Abstract
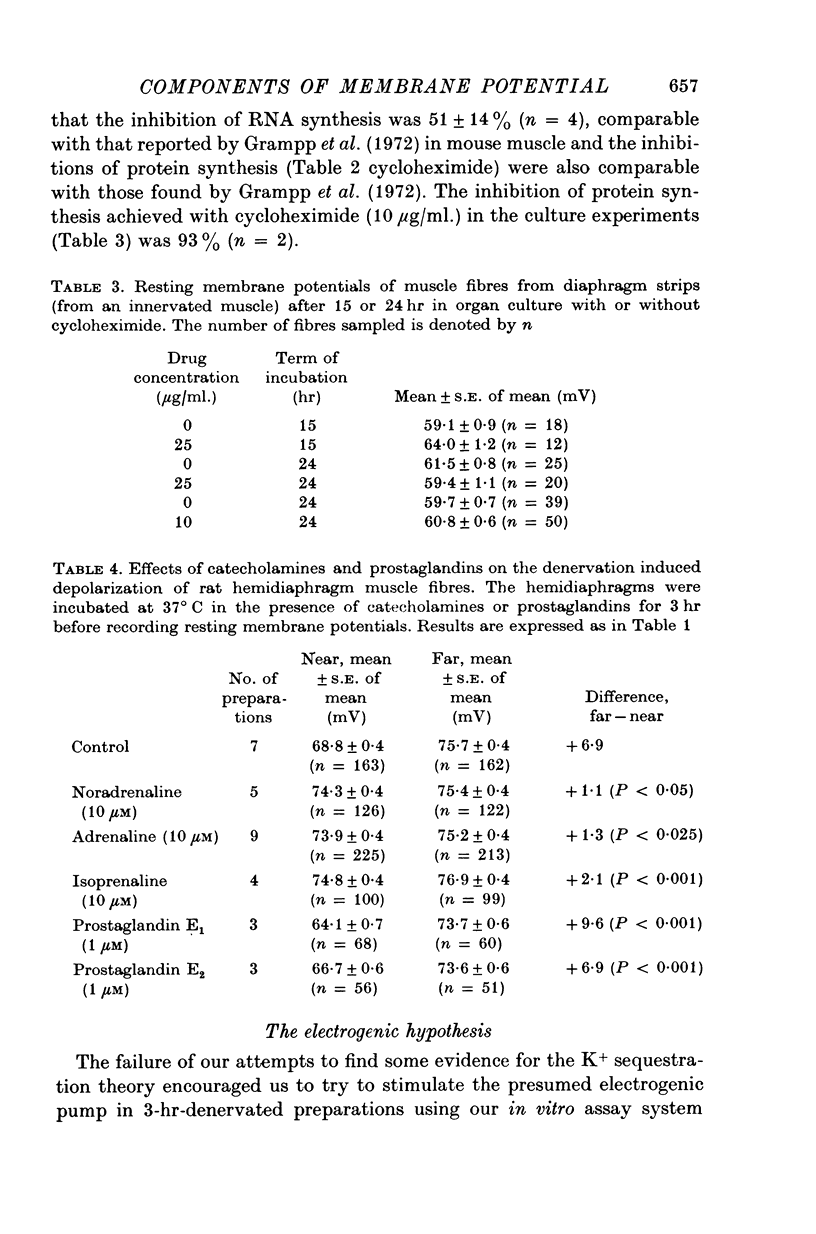
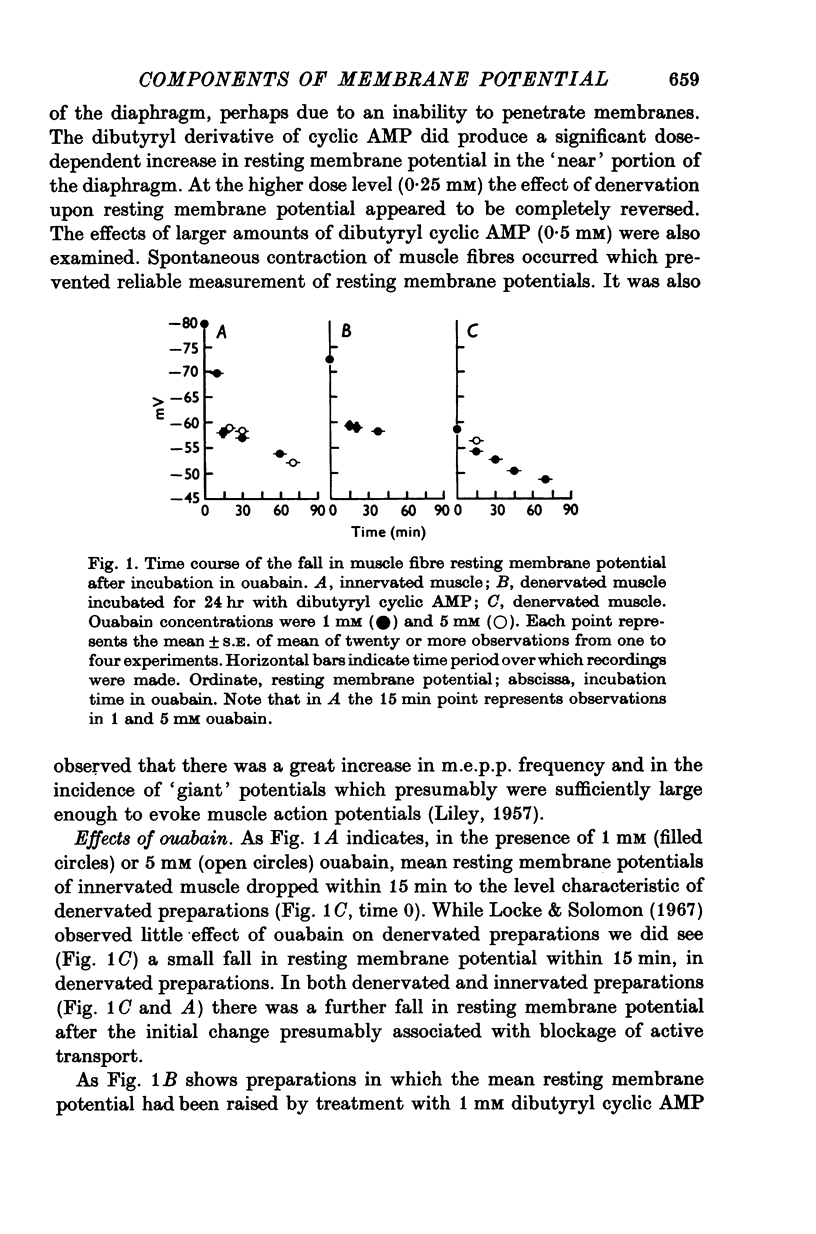
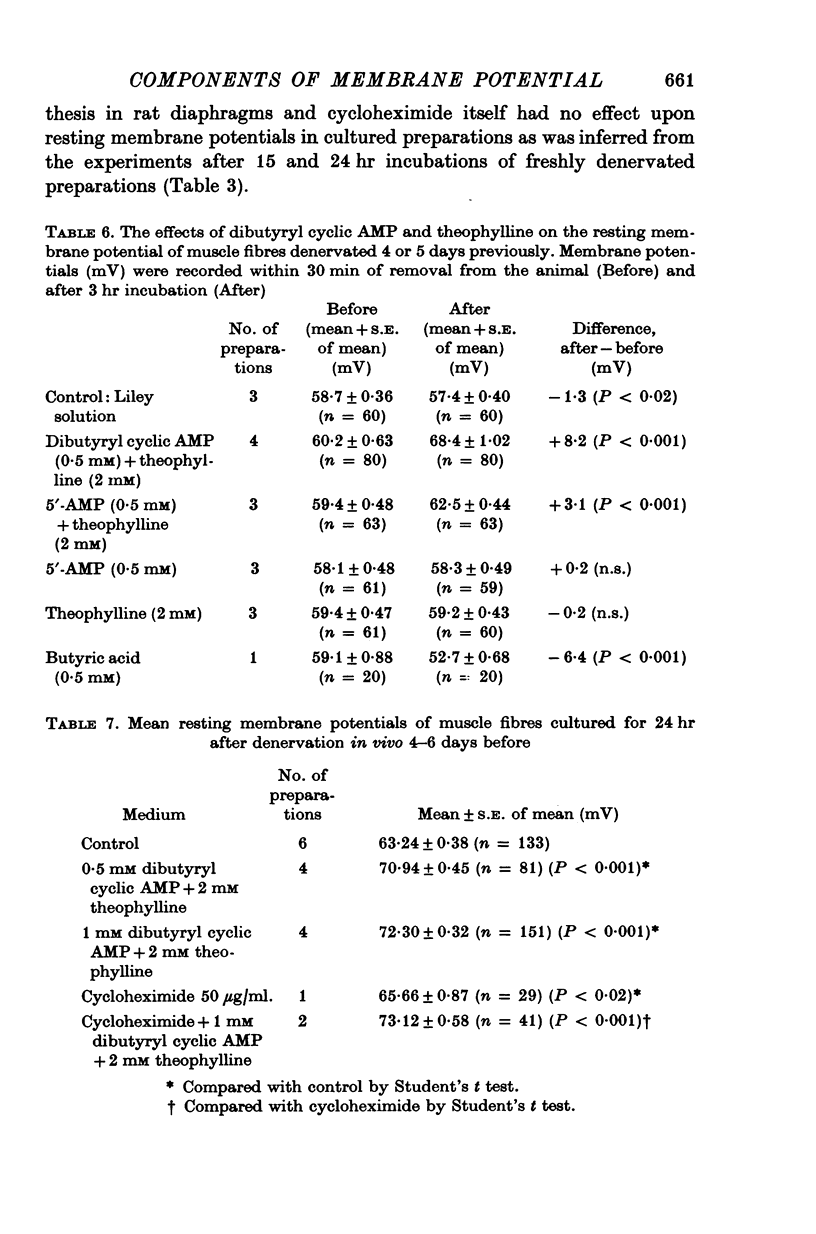
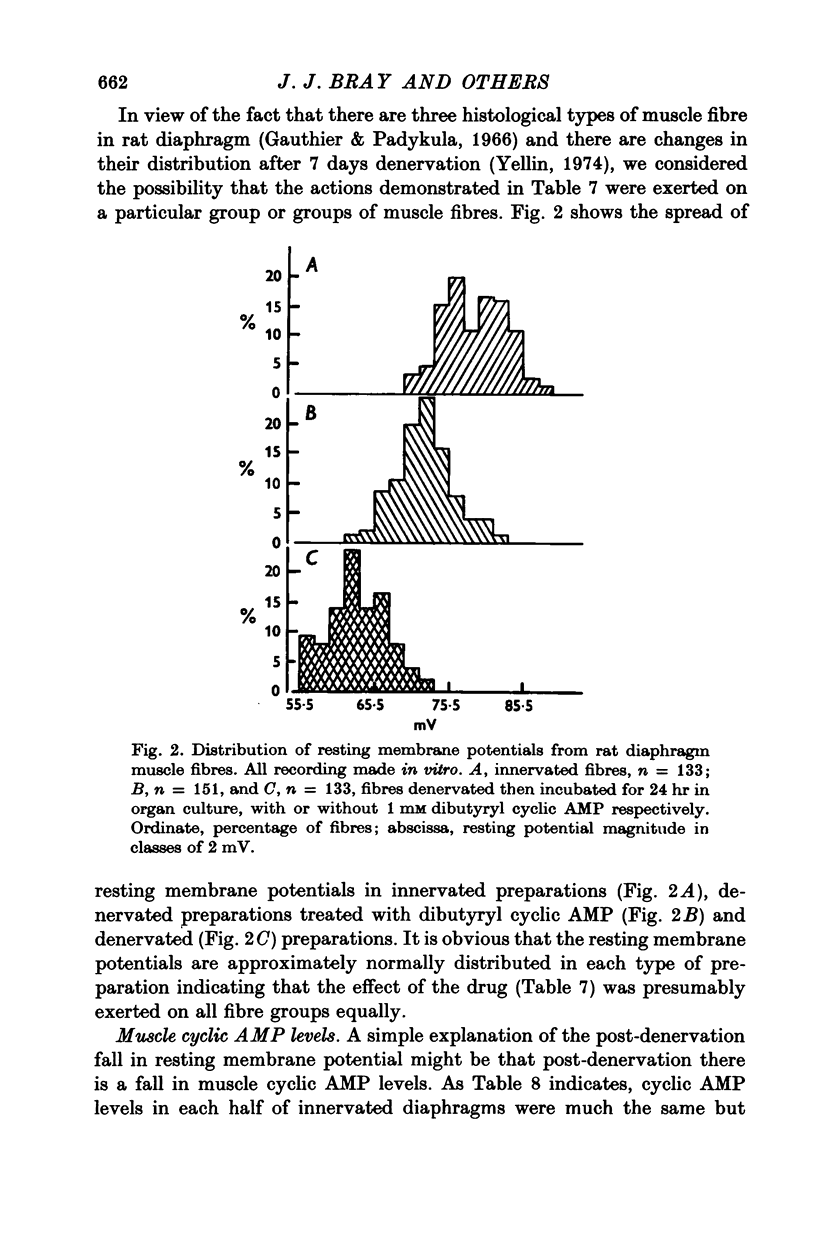
1. Resting membrane potentials of rat diaphragm muscles were measured in vitro after previous denervation for 0-10 days. In some experiments denervated muscles were incubated in vitro for 3 hr while in others they were cultured for 15-24 hr to allow adequate exposure to drugs before recording. 2. It was found that resting membrane potentials, within 2-5 mm of the site of nerve section were significantly lower, within 3 hr, than resting membrane potentials measured more than 9 mm away from site of nerve section. This difference could be reduced or abolished by bathing preparations in solutions containing adrenaline (10 muM), noradrenaline (10 muM) or isoprenaline (10 muM) or dibutyryl cyclic AMP (10 muM-0-25 mM in the presence of 2 mM theophylline). Cyclic AMP (0-5 mM) was ineffective. 3. Application of solutions containing dibutyryl cyclic AMP for 3 hr also raised the resting membrane potential of muscles denervated 4-5 days previously. Culture studies showed that this effect was sustained when the time of incubation was 24 hr. 4. Incubating freshly denervated preparations with cycloheximide (22 mug/ml.) or actinomycin D (1 mug/ml.) did not prevent the development of the early (3 hr) fall in resting membrane potential despite a concomitant inhibition of RNA or protein synthesis. Culturing freshly denervated muscles in solutions containing cycloheximide (10 or 25 mug/ml.) which blocked 93% of protein synthesis, did not prevent the expected drop in resting membrane potential after 15 or 24 hr. 5. It was found that exposure to ouabain (1 or 5 mM) produced a rapid (15 min) fall in resting membrane potential in innervated and denervated preparations treated with dibutyryl cyclic AMP but not denervated preparations. After 5 days denervation cyclic AMP levels in muscle were increased by about 40%. 6. It is suggested that upon denervation an electrogenic action of a NA+-pump is blocked and that dibutyryl cyclic AMP and catecholamines are capable of stimulating this pump.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N. Contribution of an electrogenic sodium pump to membrane potential in mammalian skeletal muscle fibres. J Physiol. 1975 Mar;245(3):499–520. doi: 10.1113/jphysiol.1975.sp010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Thesleff S. A comparative study of membrane properties of innervated and chronically denervated fast and slow skeletal muscles of the rat. Acta Physiol Scand. 1968 Aug;73(4):471–480. doi: 10.1111/j.1365-201x.1968.tb10886.x. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E., Sansone F. M., Onur R. Trophic functions of the neuron. 3. Mechanisms of neurotrophic interactions. The effects of vinblastine and colchicine on neural regulation of muscle. Ann N Y Acad Sci. 1974 Mar 22;228(0):224–243. doi: 10.1111/j.1749-6632.1974.tb20512.x. [DOI] [PubMed] [Google Scholar]
- Armstrong W. M., Lee C. O. Sodium and potassium activities in normal and "sodium-rich" frog skeletal muscle. Science. 1971 Jan 29;171(3969):413–415. doi: 10.1126/science.171.3969.413. [DOI] [PubMed] [Google Scholar]
- BOWMAN W. C., RAPER C. THE EFFECTS OF SYMPATHOMIMETIC AMINES ON CHRONICALLY DENERVATED SKELETAL MUSCLES. Br J Pharmacol Chemother. 1965 Feb;24:98–109. doi: 10.1111/j.1476-5381.1965.tb02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S., Sheppard J. R. Cyclic AMP, ATP and cell contact. Nature. 1974 Jul 5;250(461):62–64. doi: 10.1038/250062a0. [DOI] [PubMed] [Google Scholar]
- Bowman W. C., Raper C. Effects of sympathomimetic amines on neuromuscular transmission. Br J Pharmacol Chemother. 1966 Aug;27(2):313–331. doi: 10.1111/j.1476-5381.1966.tb01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Goffart M., Dias M. V. The effects of adrenaline and of sympathetic stimulation on the demarcation potential of mammalian skeletal muscle. J Physiol. 1950 Apr 15;111(1-2):184–194. doi: 10.1113/jphysiol.1950.sp004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse M. G., McMaster J., Buse J. The effect of denervation and insulin on protein synthesis in the isolated rat diaphragm. Metabolism. 1965 Nov;14(11):1220–1232. doi: 10.1016/0026-0495(65)90092-2. [DOI] [PubMed] [Google Scholar]
- Butcher R. W., Baird C. E. Effects of prostaglandins on adenosine 3',5'-monophosphate levels in fat and other tissues. J Biol Chem. 1968 Apr 25;243(8):1713–1717. [PubMed] [Google Scholar]
- CREESE R. Measurement of cation fluxes in rat diaphragm. Proc R Soc Lond B Biol Sci. 1954 Sep 27;142(909):497–513. doi: 10.1098/rspb.1954.0039. [DOI] [PubMed] [Google Scholar]
- Carlsen R. C. The possible role of cyclic AMP in the neurotrophic control of skeletal muscle. J Physiol. 1975 May;247(2):343–361. doi: 10.1113/jphysiol.1975.sp010935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. W., Rall T. W., Larner J. The influence of insulin and epinephrine on adenosine 3',5'-phosphate and glycogen transferase in muscle. Biochim Biophys Acta. 1969 Apr 1;177(2):213–219. doi: 10.1016/0304-4165(69)90130-5. [DOI] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockry M., Kernan R. P., Tangney A. Active transport of sodium and potassium in mammalian skeletal muscle and its modification by nerve and by cholinergic and adrenergic agents. J Physiol. 1966 Sep;186(1):187–200. doi: 10.1113/jphysiol.1966.sp008028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Padykula H. A. Cytological studies of fiber types in skeletal muscle. A comparative study of the mammalian diaphragm. J Cell Biol. 1966 Feb;28(2):333–354. doi: 10.1083/jcb.28.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grampp W., Harris J. B., Thesleff S. Inhibition of denervation changes in skeletal muscle by blockers of protein synthesis. J Physiol. 1972 Mar;221(3):743–754. doi: 10.1113/jphysiol.1972.sp009780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTCHISON W. C., MUNRO H. N. The determination of nucleic acids in biological materials. A review. Analyst. 1961 Dec;86:768–813. doi: 10.1039/an9618600768. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Manchester K. L. The effects of potassium ions and denervation on protein synthesis and the transport of amino acids in muscle. Biochem J. 1966 Oct;101(1):135–145. doi: 10.1042/bj1010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Ochs S. Effects of sodium extrusion and local anaesthetics on muscle membrane resistance and potential. J Physiol. 1966 Nov;187(1):5–21. doi: 10.1113/jphysiol.1966.sp008072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y. Resting potentials of Na-loaded sartorius muscle fibres of toads during recovery in high K ringer. Kumamoto Med J. 1965 Mar 31;18(1):23–30. [PubMed] [Google Scholar]
- KERNAN R. P. RESTING POTENTIAL OF ISOLATED RAT MUSCLES MEASURED IN PLASMA. Nature. 1963 Nov 2;200:474–475. doi: 10.1038/200474a0. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. The role of lactate in the active excretion of sodium by frog muscle. J Physiol. 1962 Jun;162:129–137. doi: 10.1113/jphysiol.1962.sp006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLAUS W., LUELLMANN H., MUSCHOLL E. [Potassium flux of normal and denervated rat diaphragm]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:761–775. [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Some effects produced by adrenaline upon neuromuscular propagation in rats. J Physiol. 1958 Apr 30;141(2):291–304. doi: 10.1113/jphysiol.1958.sp005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan R. P., MacDermott M. Proceedings: Changes in potassium activity within frog sartorius muscle fibres during sodium enrichment in potassium-free Ringer fluid. J Physiol. 1975 Jul;249(1):25P–26P. [PubMed] [Google Scholar]
- Kernan R. P. Studies of caesium uptake by rat soleus and vastus lateralis muscles in vivo and of its efflux rate relative to potassium in vitro. Pflugers Arch. 1972;333(2):95–110. doi: 10.1007/BF00586910. [DOI] [PubMed] [Google Scholar]
- Kuba K. Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J Physiol. 1970 Dec;211(3):551–570. doi: 10.1113/jphysiol.1970.sp009293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. L., SHY G. M., WELLS J. Some properties of mammalian skeletal muscle fibres with particular reference to fibrillation potentials. J Physiol. 1957 Mar 11;135(3):522–535. doi: 10.1113/jphysiol.1957.sp005727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. Spontaneous release of transmitter substance in multiquantal units. J Physiol. 1957 May 23;136(3):595–605. doi: 10.1113/jphysiol.1957.sp005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LULLMANN H. Uber die Konstanz des Membranpotentials bei spontanen Anderungen der Ionengradienten am normalen und denervierten Rattenzwerchfell. Pflugers Arch. 1958;267(2):188–189. doi: 10.1007/BF00362985. [DOI] [PubMed] [Google Scholar]
- Lentz T. L. A role of cyclic AMP in a neurotrophic process. Nat New Biol. 1972 Aug 2;238(83):154–155. doi: 10.1038/newbio238154a0. [DOI] [PubMed] [Google Scholar]
- Lentz T. L. Trophic functions of the neuron. VI. Other trophic systems. Neurotrophic regulation at the neuromuscular junction. Ann N Y Acad Sci. 1974 Mar 22;228(0):323–337. doi: 10.1111/j.1749-6632.1974.tb20521.x. [DOI] [PubMed] [Google Scholar]
- Locke S., Solomon H. C. Relation of resting potential of rat gastrocnemius and soleus muscles to innervation, activity, and the Na-K pump. J Exp Zool. 1967 Dec;166(3):377–386. doi: 10.1002/jez.1401660310. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchnik S., Ruarte A. C., Kotsias B. A. Effects of actinomycin D on fibrillation activity in denervated skeletal muscles of the rat. Life Sci. 1973 Dec 16;13(12):1763–1770. doi: 10.1016/0024-3205(73)90123-9. [DOI] [PubMed] [Google Scholar]
- Purves D., Sakmann B. The effect of contractile activity on fibrillation and extrajunctional acetylcholine-sensitivity in rat muscle maintained in organ culture. J Physiol. 1974 Feb;237(1):157–182. doi: 10.1113/jphysiol.1974.sp010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. I. Quantitative aspects. Acta Physiol Scand. 1971 Apr;81(4):557–564. doi: 10.1111/j.1748-1716.1971.tb04932.x. [DOI] [PubMed] [Google Scholar]
- TROWELL O. A. The culture of mature organs in a synthetic medium. Exp Cell Res. 1959 Jan;16(1):118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]
- Thesleff S. Trophic functions of the neuron. II. Denervation and regulation of muscle. Physiological effects of denervation of muscle. Ann N Y Acad Sci. 1974 Mar 22;228(0):89–104. doi: 10.1111/j.1749-6632.1974.tb20504.x. [DOI] [PubMed] [Google Scholar]
- Yellin H. Changes in fiber types of the hypertrophying denervated hemidiaphragm. Exp Neurol. 1974 Feb;42(2):412–428. doi: 10.1016/0014-4886(74)90035-1. [DOI] [PubMed] [Google Scholar]


