Abstract
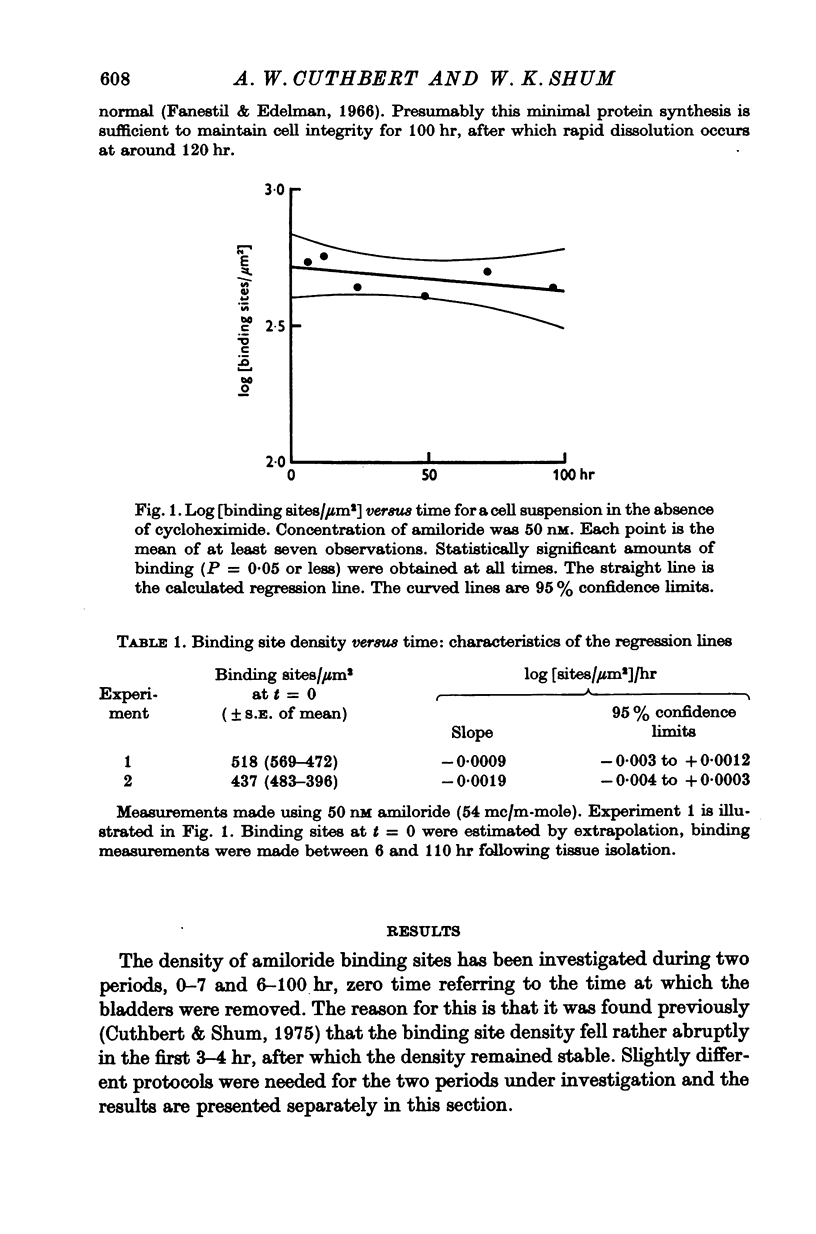
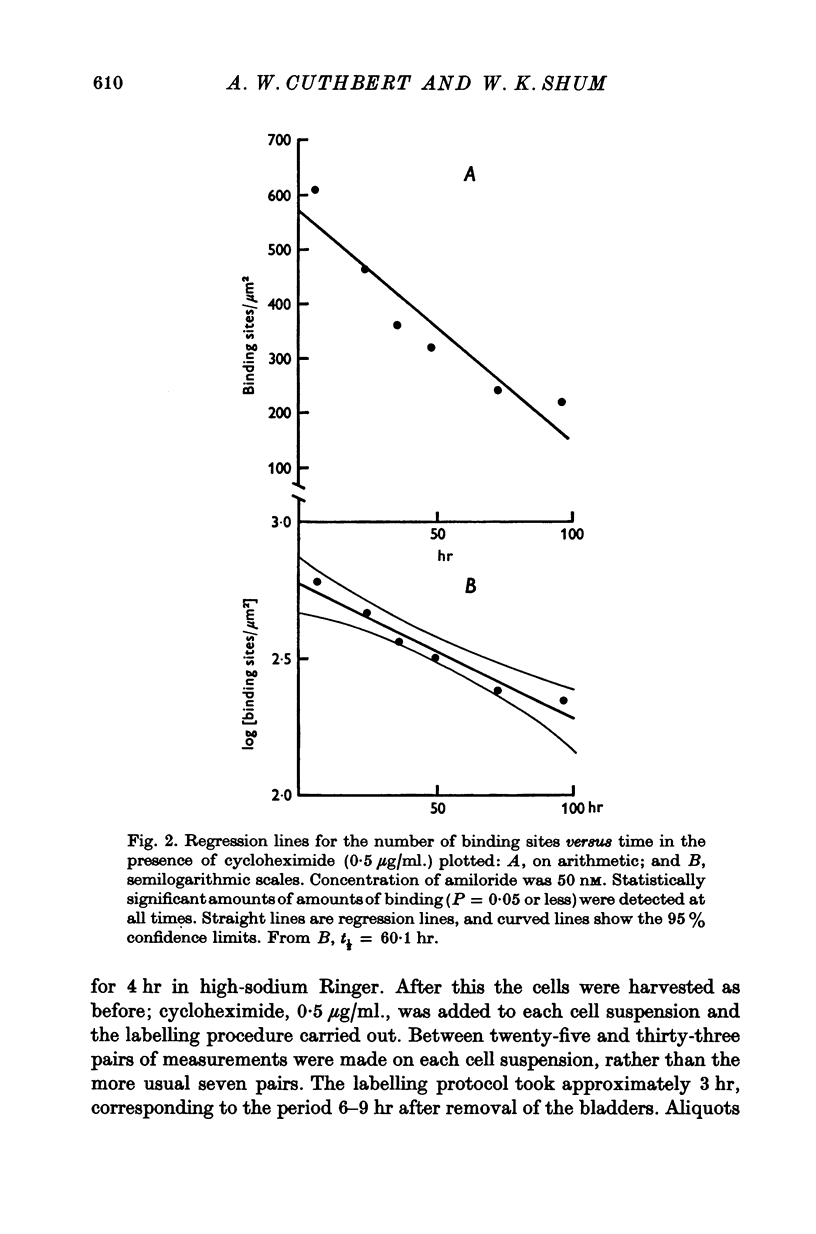
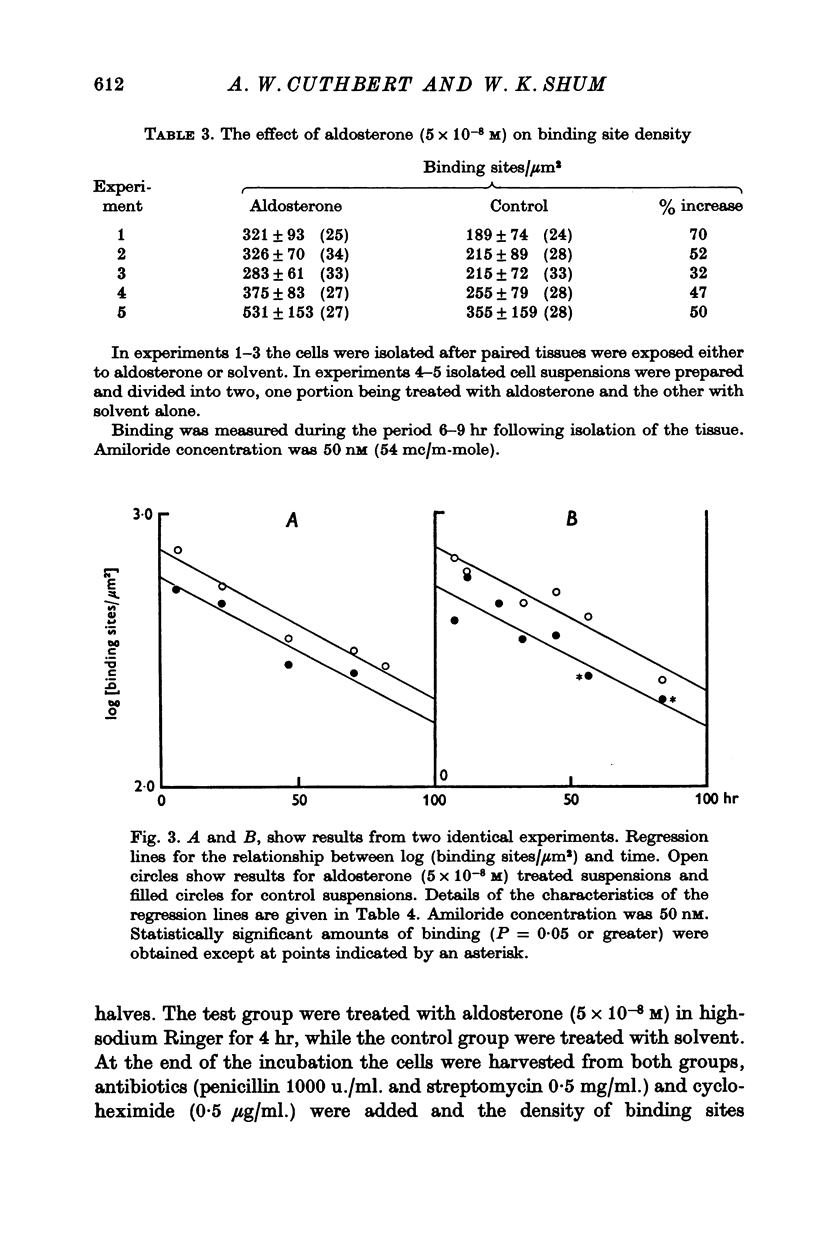
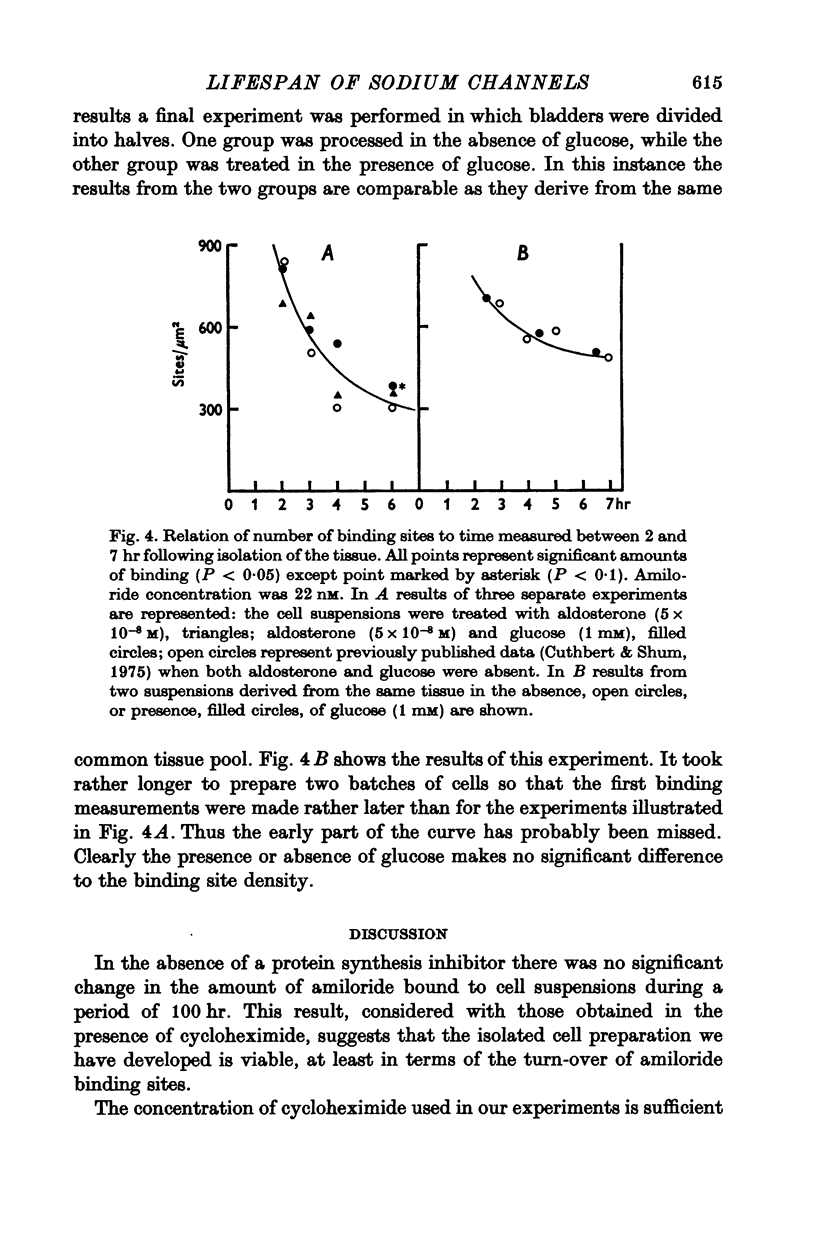
1. Sodium entry sites in the membranes of isolated epithelial cells prepared from bladders of toads (Bufo marinus) have been labelled with amiloride. The number of binding sites remained constant in suspensions for up to 100 hr. 2. In the presence of a protein synthesis inhibitor (cycloheximide, 0-5 mug/ml.) there was a decline in the density of binding sites was approximately exponential. Regression analysis gave a half-life of approximately 60 hr. 3. Aldosterone (5 X 10(-8) M) caused a significant (P less than 0-001) increase (50%) in the density of amiloride binding sites. Cells which had been treated with aldosterone had populations of binding sites which declined, in the presence of cycloheximide, at rates indistinguishable from those of untreated cells.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuthbert A. W. An upper limit to the number of sodium channels in frog skin epithelium. J Physiol. 1973 Feb;228(3):681–692. doi: 10.1113/jphysiol.1973.sp010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Okpako D., Shwm W. K. Proceedings: Aldosterone, moulting and the number of sodium channels in frog skin. Br J Pharmacol. 1974 May;51(1):128P–129P. [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Amiloride and the sodium channel. Naunyn Schmiedebergs Arch Pharmacol. 1974;281(3):261–269. doi: 10.1007/BF00500595. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Characteristics of the entry process for sodium in transporting epithelia as revealed with amiloride. J Physiol. 1976 Mar;255(3):587–604. doi: 10.1113/jphysiol.1976.sp011297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Effects of vasopressin and aldosterone on amiloride binding in toad bladder epithelial cells. Proc R Soc Lond B Biol Sci. 1975 Jun 17;189(1097):543–575. doi: 10.1098/rspb.1975.0072. [DOI] [PubMed] [Google Scholar]
- Fanestil D. D., Edelman I. S. On the mechanism of action of aldosterone on sodium transport: effects of inhibitors of RNA and of protein synthesis. Fed Proc. 1966 May-Jun;25(3):912–916. [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetycholine receptor production and incorporation into membranes of developing muscle fibers. Dev Biol. 1973 Jan;30(1):153–165. doi: 10.1016/0012-1606(73)90054-7. [DOI] [PubMed] [Google Scholar]
- Lipton P., Edelman I. S. Effects of aldosterone and vasopressin on electrolytes of toad bladder epithelial cells. Am J Physiol. 1971 Sep;221(3):733–741. doi: 10.1152/ajplegacy.1971.221.3.733. [DOI] [PubMed] [Google Scholar]
- Rossier B. C., Wilce P. A., Edelman I. S. Kinetics of RNA labeling in toad bladder epithelium: effects of aldosterone and related steroids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3101–3105. doi: 10.1073/pnas.71.8.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP G. W., LEAF A. THE CENTRAL ROLE OF PYRUVATE IN THE STIMULATION OF SODIUM TRANSPORT BY ALDOSTERONE. Proc Natl Acad Sci U S A. 1964 Oct;52:1114–1121. doi: 10.1073/pnas.52.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. N., Sapirstein V. S. Identification of aldosterone-induced proteins in the toad's urinary bladder. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4056–4060. doi: 10.1073/pnas.72.10.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Komack C. L., Leaf A. Studies on the binding of aldosterone in the toad bladder. J Clin Invest. 1966 Apr;45(4):450–459. doi: 10.1172/JCI105359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Leaf A. Mechanism of action of aldosterone. Physiol Rev. 1966 Oct;46(4):593–633. doi: 10.1152/physrev.1966.46.4.593. [DOI] [PubMed] [Google Scholar]


