Abstract
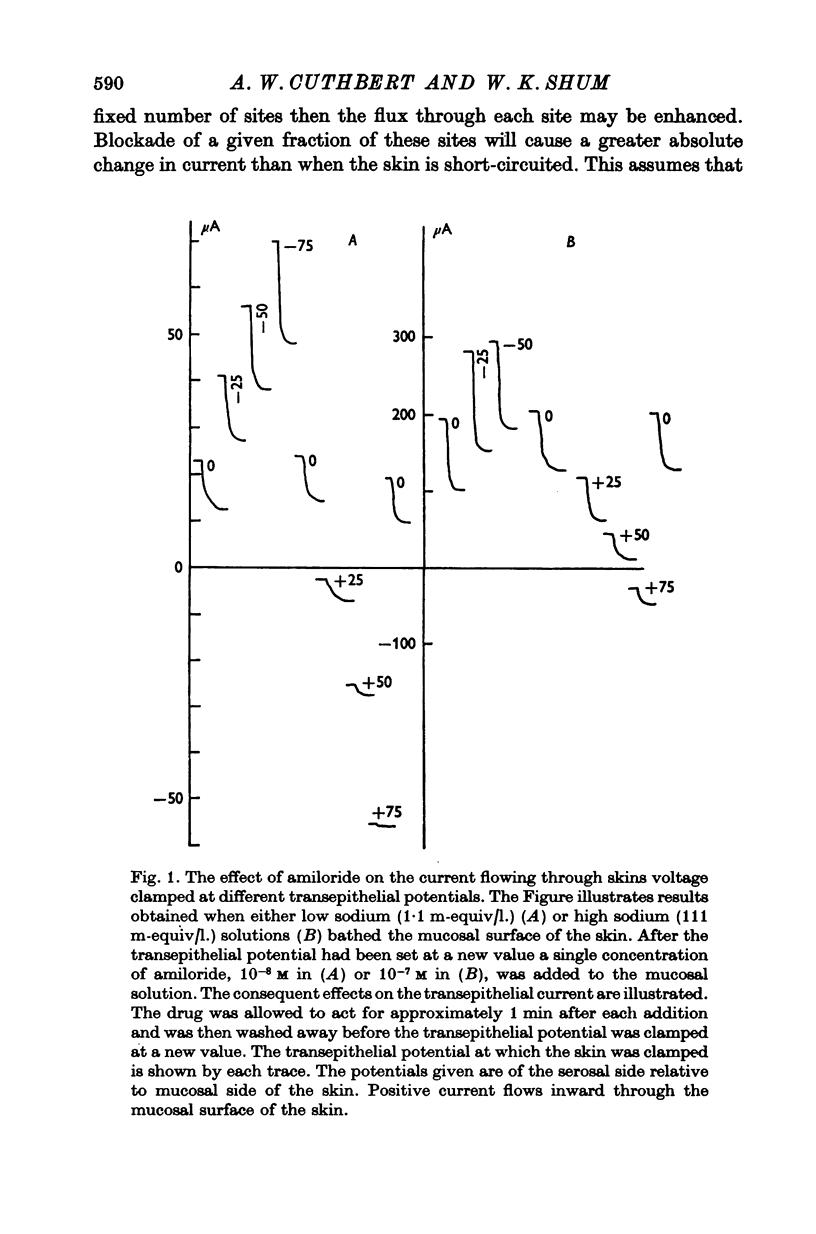
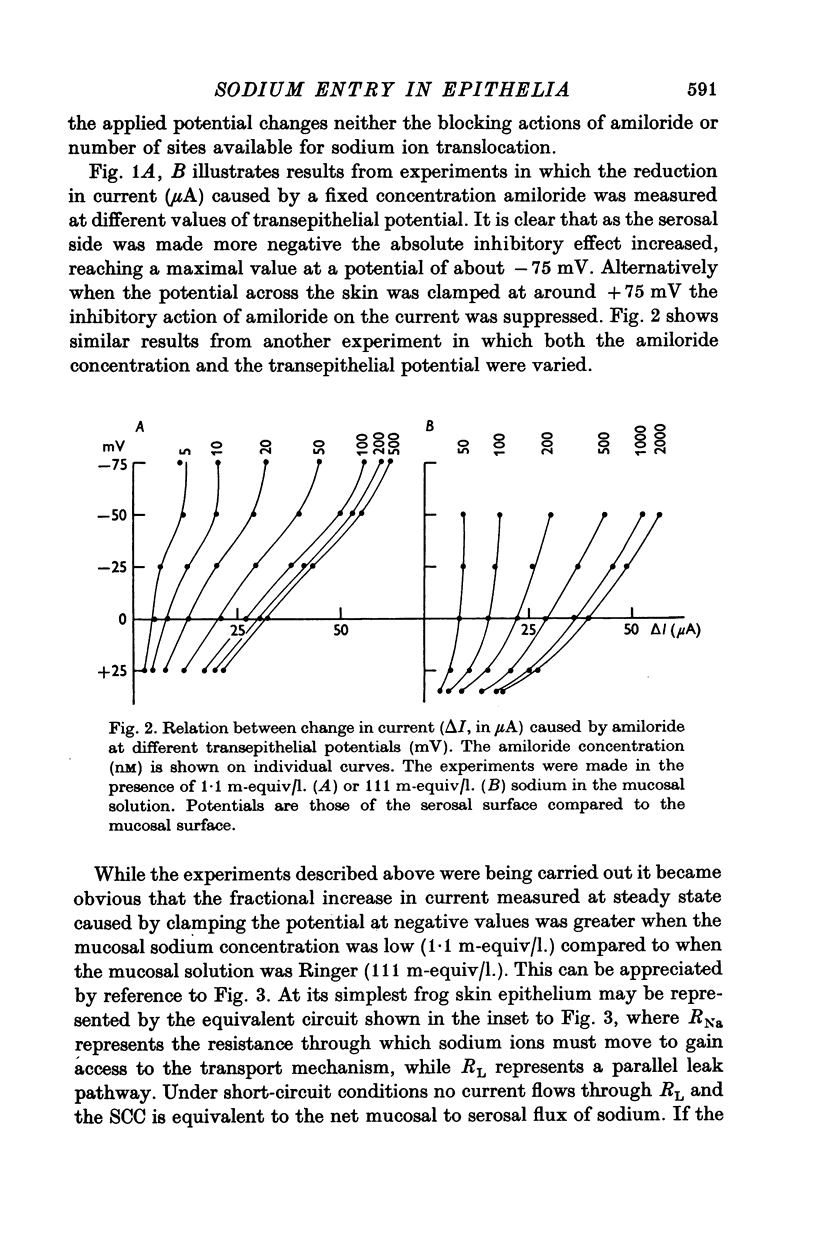
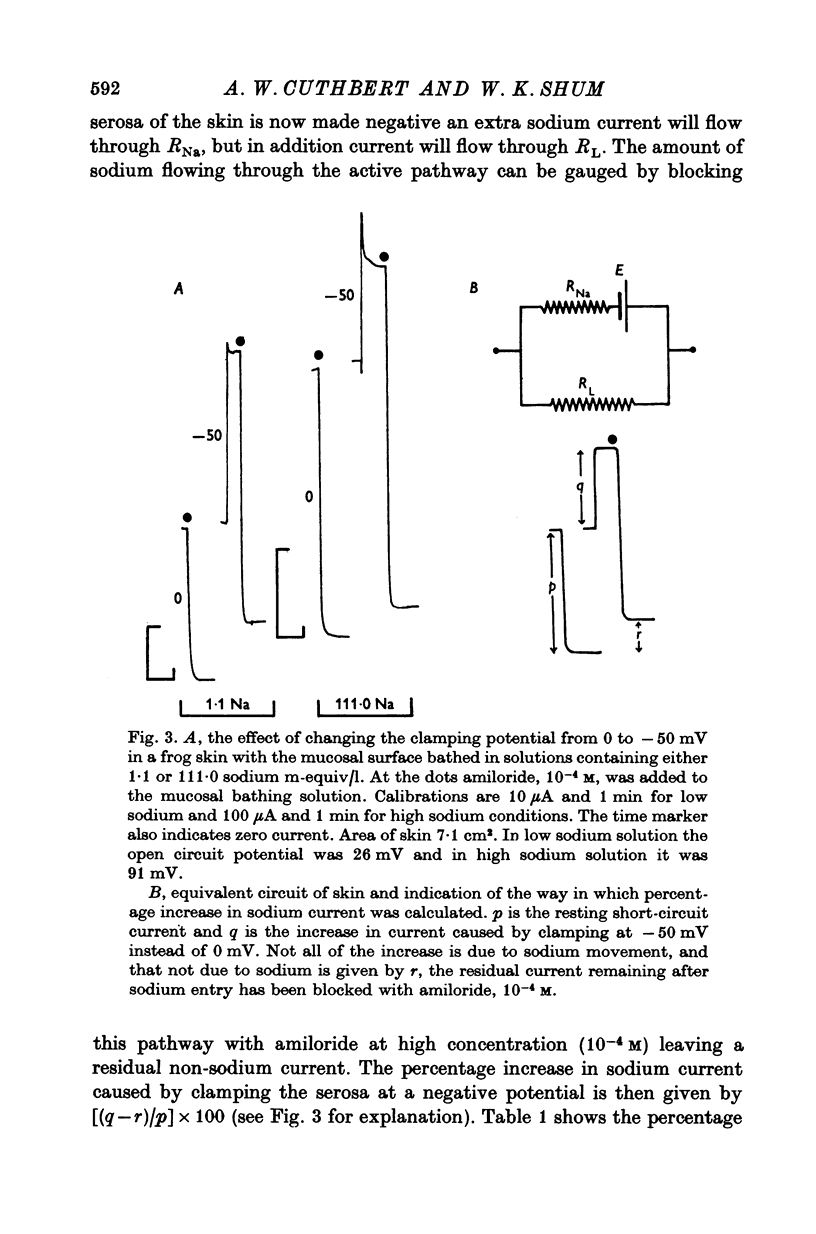
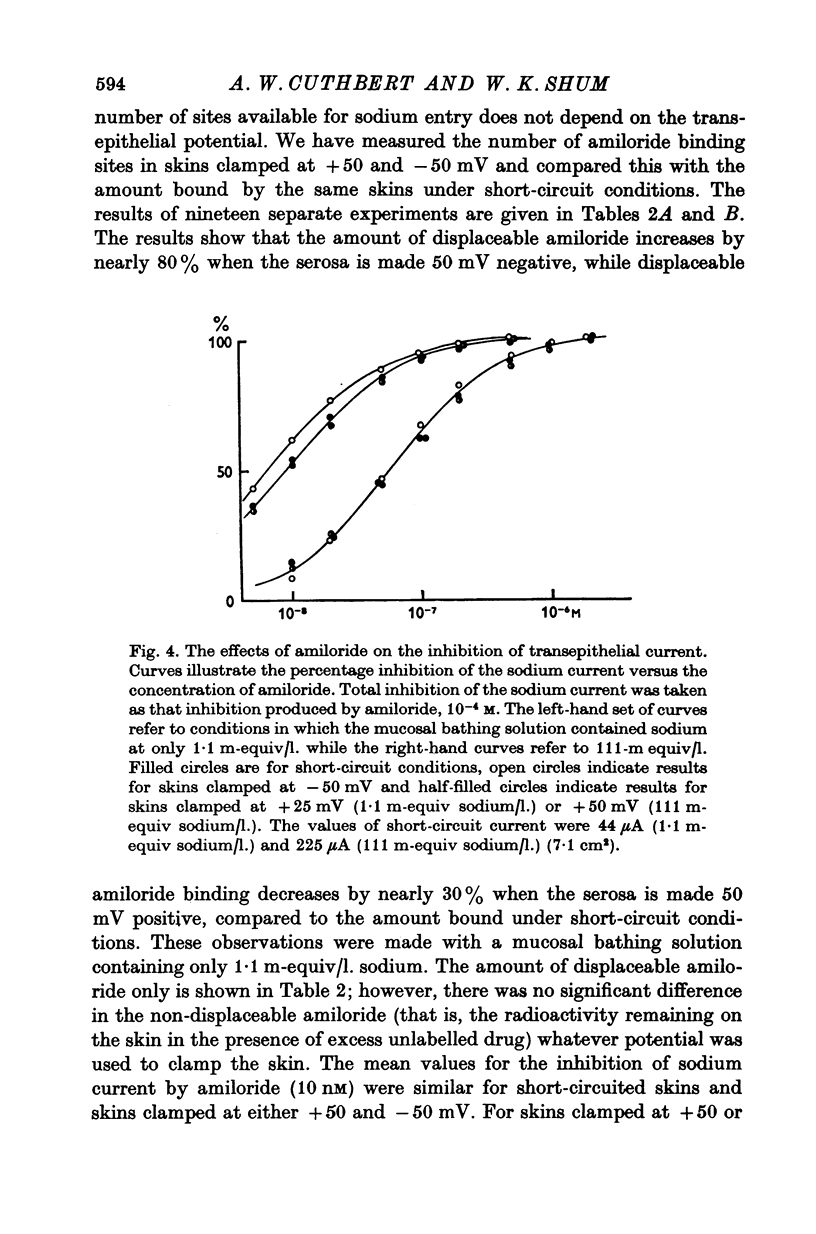
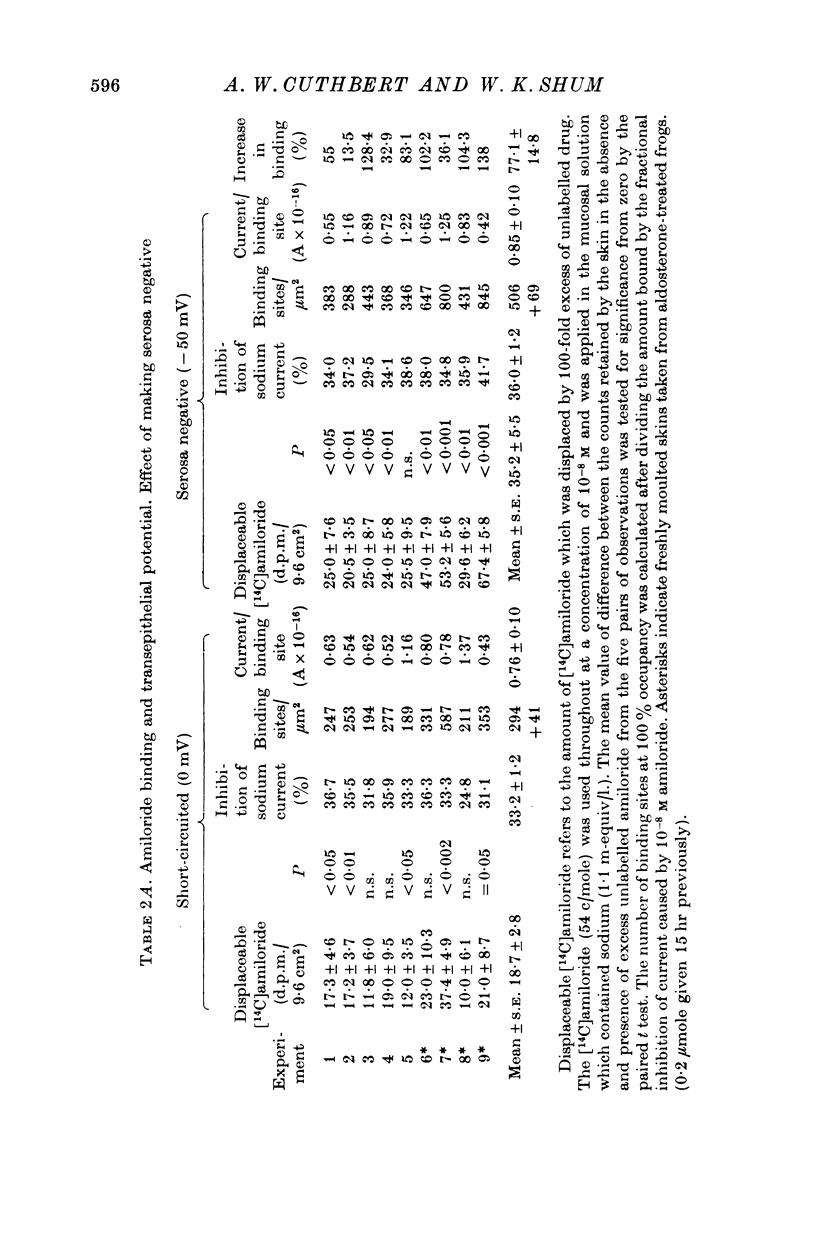
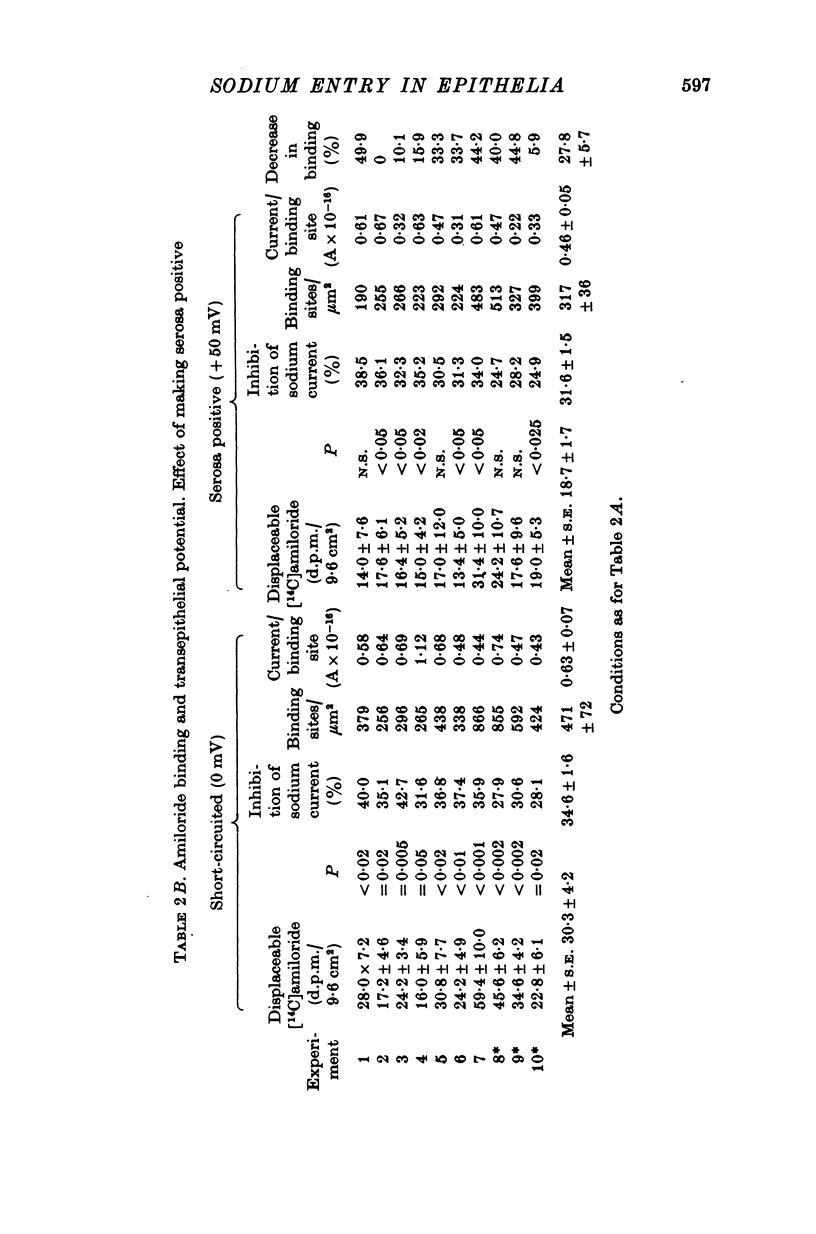
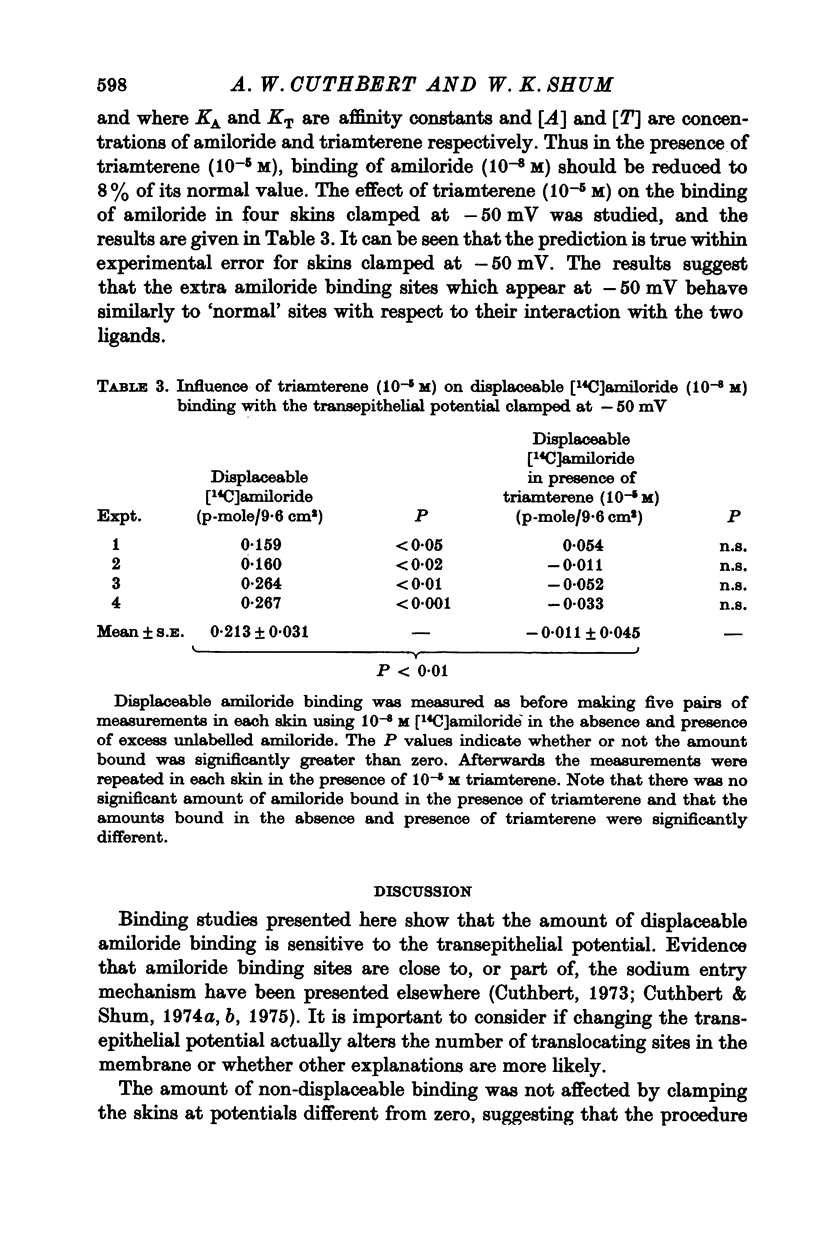
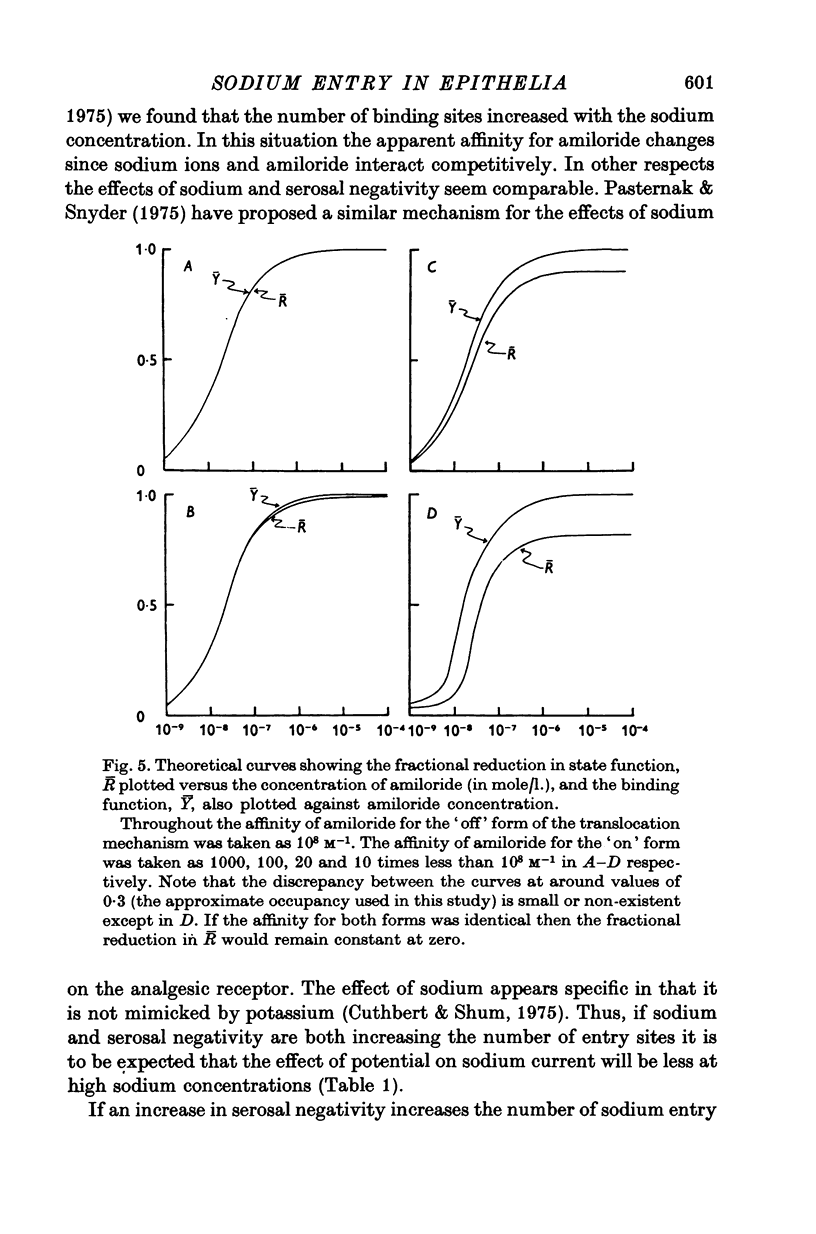
1. The permeation of sodium ions trhough the mucosal surface of frog skin epithelium at different transepithelial potentials has been investigated using the blocking drug amiloride. 2. An increase in serosal negativity in voltage-clamped skins was associated with an increase in the absolute amount of inhibition caused by a fixed concentration of amiloride. Hyperpolarizing or depolarizing skins with respect to the short-circuited condition did not affect the apparent affinity of amiloride for the entry sites. 3. When skins were voltage clamped at -50 mV (serosa negative) the specific binding of amiloride to sodium entry sites was increased by 77% compared to the short-circuited condition. Skins clamped at +50 mV had only 72% of the specific binding found in short-circuited skins. Experiments with a second blocking drug, triamterene, indicated that the extra binding sites appearing at -50mV were similar to those found under short-circuit conditions. The appearance and disappearance of binding sites may reflect changes in cell volume. 4. The findings suggest that the increased sodium current which flows when skins are clamped at -50 mV results from an increase in the number of entry sites, and perhaps also to a voltage sensitive increase in flux through each entry site.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biber T. U., Curran P. F. Direct measurement of uptake of sodium at the outer surface of the frog skin. J Gen Physiol. 1970 Jul;56(1):83–99. doi: 10.1085/jgp.56.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber T. U., Sanders M. L. Influence of transepithelial potential difference on the sodium uptake at the outer surface of the isolated frog skin. J Gen Physiol. 1973 May;61(5):529–551. doi: 10.1085/jgp.61.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia O. A., Chiarandini D. J. Transport of lithium and rectification by frog skin. Biochim Biophys Acta. 1973 May 25;307(3):578–589. doi: 10.1016/0005-2736(73)90302-7. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Rotunno C. A. The effect of antidiuretic hormone on Na movement across frog skin. J Physiol. 1971 Feb;213(1):119–133. doi: 10.1113/jphysiol.1971.sp009372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M. Effects of active sodium transport on current-voltage relationship of toad bladder. Am J Physiol. 1970 Jul;219(1):234–245. doi: 10.1152/ajplegacy.1970.219.1.234. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W. An upper limit to the number of sodium channels in frog skin epithelium. J Physiol. 1973 Feb;228(3):681–692. doi: 10.1113/jphysiol.1973.sp010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Amiloride and the sodium channel. Naunyn Schmiedebergs Arch Pharmacol. 1974;281(3):261–269. doi: 10.1007/BF00500595. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Effects of vasopressin and aldosterone on amiloride binding in toad bladder epithelial cells. Proc R Soc Lond B Biol Sci. 1975 Jun 17;189(1097):543–575. doi: 10.1098/rspb.1975.0072. [DOI] [PubMed] [Google Scholar]
- Erlij D., Smith M. W. Sodium uptake by frog skin and its modification by inhibitors of transepithelial sodium transport. J Physiol. 1973 Jan;228(1):221–239. doi: 10.1113/jphysiol.1973.sp010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D. R., Smith M. W. Direct measurement of sodium uptake by toad bladder mucosal cells. J Endocrinol. 1972 Oct;55(1):195–201. doi: 10.1677/joe.0.0550195. [DOI] [PubMed] [Google Scholar]
- Finn A. L., Reuss L. Effects of changes in the composition of the serosal solution on the electrical properties of the toad urinary bladder epithelium. J Physiol. 1975 Sep;250(3):541–558. doi: 10.1113/jphysiol.1975.sp011069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A. L. Transepithelial potential difference in toad urinary bladder is not due to ionic diffusion. Nature. 1974 Aug 9;250(5466):495–496. doi: 10.1038/250495a0. [DOI] [PubMed] [Google Scholar]
- GATZY J. T., CLARKSON T. W. THE EFFECT OF MUCOSAL AND SEROSAL SOLUTION CATIONS ON BIOELECTRIC PROPERTIES OF THE ISOLATED TOAD BLADDER. J Gen Physiol. 1965 Mar;48:647–671. doi: 10.1085/jgp.48.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Leb D. E., Edwards C., Lindley B. D., Hoshiko T. Interaction between the effects of inside and outside Na and K on bullfrog skin potential. J Gen Physiol. 1965 Nov;49(2):309–320. doi: 10.1085/jgp.49.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel L. J., Curran P. F. Response of the frog skin to steady-state voltage clamping. I. The shunt pathway. J Gen Physiol. 1972 May;59(5):503–518. doi: 10.1085/jgp.59.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. H., Reisin I. L., Rodríguez Boulan E., Rotunno C. A., Cereijido M. Barriers to sodium movement across frog skin. J Membr Biol. 1973;11(2):99–115. doi: 10.1007/BF01869815. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W., Snyder S. H. Identification of novel high affinity opiate receptor binding in rat brain. Nature. 1975 Feb 13;253(5492):563–565. doi: 10.1038/253563a0. [DOI] [PubMed] [Google Scholar]
- Rotunno C. A., Vilallonga F. A., Fernández M., Cereijido M. The penetration of sodium into the epithelium of the frog skin. J Gen Physiol. 1970 Jun;55(6):716–735. doi: 10.1085/jgp.55.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Lief P. D., Essig A. Conductance of active and passive pathways in the toad bladder. Am J Physiol. 1974 Jun;226(6):1265–1271. doi: 10.1152/ajplegacy.1974.226.6.1265. [DOI] [PubMed] [Google Scholar]
- Salako L. A., Smith A. J. Effects of the diuretics, triamterene and mersalyl on active sodium transport mechanisms in isolated frog skin. Br J Pharmacol. 1971 Mar;41(3):552–557. doi: 10.1111/j.1476-5381.1971.tb08053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voûte C. L., Ussing H. H. Some morphological aspects of active sodium transport. The epithelium of the frog skin. J Cell Biol. 1968 Mar;36(3):625–638. doi: 10.1083/jcb.36.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]


