Abstract
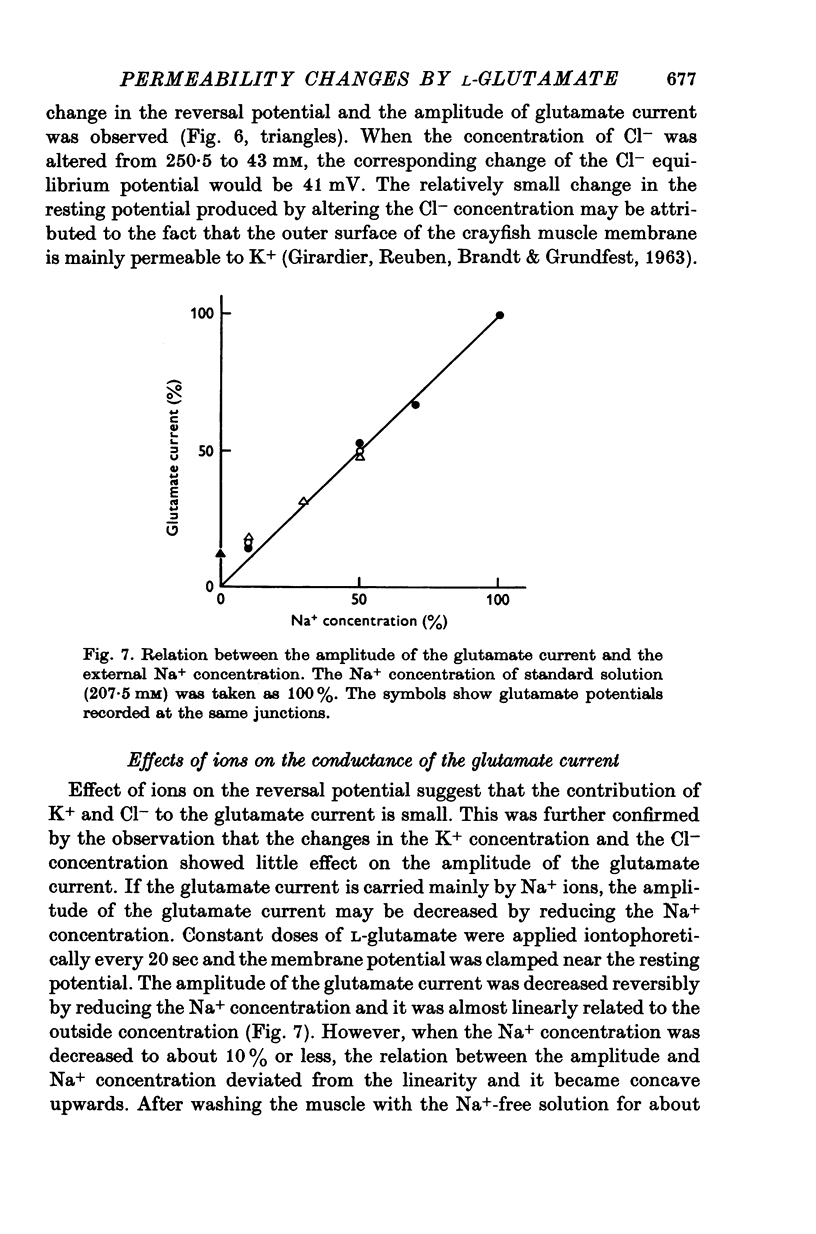
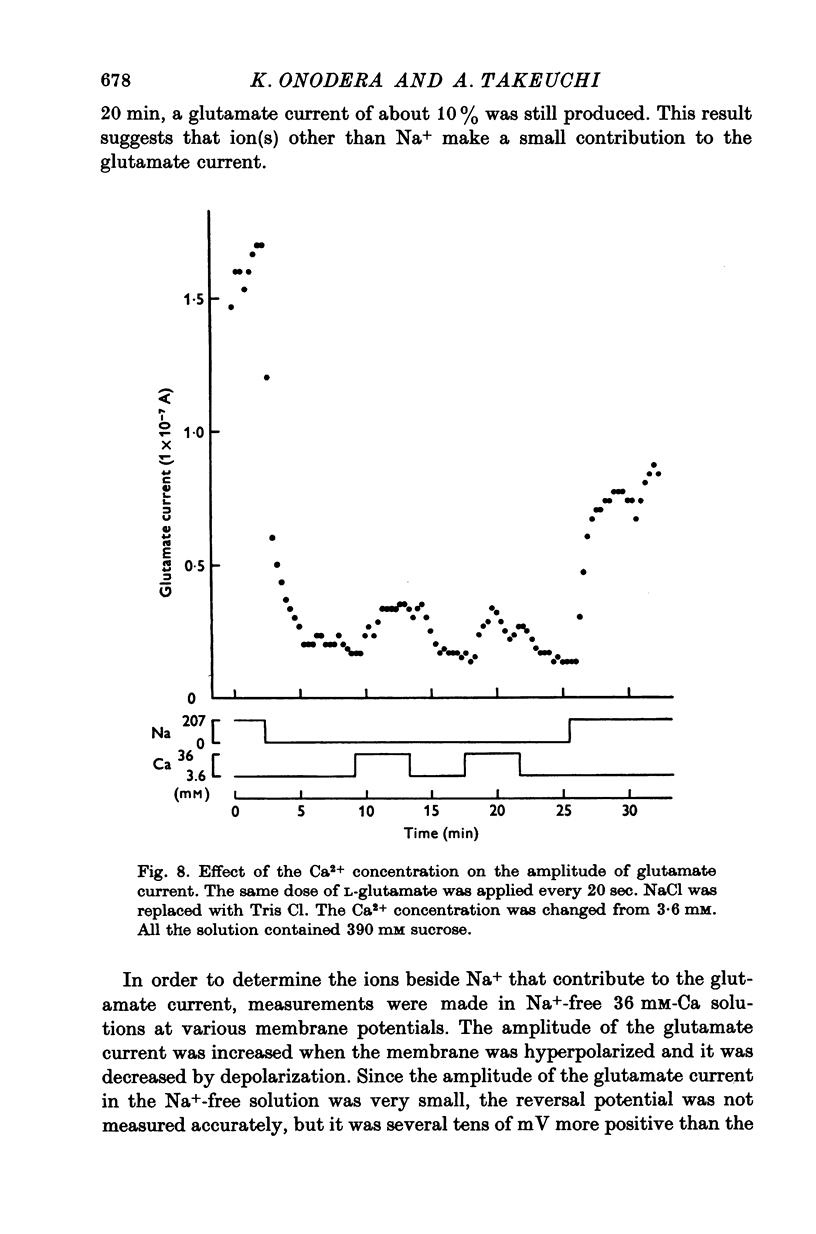
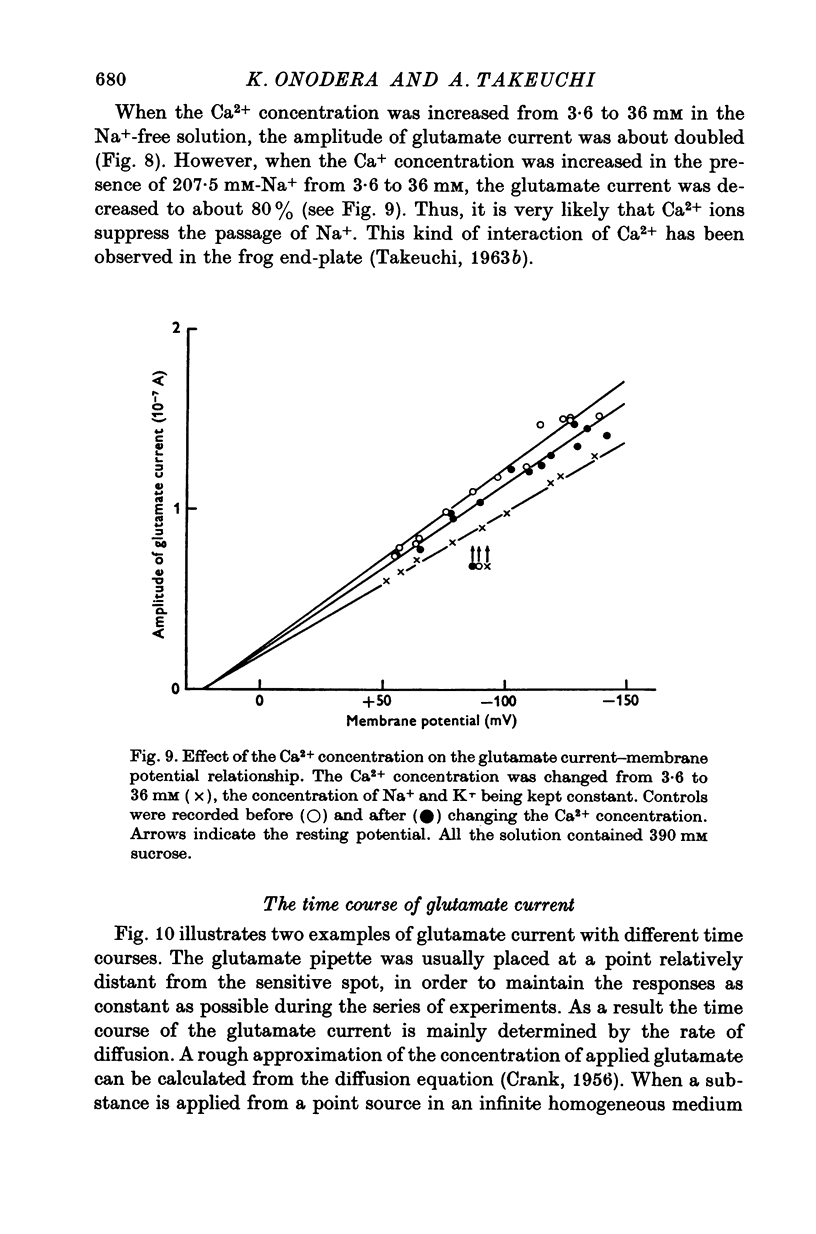
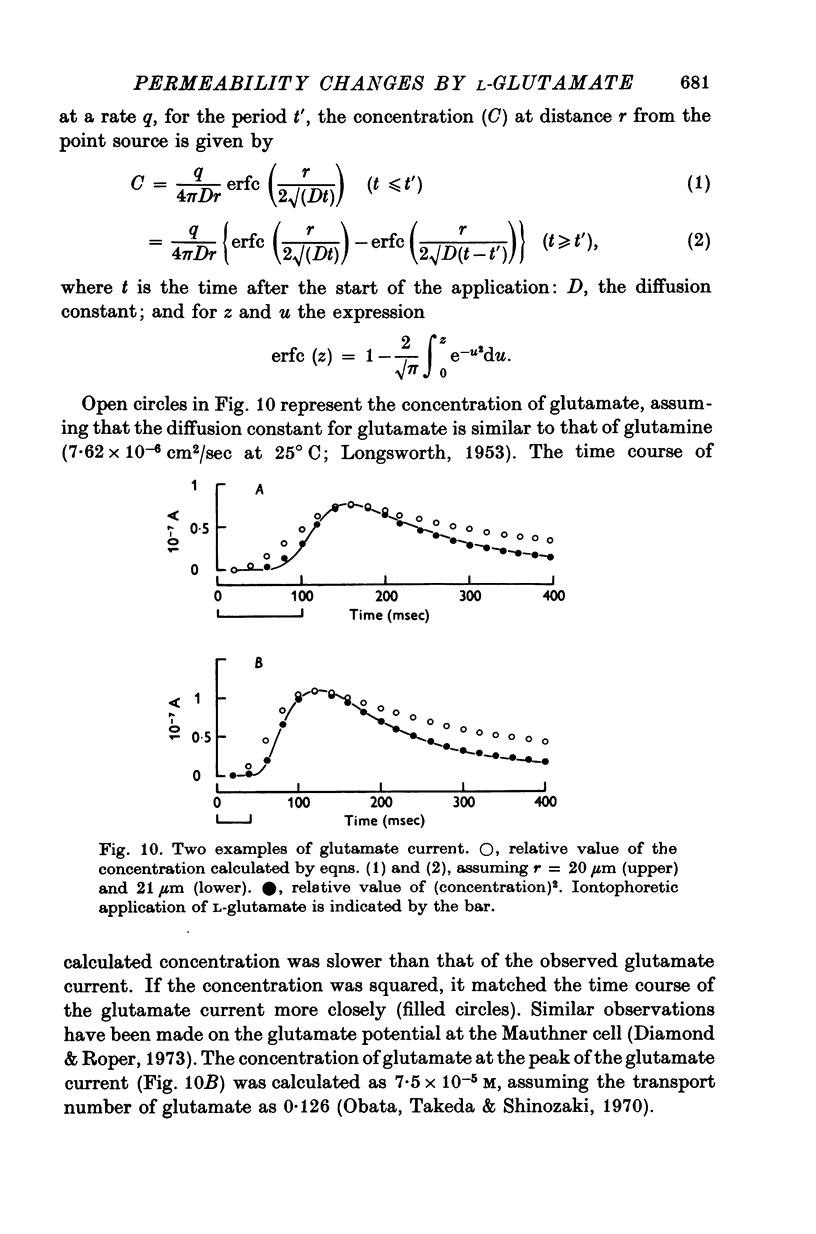
1. Permeability changes produced by L-glutamate at the neuromuscular junction of the crayfish (Cambarus clarkii) were investigated by application of the drug iontophoretically to the voltage-clamped junction and measuring the resulting 'glutamate current'. 2. Reversal potentials were determined by measuring the glutamate current at different membrane potentials. They were +39-1 +/- 3-6 mV (mean +/- S.E. of mean) in normal solution and +16-5 +/- 2-0 mV in solutions made twice as hypertonic by the addition of sucrose. 3. Decreasing external Na+ concentration shifted the reversal potential in the negative direction; increased Na+ in the positive direction. 4. The relation between the amplitude of the glutamate current and extracellular Na+ concentration was approximately linear. 5. Alteration of the external K+ or Cl- concentration did not affect the amplitude or reversal potential of glutamate current. 6. In Na+-free solution the application of L-glutamate produced a small inward current at the resting potential and its amplitude was augmented by increasing the external Ca2+ concentration. 7. Increasing the Ca2+ concentration in the normal Na+ media produced no appreciable effect on the reversal potential but decreased the amplitude of glutamate current. 8. The results indicate that L-glutamate increases the membrane permeability mainly to Na+ and slightly to Ca2+. 9. The time course of glutamate current was shorter than that of the concentration calculated from the diffusion equation and it was simulated more closely by the square of the concentration.
Full text
PDF

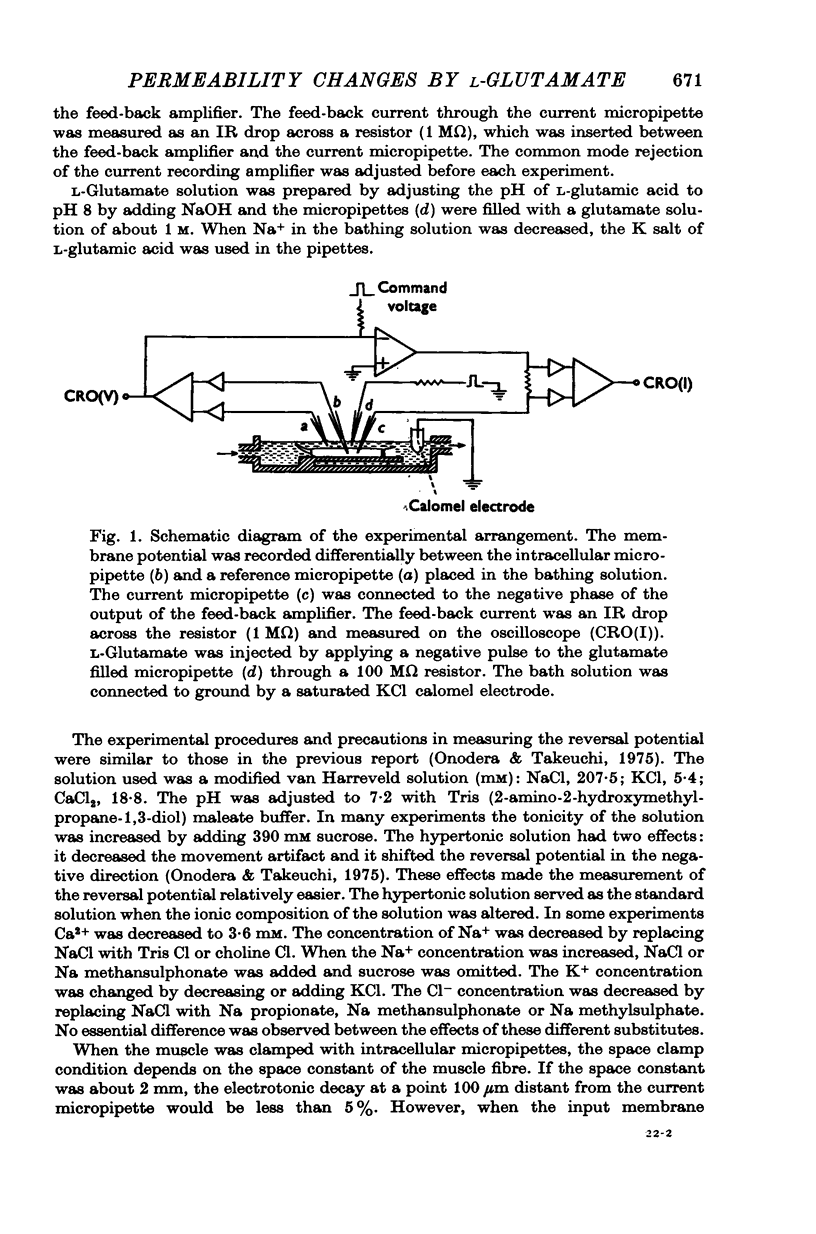

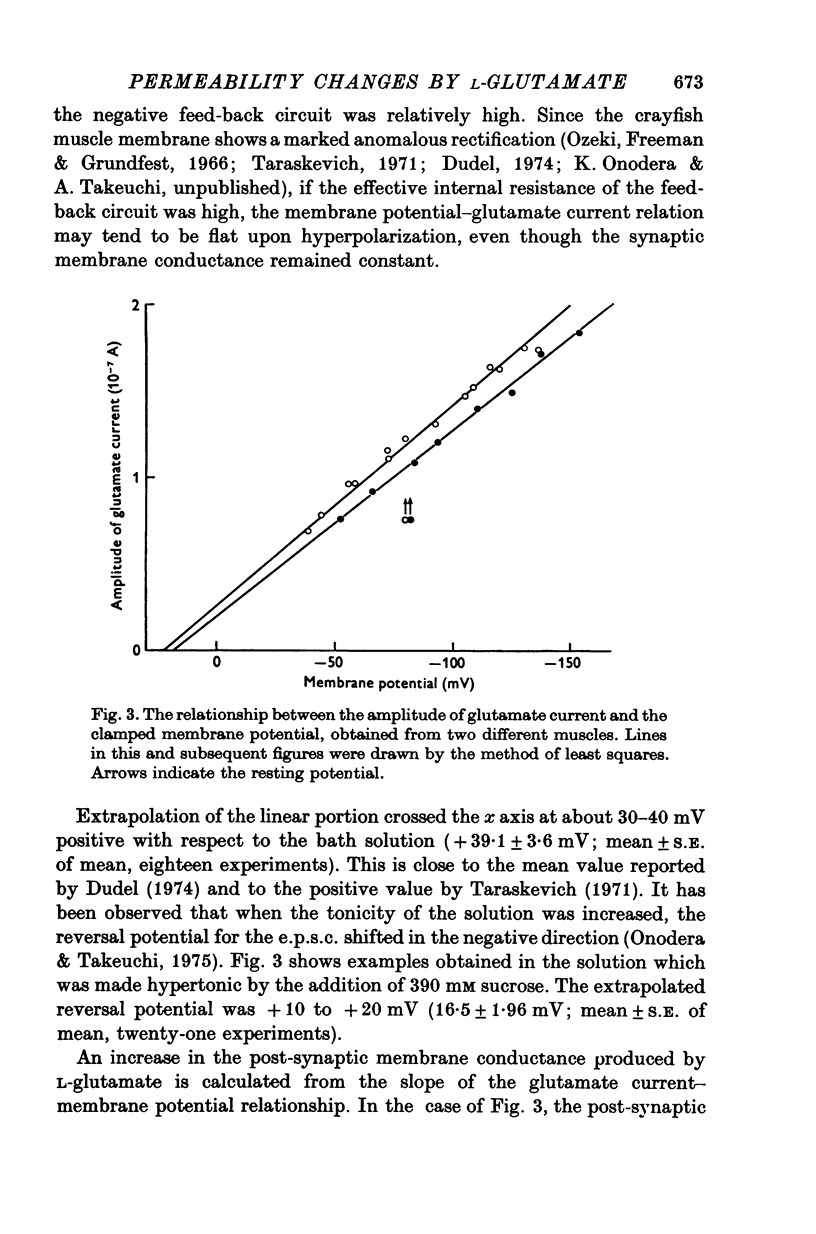
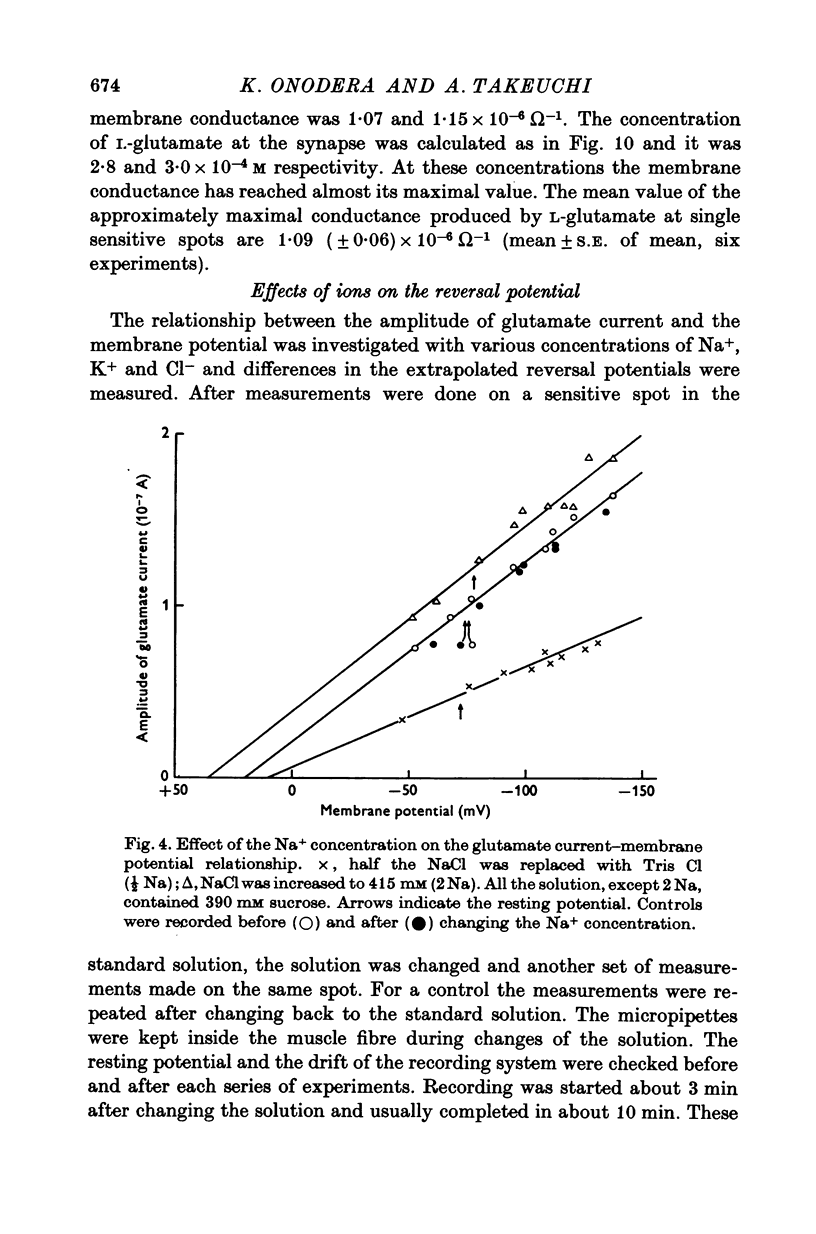

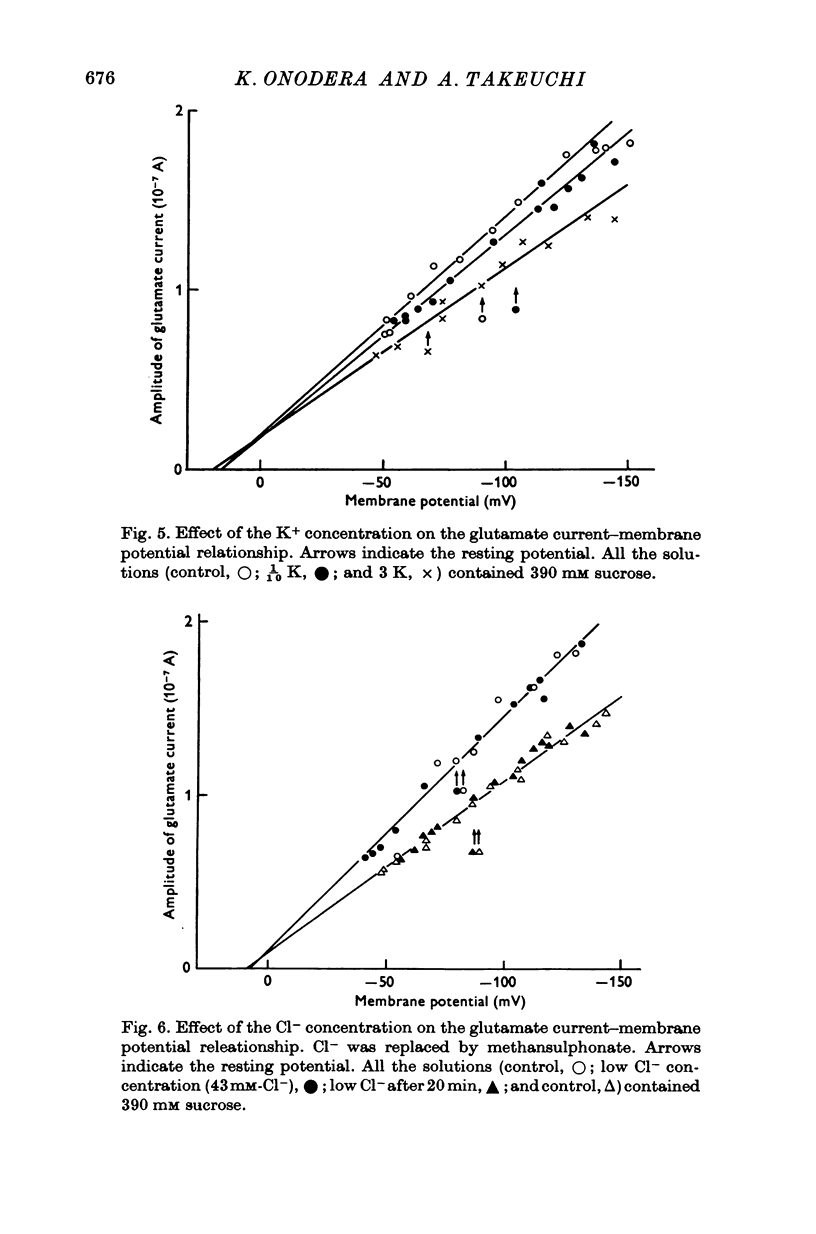





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Diamond J., Roper S. Analysis of Mauthner cell responses to iontophoretically delivered pulses of GABA, glycine and L-glutamate. J Physiol. 1973 Jul;232(1):113–128. doi: 10.1113/jphysiol.1973.sp010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. Nonlinear voltage dependence of excitatory synaptic current in crayfish muscle. Pflugers Arch. 1974;352(3):227–241. doi: 10.1007/BF00590488. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARDIER L., REUBEN J. P., BRANDT P. W., GRUNDFEST H. EVIDENCE FOR ANION-PERMSELECTIVE MEMBRANE IN CRAYFISH MUSCLE FIBERS AND ITS POSSIBLE ROLE IN EXCITATION-CONTRACTION COUPLING. J Gen Physiol. 1963 Sep;47:189–214. doi: 10.1085/jgp.47.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Acetylcholine in mammalian neuromuscular transmission. Nature. 1958 Sep 20;182(4638):805–806. doi: 10.1038/182805b0. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K., Takeda K., Shinozaki H. Electrophoretic release of gamma-aminobutyric acid and glutamic acid from micropipettes. Neuropharmacology. 1970 May;9(3):191–194. doi: 10.1016/0028-3908(70)90066-3. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Ionic mechanism of the excitatory synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1975 Oct;252(1):295–318. doi: 10.1113/jphysiol.1975.sp011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki M., Freeman A. R., Grundfest H. The membrane components of crustacean neuromuscular systems. II. Analysis of interactions among the electrogenic components. J Gen Physiol. 1966 Jul;49(6):1335–1349. doi: 10.1085/jgp.0491335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki M., Grundfest H. Crayfish muscle fiber: ionic requirements for depolarizing synaptic electrogenesis. Science. 1967 Jan 27;155(3761):478–481. doi: 10.1126/science.155.3761.478. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. LOCALIZED ACTION OF GAMMA-AMINOBUTYRIC ACID ON THE CRAYFISH MUSCLE. J Physiol. 1965 Mar;177:225–238. doi: 10.1113/jphysiol.1965.sp007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI N. Effects of calcium on the conductance change of the end-plate membrane during the action of transmitter. J Physiol. 1963 Jun;167:141–155. doi: 10.1113/jphysiol.1963.sp007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI N. Some properties of conductance changes at the end-plate membrane during the action of acetylcholine. J Physiol. 1963 Jun;167:128–140. doi: 10.1113/jphysiol.1963.sp007136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Onodera K. Reversal potentials of the excitatory transmitter and L-glutamate at the crayfish neuromuscular junction. Nat New Biol. 1973 Mar 28;242(117):124–126. doi: 10.1038/newbio242124a0. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Actions of transmitter substances on the neuromuscular junctions of vertebrates and invertebrates. Adv Biophys. 1972;3:45–95. [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion permeability of the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1967 Aug;191(3):575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraskevich P. S. Reversal potentials of L-glutamate and the excitatory transmitter at the neuromuscular junction of the crayfish. Biochim Biophys Acta. 1971 Aug 13;241(2):700–703. doi: 10.1016/0005-2736(71)90071-x. [DOI] [PubMed] [Google Scholar]


