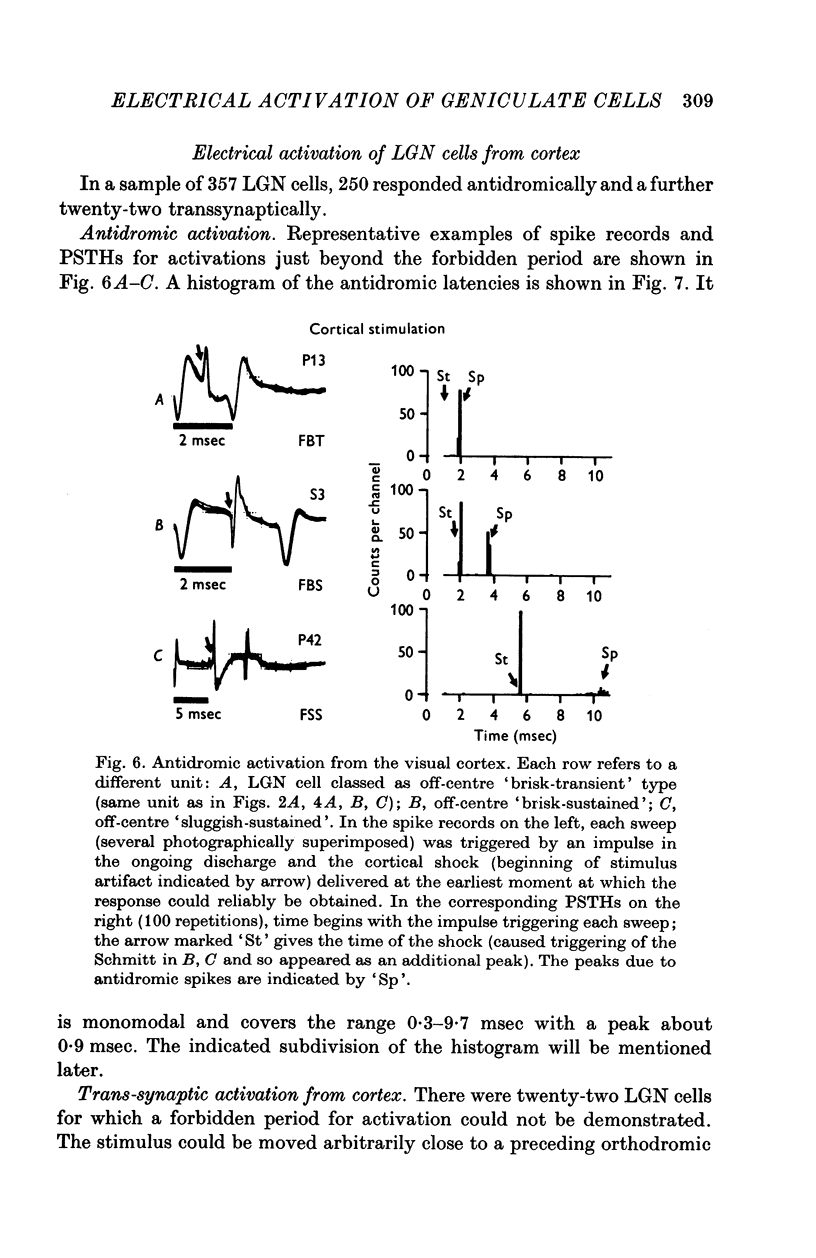
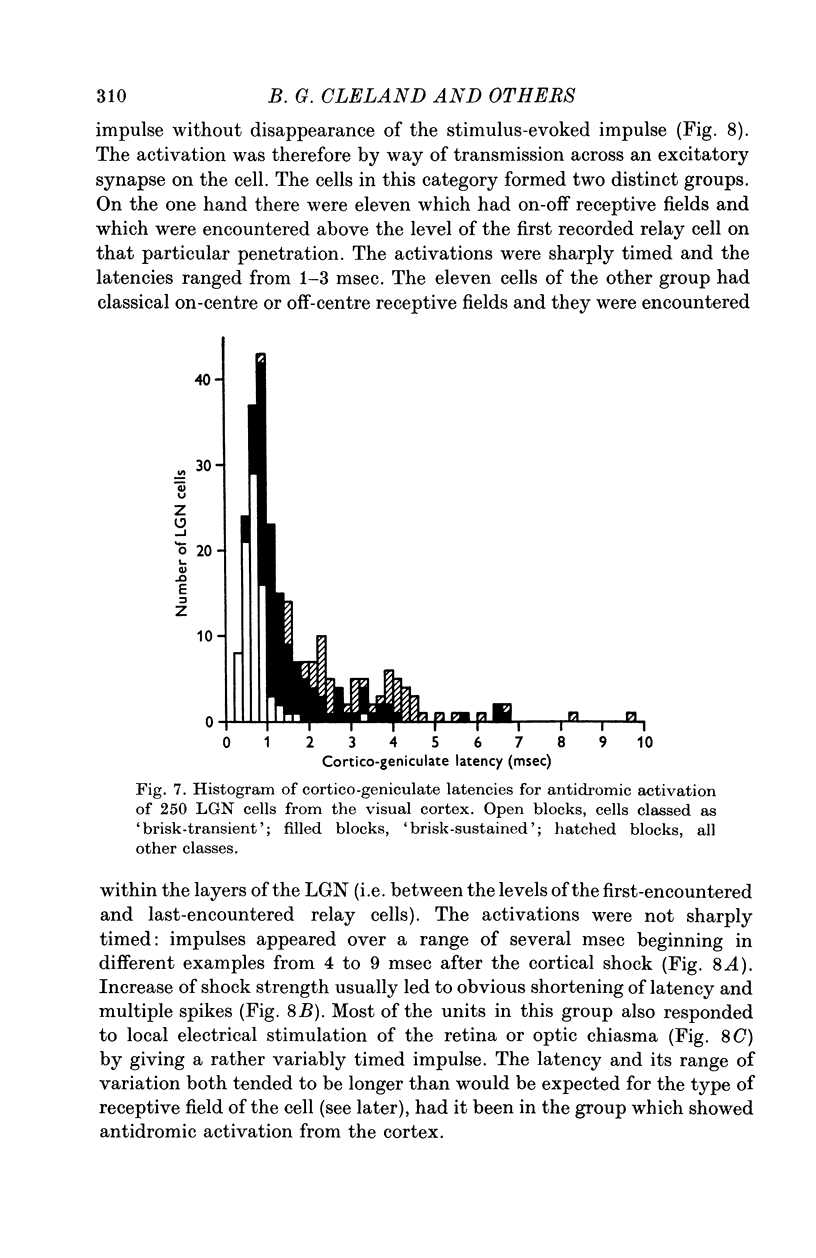
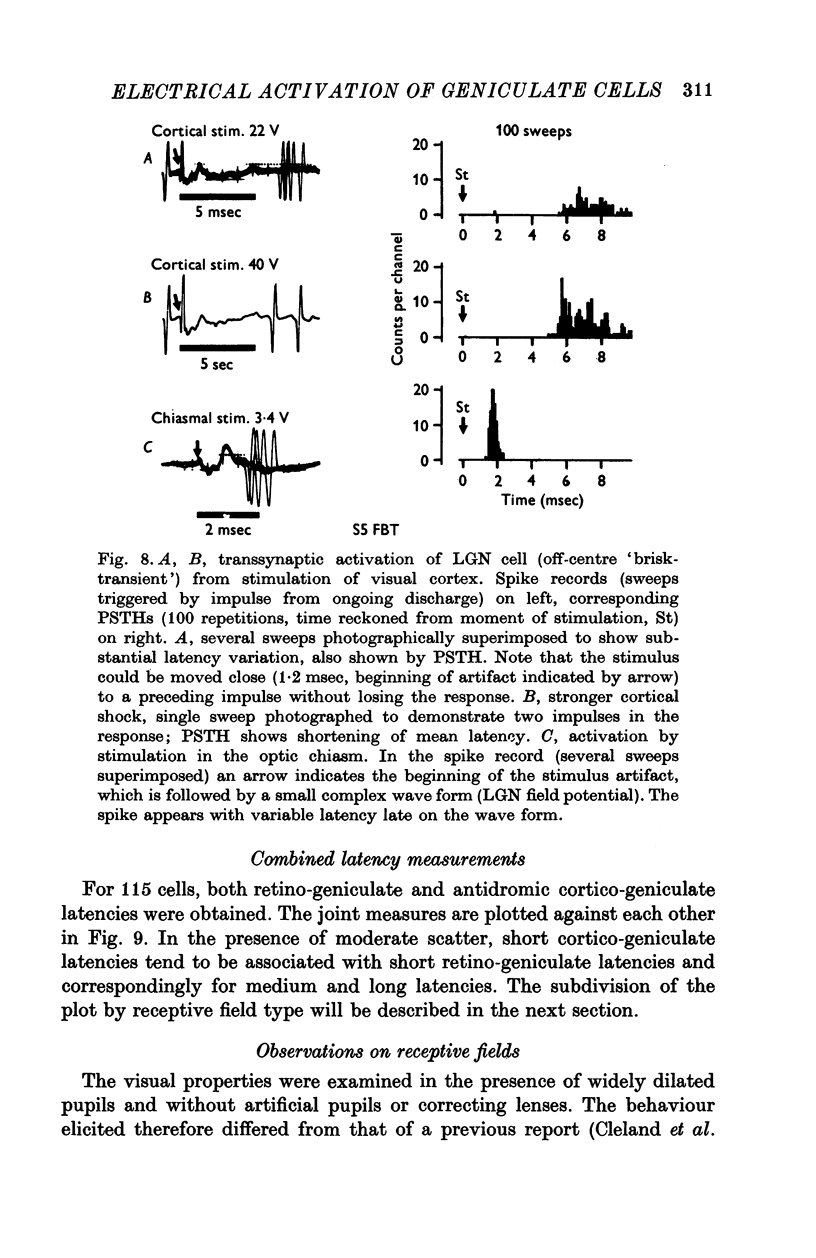
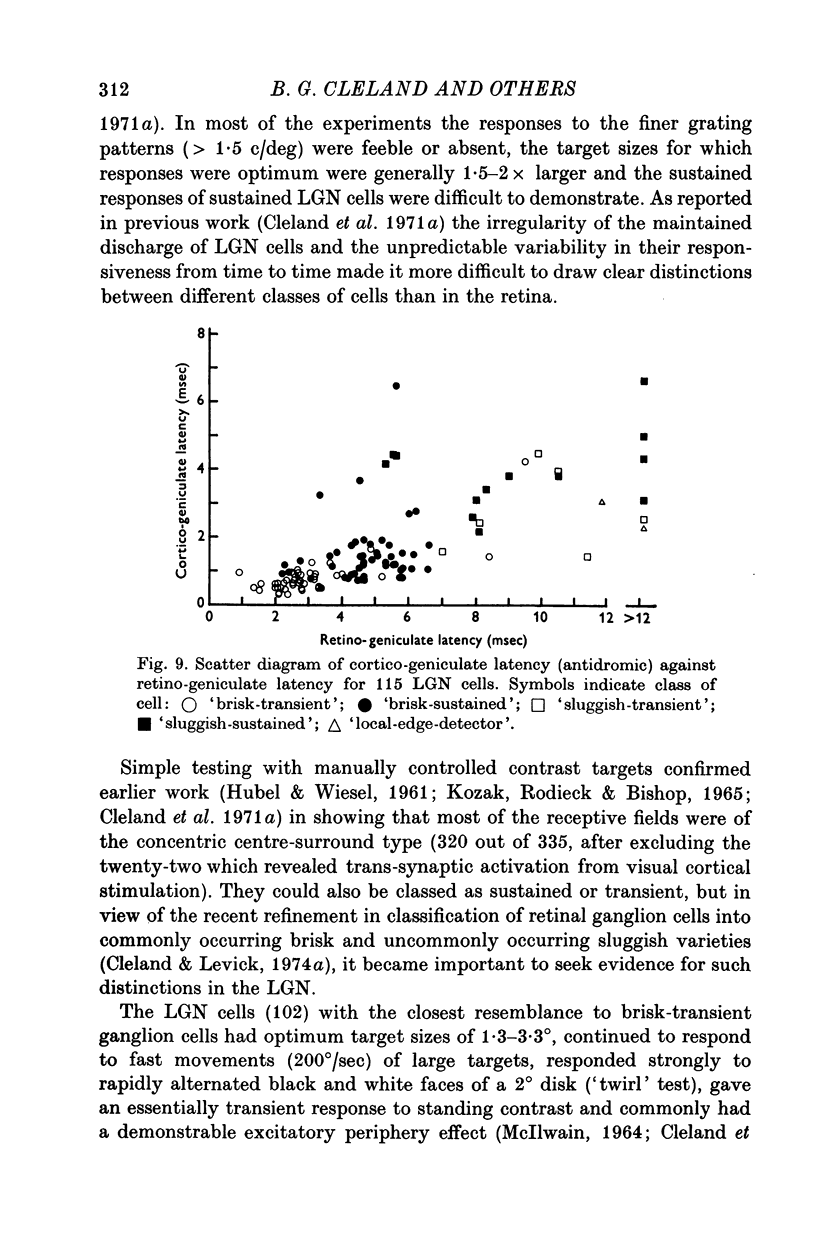
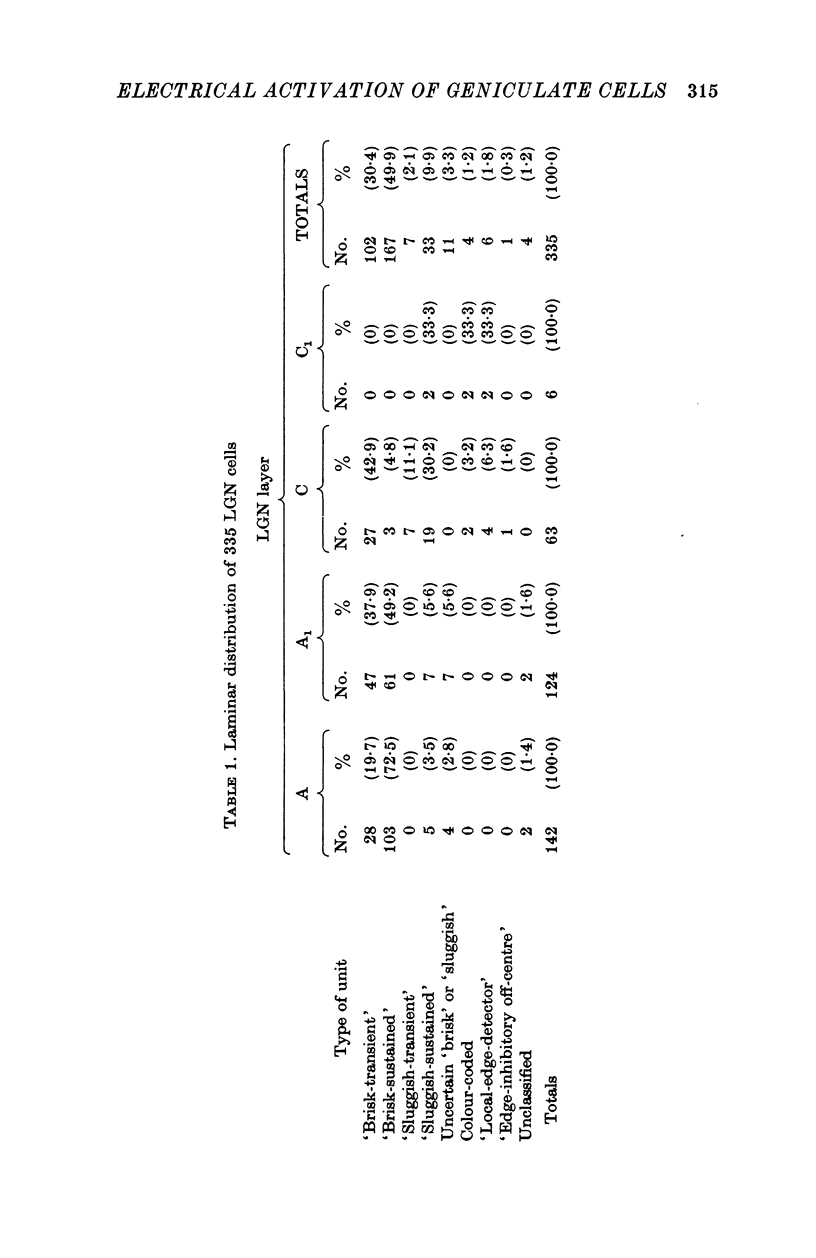
1. Lateral geniculate neurones of the cat were studied in terms of the latency for activation by local electrical stimulation of the retina, the latency of electrical activation from the visual cortex and properties of receptive fields. Most of the units were relay cells (antidromic activation from visual cortex) but a small proportion were trans-synaptically activated from the cortex. The latter group included units with on-off, on-centre or off-centra receptive fields. 2. Direct activation of lateral geniculate neurones from local electrical stimulation of retinal ganglion cells or their axons in the retina was identified by the sharpness of timing of the elicited impulses. This procedure revealed the existence of slowly conducting axons relaying in the lateral geniculate nucleus. 3. The distribution of latencies for direct activation from the retina was bimodal with an extended tail of long values. It is similar to the distribution of antidromic latencies of retinal ganglion cells following stimulation of the optic tract. 4. There was a tendency for geniculate neurones with fast input from the retina to have fast axons to the visual cortex and correspondingly for medium-speed and slow input. 5. The previous classification of geniculate receptive fields into sustained and transient types was extended to include commonly encountered 'brisk' and uncommonly encountered 'sluggish' varieties of each. The extension was based on visual properties and latency for direct electrical activation from the retina. Units with receptive fields differing from the familiar on-centre or off-centre concentric pattern were encountered rarely; they included colour-coded fields, local-edge-detectors and one edge-inhibitory off-centre type.
Full text
PDF




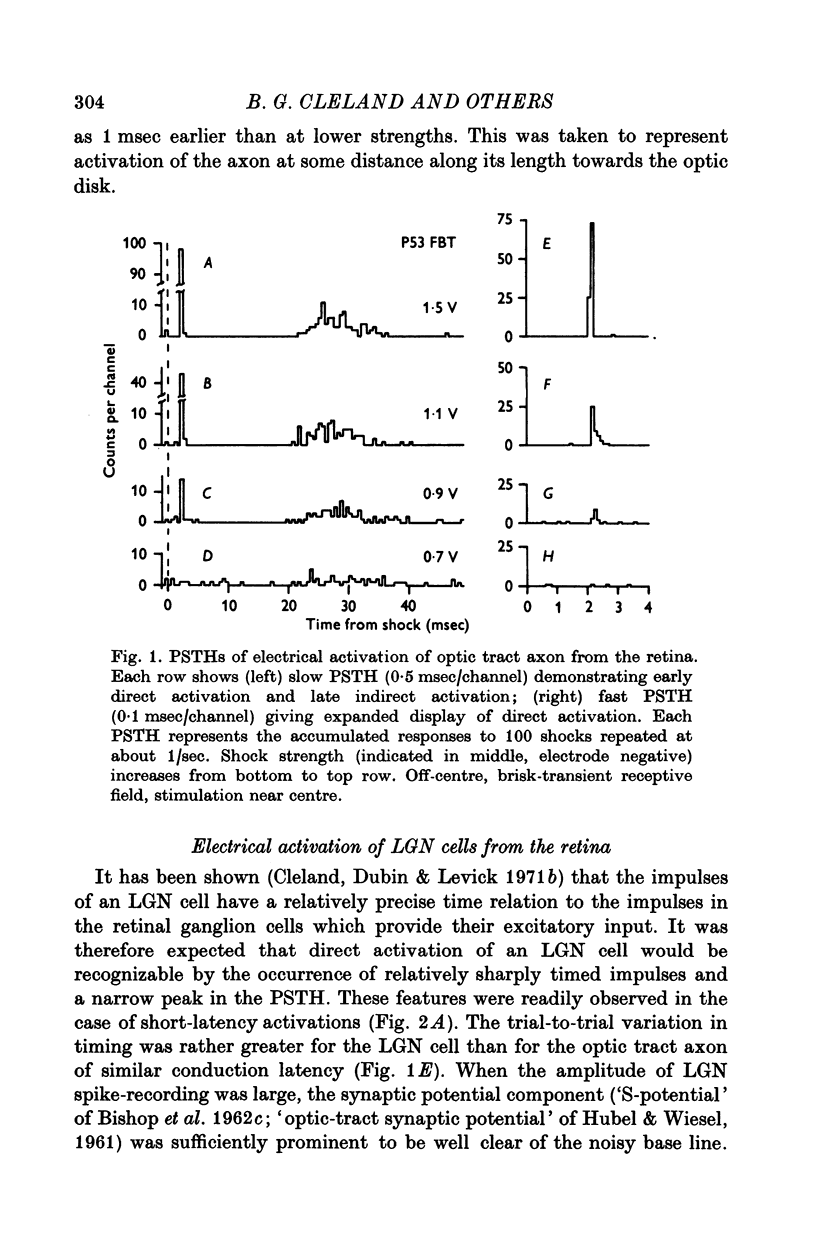
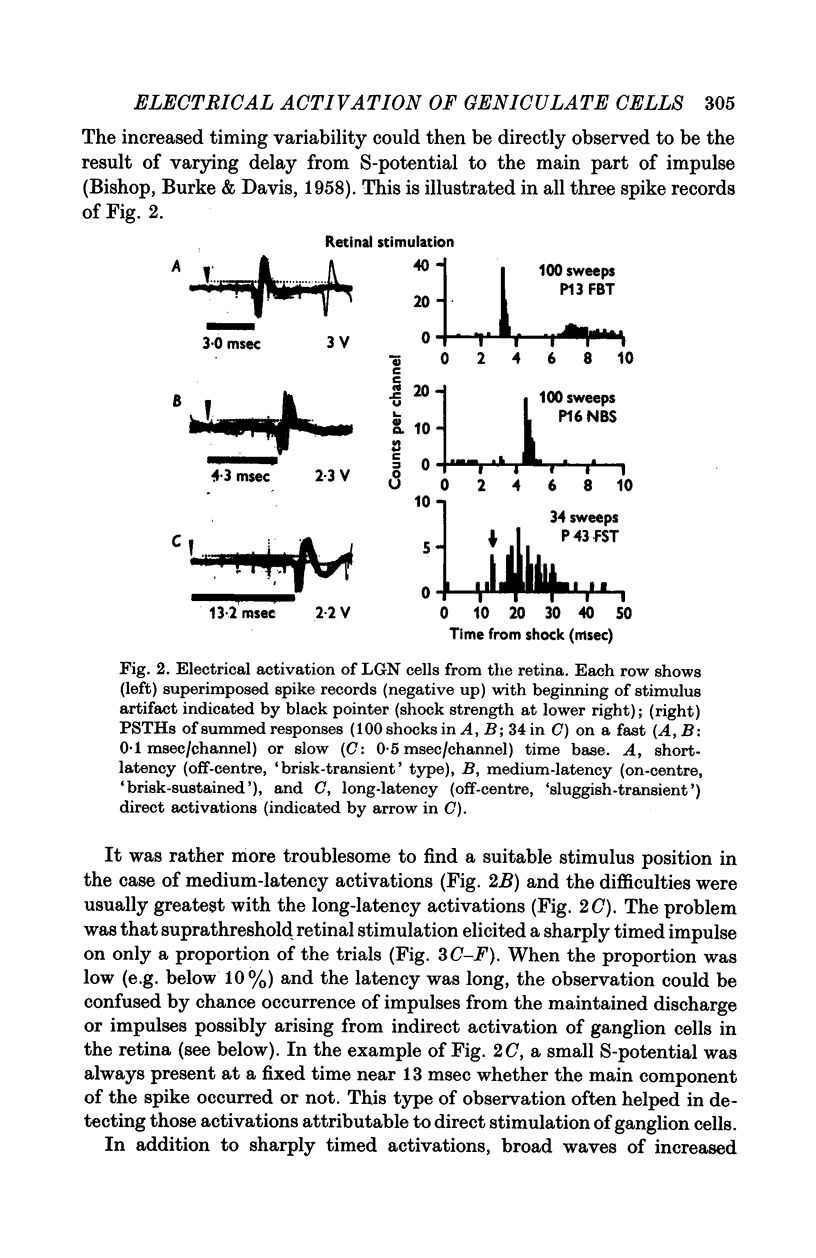
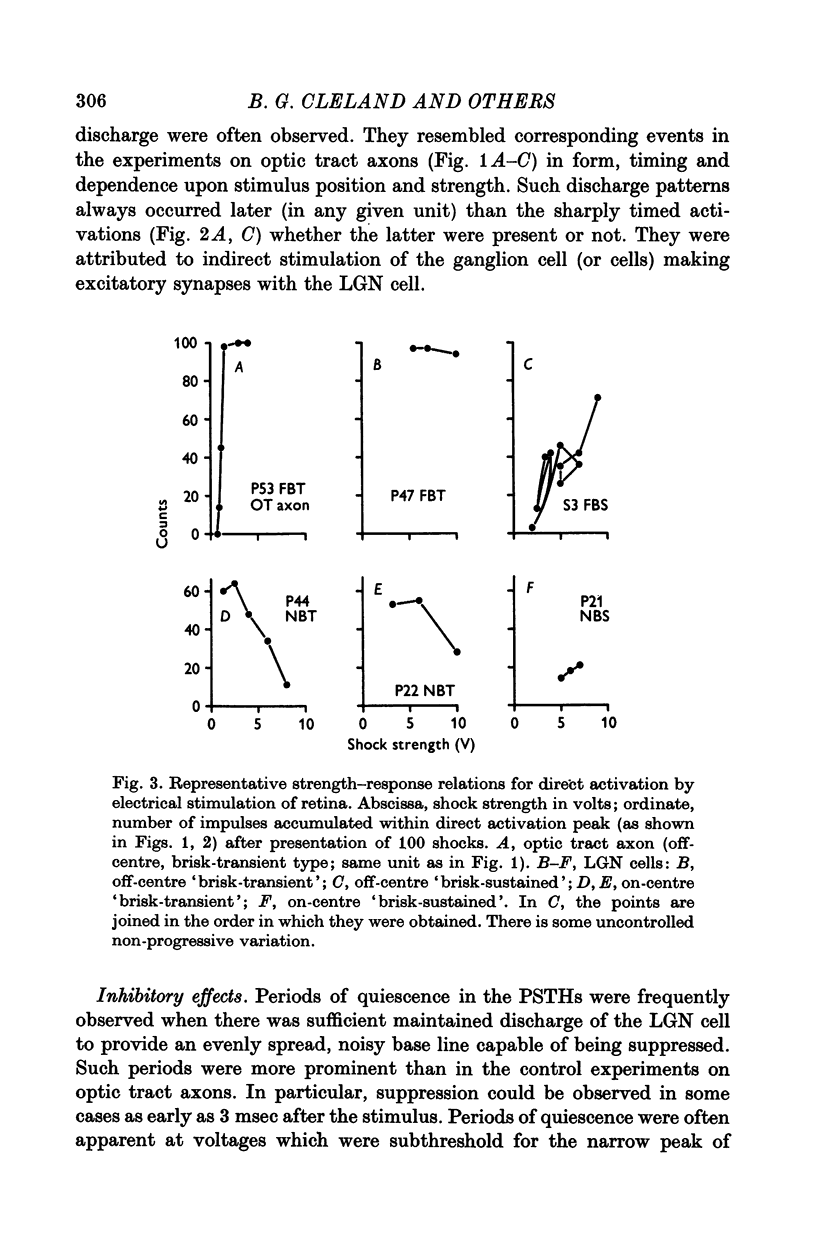
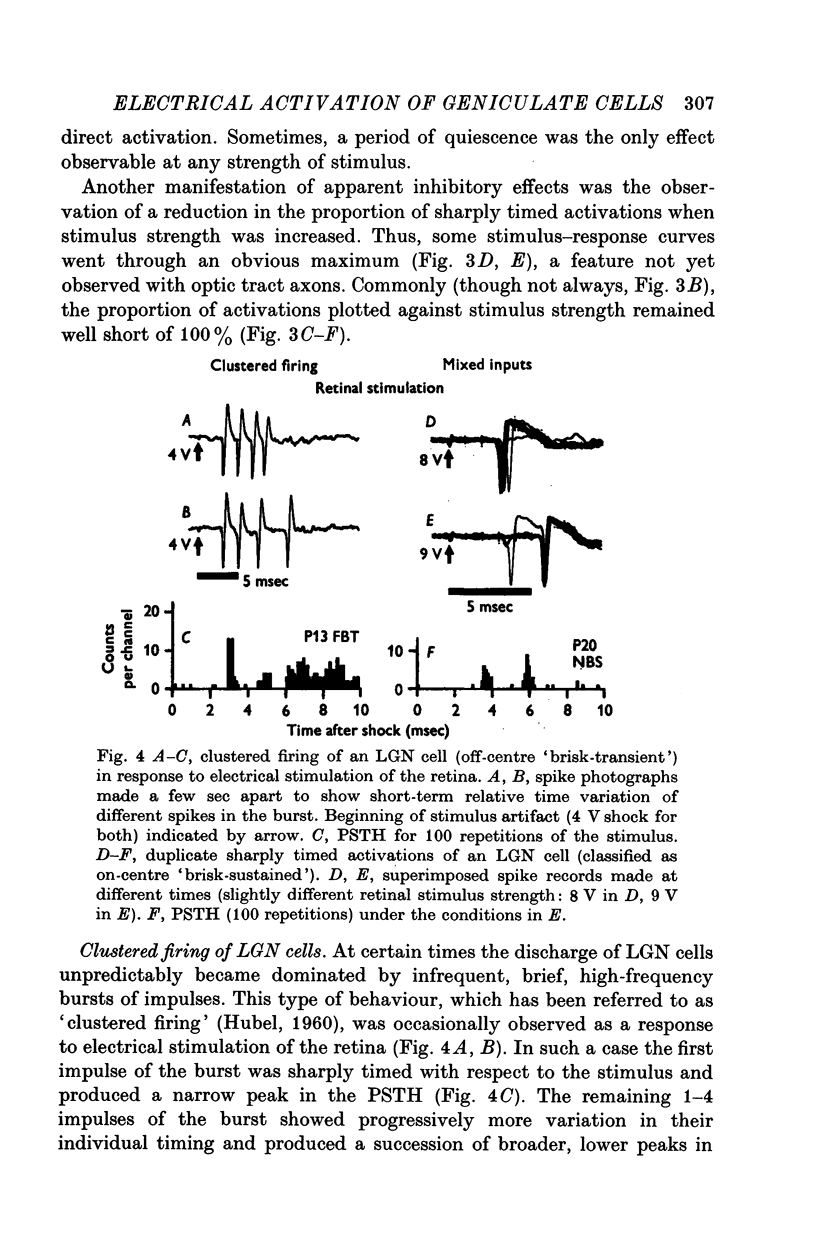







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., BURKE W., DAVIS R. Single-unit recording from antidromically activated optic radiation neurones. J Physiol. 1962 Aug;162:432–450. doi: 10.1113/jphysiol.1962.sp006943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. Synapse discharge by single fibre in mammalian visual system. Nature. 1958 Sep 13;182(4637):728–730. doi: 10.1038/182728b0. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The interpretation of the extracellular response of single lateral geniculate cells. J Physiol. 1962 Aug;162:451–472. doi: 10.1113/jphysiol.1962.sp006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., McLEOD J. G. Nature of potentials associated with synaptic transmission in lateral geniculate of cat. J Neurophysiol. 1954 Jul;17(4):387–414. doi: 10.1152/jn.1954.17.4.387. [DOI] [PubMed] [Google Scholar]
- Bishop G. H., Clare M. H., Landau W. M. Further analysis of fiber groups in the optic tract of the cat. Exp Neurol. 1969 Jul;24(3):386–399. doi: 10.1016/0014-4886(69)90144-7. [DOI] [PubMed] [Google Scholar]
- Boycott B. B., Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974 Jul;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke W., Jervie Sefton A. Discharge patterns of principal cells and interneurones in lateral geniculate nucleus of rat. J Physiol. 1966 Nov;187(1):201–212. doi: 10.1113/jphysiol.1966.sp008083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAPPER D. R., NOELL W. K. RETINAL EXCITATION AND INHIBITION FROM DIRECT ELECTRICAL STIMULATION. J Neurophysiol. 1963 Nov;26:924–947. doi: 10.1152/jn.1963.26.6.924. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Simultaneous recording of input and output of lateral geniculate neurones. Nat New Biol. 1971 Jun 9;231(23):191–192. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974 Jul;240(2):457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Wässle H. Physiological identification of a morphological class of cat retinal ganglion cells. J Physiol. 1975 Jun;248(1):151–171. doi: 10.1113/jphysiol.1975.sp010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Morstyn R., Wagner H. G., Levick W. R. Long-latency retinal input to lateral geniculate neurones of the cat. Brain Res. 1975 Jun 27;91(2):306–310. doi: 10.1016/0006-8993(75)90553-3. [DOI] [PubMed] [Google Scholar]
- DOTY R. W., GRIMM F. R. Cortical responses to local electrical stimulation of retina. Exp Neurol. 1962 Apr;5:319–334. doi: 10.1016/0014-4886(62)90041-9. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: evidence for more than one cone process. J Physiol. 1970 Nov;211(1):125–137. doi: 10.1113/jphysiol.1970.sp009270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y., Saito H. Phasic and tonic cells in the cat's lateral geniculate nucleus. Tohoku J Exp Med. 1972 Feb;106(2):209–210. doi: 10.1620/tjem.106.209. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Stone J. Retinal distribution and central projections of Y-, X-, and W-cells of the cat's retina. J Neurophysiol. 1974 Jul;37(4):749–772. doi: 10.1152/jn.1974.37.4.749. [DOI] [PubMed] [Google Scholar]
- Guillery RW THE LAMINAR D. a new interpretation. J Comp Neurol. 1970 Mar;138(3):339–366. doi: 10.1002/cne.901380307. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. A study of Golgi preparations from the dorsal lateral geniculate nucleus of the adult cat. J Comp Neurol. 1966 Sep;128(1):21–50. doi: 10.1002/cne.901280104. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Sumitomo I., Iwama K. Activation of lateral geniculate neurons by electrical stimulation of superior colliculus in cats. Jpn J Physiol. 1967 Dec 15;17(6):638–651. doi: 10.2170/jjphysiol.17.638. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P. Conduction velocity in pathways from retina to superior colliculus in the cat: a correlation with receptive-field properties. J Neurophysiol. 1973 May;36(3):409–424. doi: 10.1152/jn.1973.36.3.409. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Stone J., Sherman S. M. Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1972 Jul;35(4):518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- KOZAK W., RODIECK R. W., BISHOP P. O. RESPONSES OF SINGLE UNITS IN LATERAL GENICULATE NUCLEUS OF CAT TO MOVING VISUAL PATTERNS. J Neurophysiol. 1965 Jan;28:19–47. doi: 10.1152/jn.1965.28.1.19. [DOI] [PubMed] [Google Scholar]
- Kato H., Yamamoto M., Nakahama H. Intracellular recordings from the lateral geniculate neurons of cats. Jpn J Physiol. 1971 Jun;21(3):307–323. doi: 10.2170/jjphysiol.21.307. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Cleland B. G. Receptive fields of cat retinal ganglion cells having slowly conducting axons. Brain Res. 1974 Jul 5;74(1):156–160. doi: 10.1016/0006-8993(74)90119-x. [DOI] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- Pearlman A. L., Daw N. W. Opponent color cells in the cat lateral geniculate nucleus. Science. 1970 Jan 2;167(3914):84–86. doi: 10.1126/science.167.3914.84. [DOI] [PubMed] [Google Scholar]
- Stone J., Hoffman K. P. Conduction velocity as a parameter in the organisation of the afferent relay in the cat's lateral geniculate nucleus. Brain Res. 1971 Sep 24;32(2):454–459. doi: 10.1016/0006-8993(71)90339-8. [DOI] [PubMed] [Google Scholar]
- Stone J., Hoffmann K. P. Very slow-conducting ganglion cells in the cat's retina: a major, new functional type? Brain Res. 1972 Aug 25;43(2):610–616. doi: 10.1016/0006-8993(72)90416-7. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kato E. Binocular interaction at cat's lateral geniculate body. J Neurophysiol. 1966 Sep;29(5):909–920. doi: 10.1152/jn.1966.29.5.909. [DOI] [PubMed] [Google Scholar]
- Tömböl T. Short neurons and their synaptic relations in the specific thalamic nuclei. Brain Res. 1967 Jan 20;3(4):307–326. doi: 10.1016/0006-8993(67)90095-9. [DOI] [PubMed] [Google Scholar]
- Tömböl T. Two types of short axon (Golgi 2nd) interneurons in the specific thalamic nuclei. Acta Morphol Acad Sci Hung. 1969;17(3):285–297. [PubMed] [Google Scholar]
- VASTOLA E. F. Conduction velocities in single fibers of the visual radiation. Exp Neurol. 1963 Jan;7:1–12. doi: 10.1016/0014-4886(63)90089-x. [DOI] [PubMed] [Google Scholar]
- Wässle H., Levick W. R., Cleland B. G. The distribution of the alpha type of ganglion cells in the cat's retina. J Comp Neurol. 1975 Feb 1;159(3):419–438. doi: 10.1002/cne.901590308. [DOI] [PubMed] [Google Scholar]


