Abstract
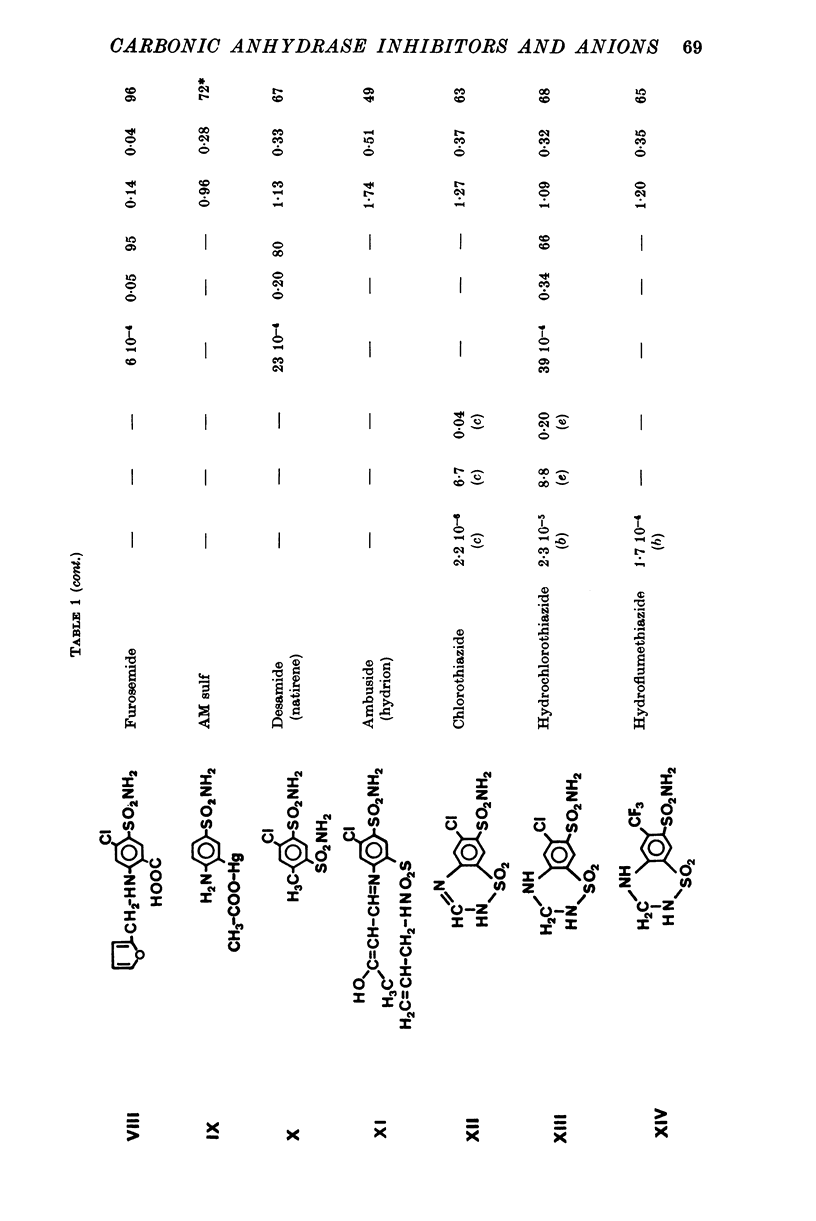
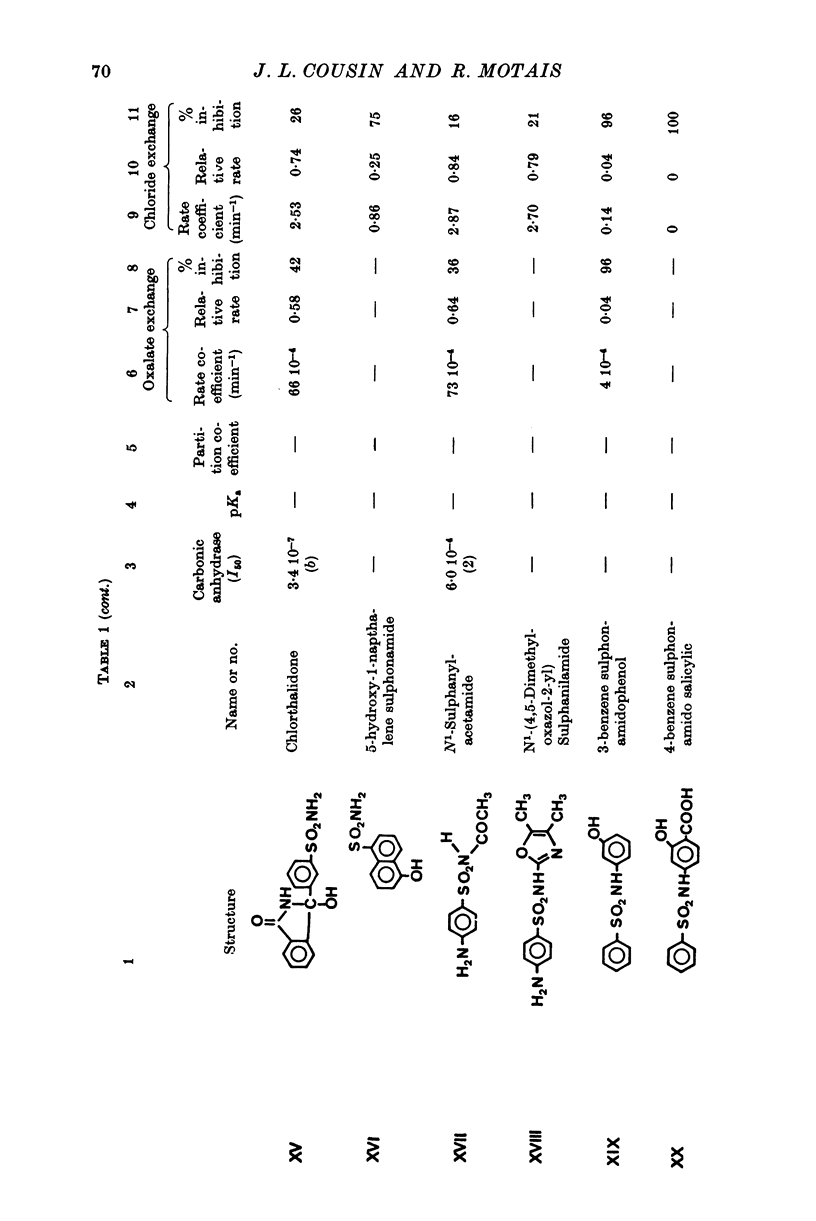
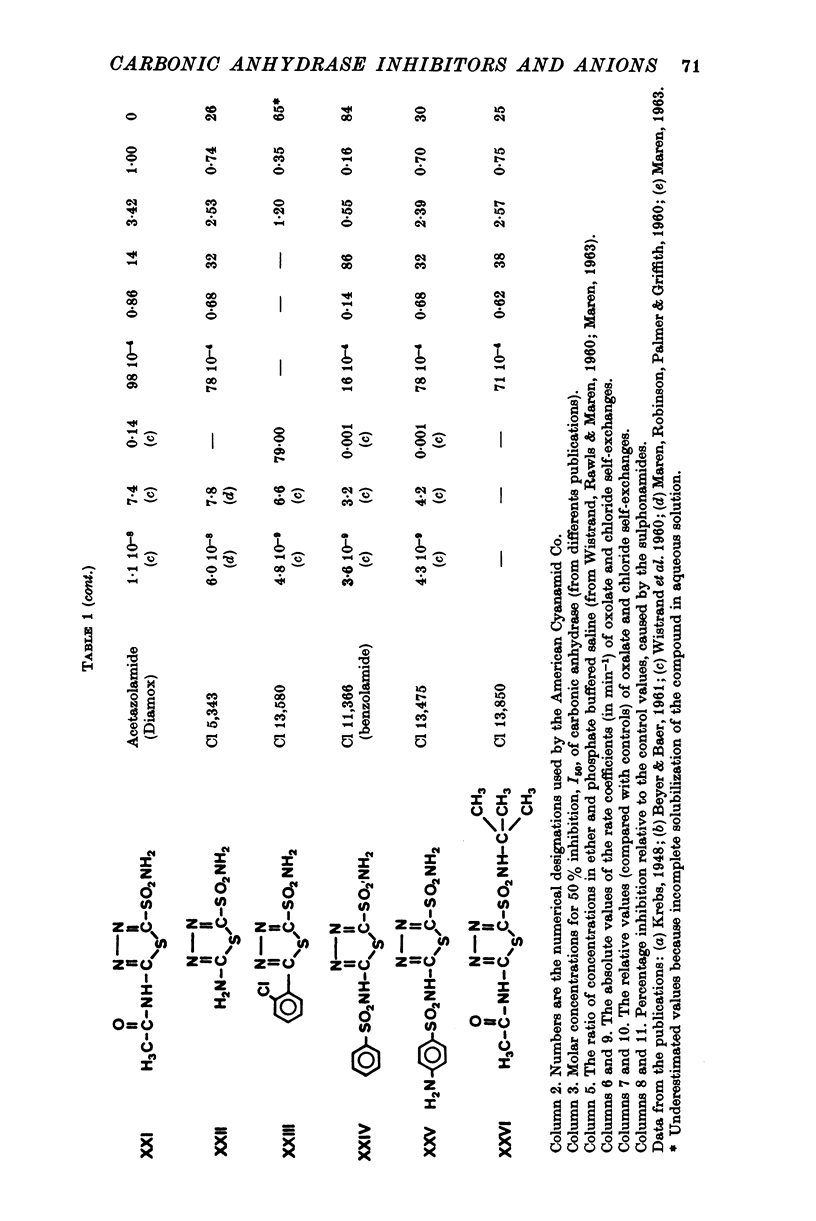
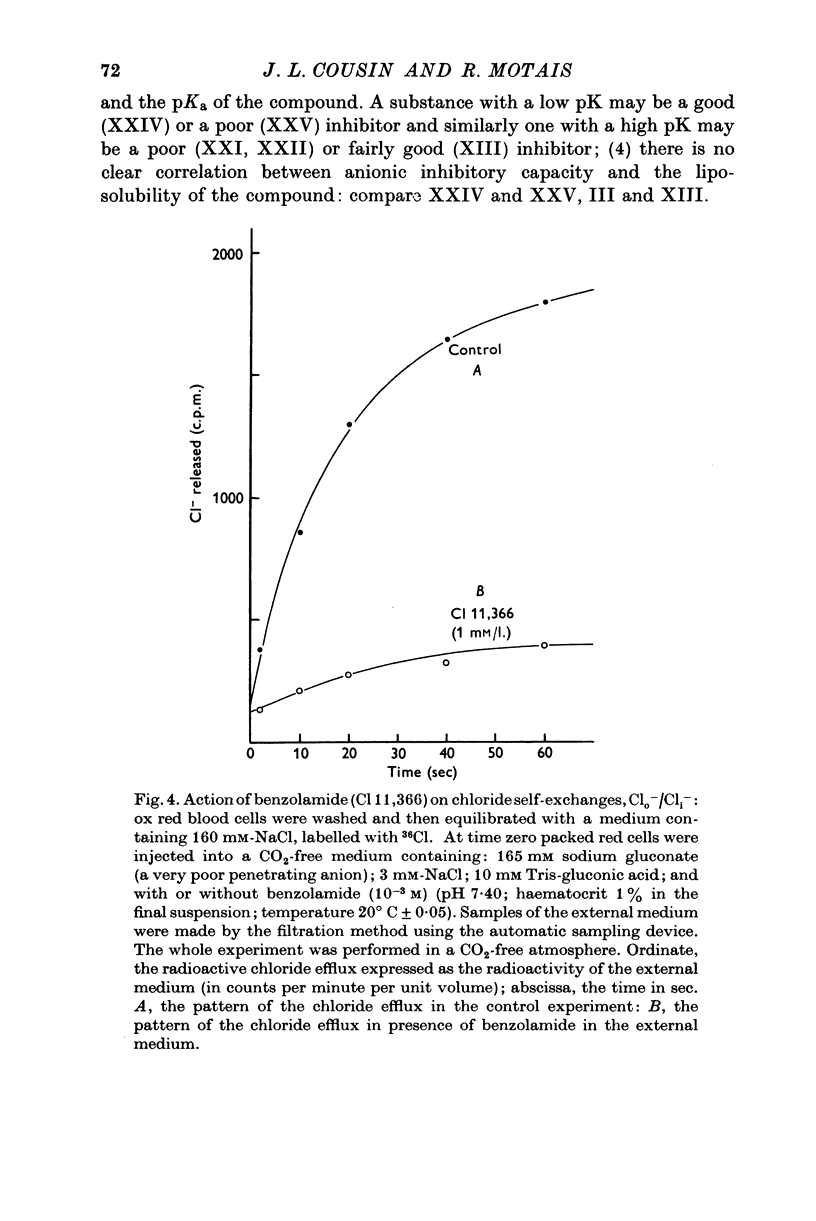
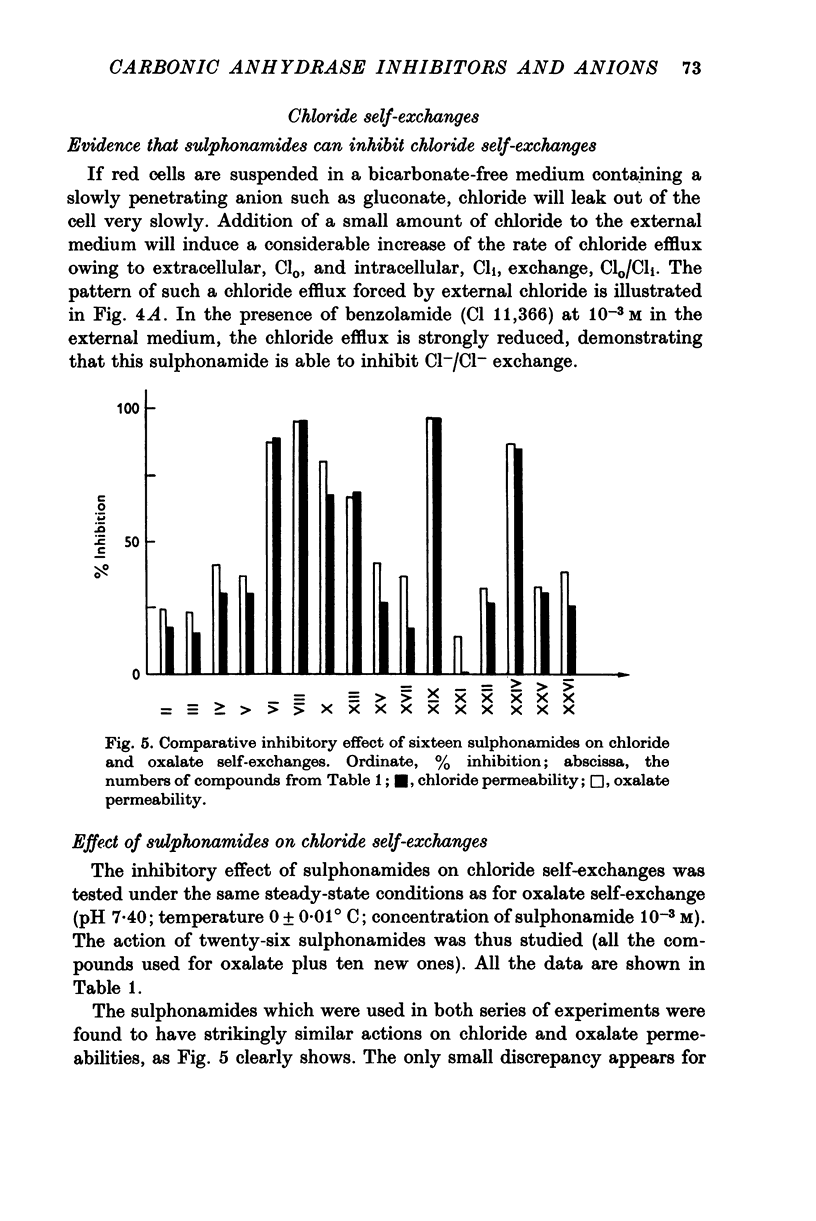
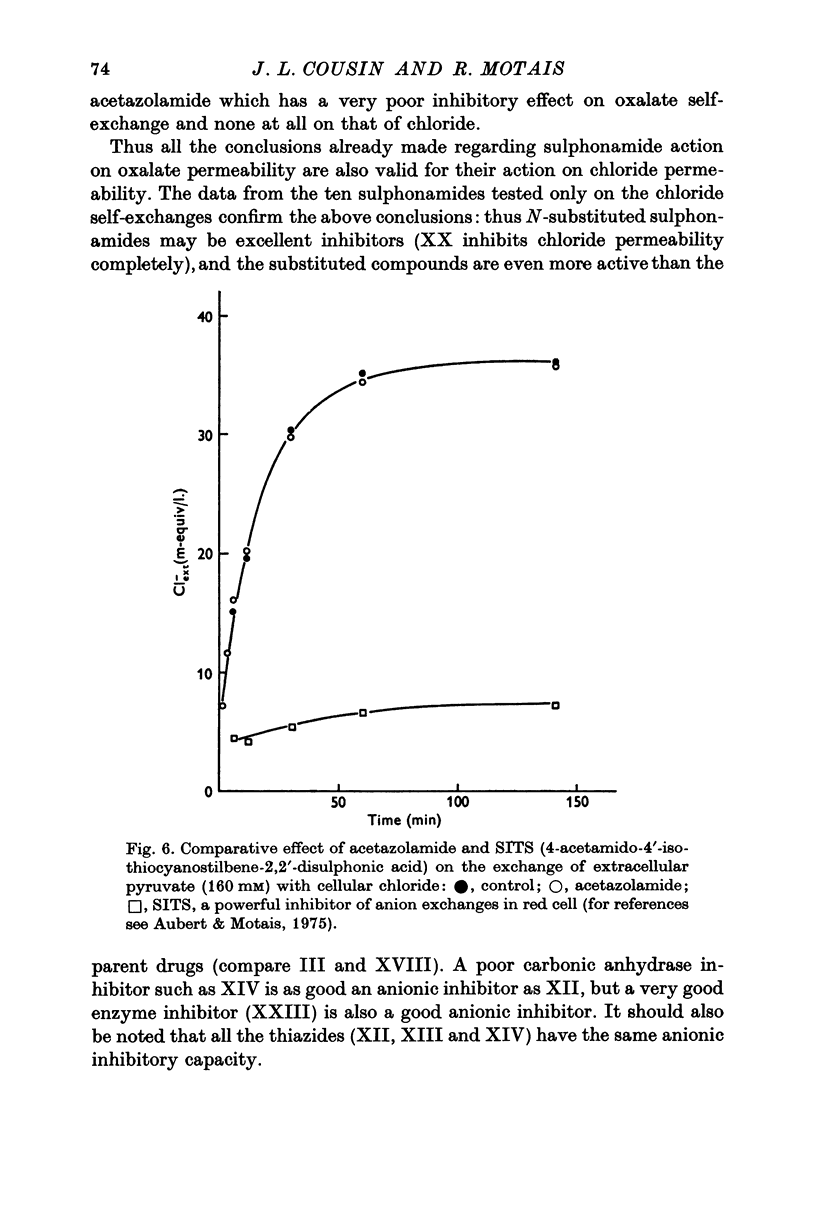
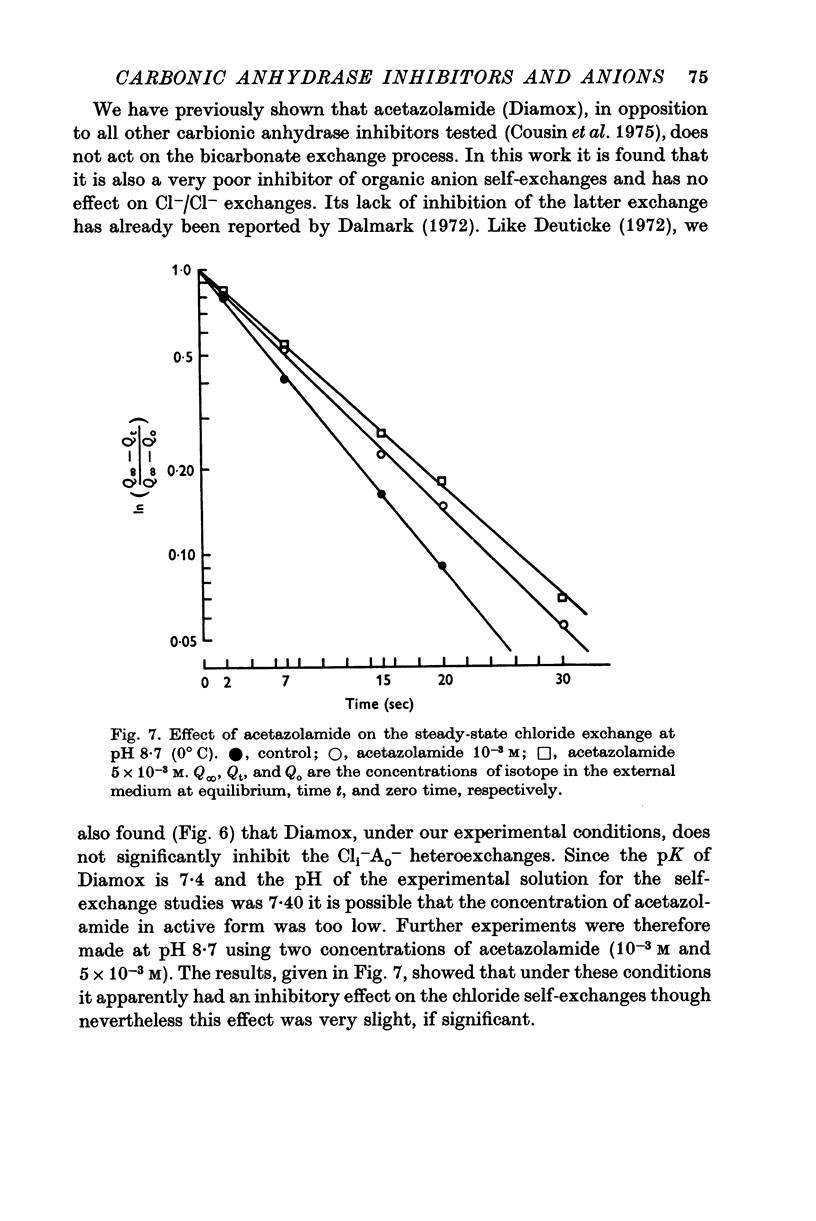
1. Organic anion permeability in ox red blood cell was measured by studying steady-state self-exchange of oxalate, chosen as a prototypical substrate of the organic anion transport system previously described; chloride self-exchange measured the inorganic anion permeability. 2. Carbonic anhydrase inhibitors of the sulphonamide class inhibit both organic anion self-exchange (A-/A-) and chloride self-exchang (CL-/CL-) although carbonic anhydrase plays no role in these exchanges. These results confirm the conclusions already published that sulphonamides can act directly on the cellular membrane as specific inhibitors of anion transport. 3. There is a correlation between the chemical structure of the sulphonamides and their capacity for inhibiting transmembrane anionic exchange. It is of significance that N-sulphamyl substitution, which abolishes the carbonic anhydrase inhibitory potency, does not destroy anionic inhibitory capacity and may even increase it. 4. For each sulphonamide the capacities for inhibiting chloride transport and oxalate transport are strictly identical. Inhibition appears non-competitive. 5. The temperature sensitivity of oxalate self-exchange is exactly the same as that of chloride self-exchange. From this, and from the nature of their inhibition by sulphonamides, it is proposed that chloride and organic anions share the same transport mechanism. 6. In the light of the present results the chloruretic action of sulphonamides in various tissues, in particular the kidney, is discussed.
Full text
PDF

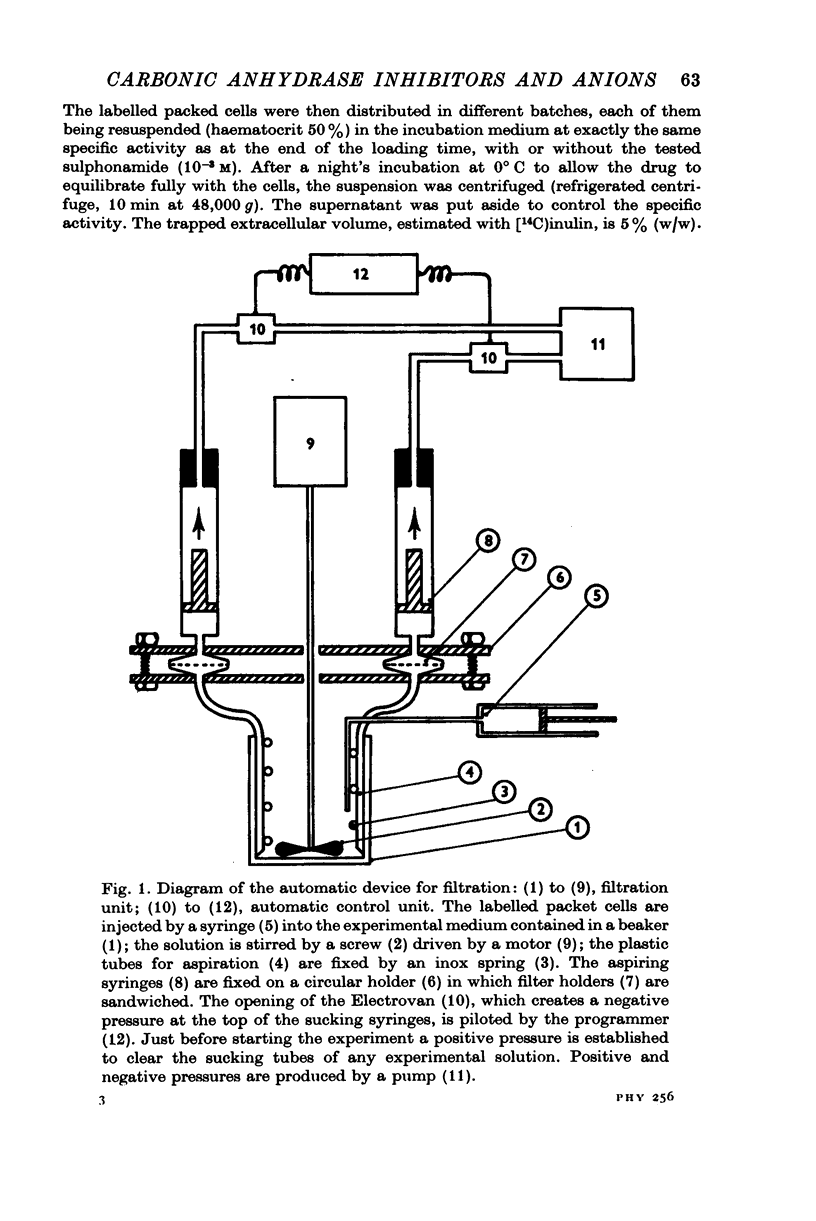


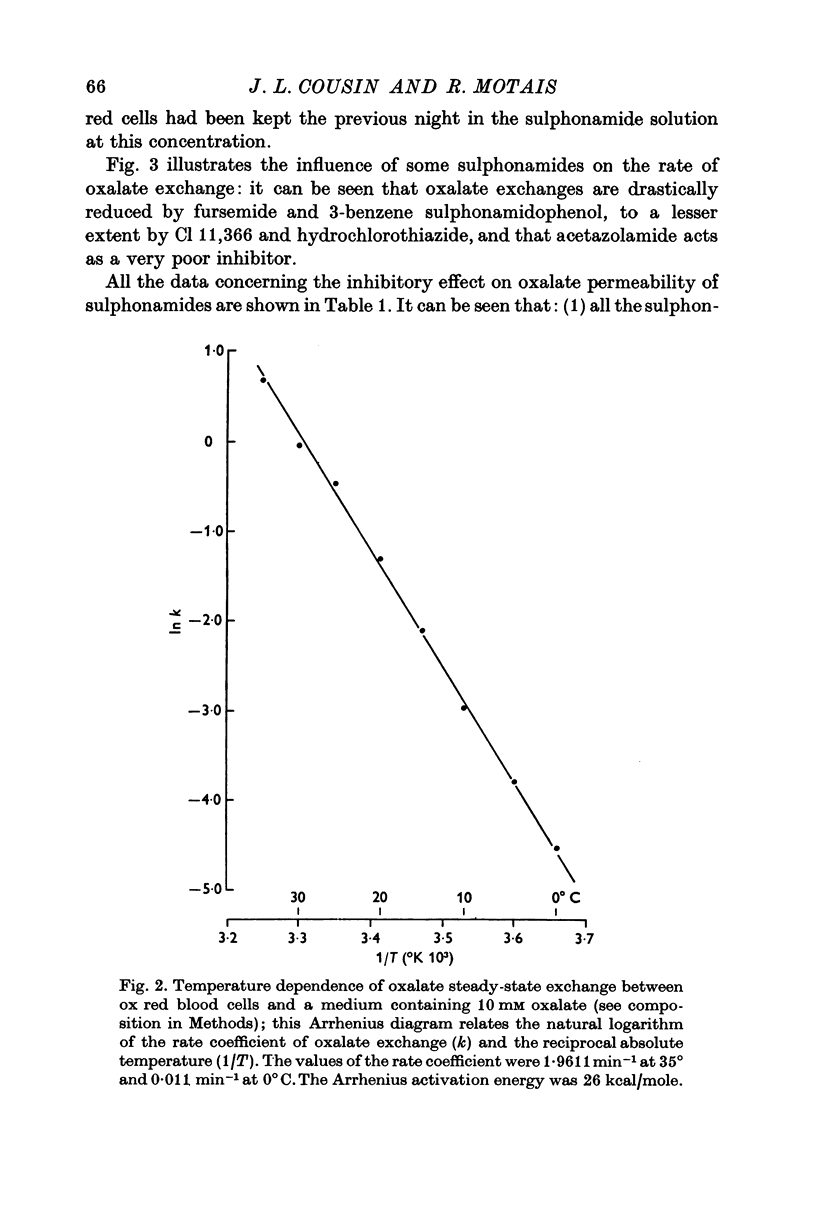
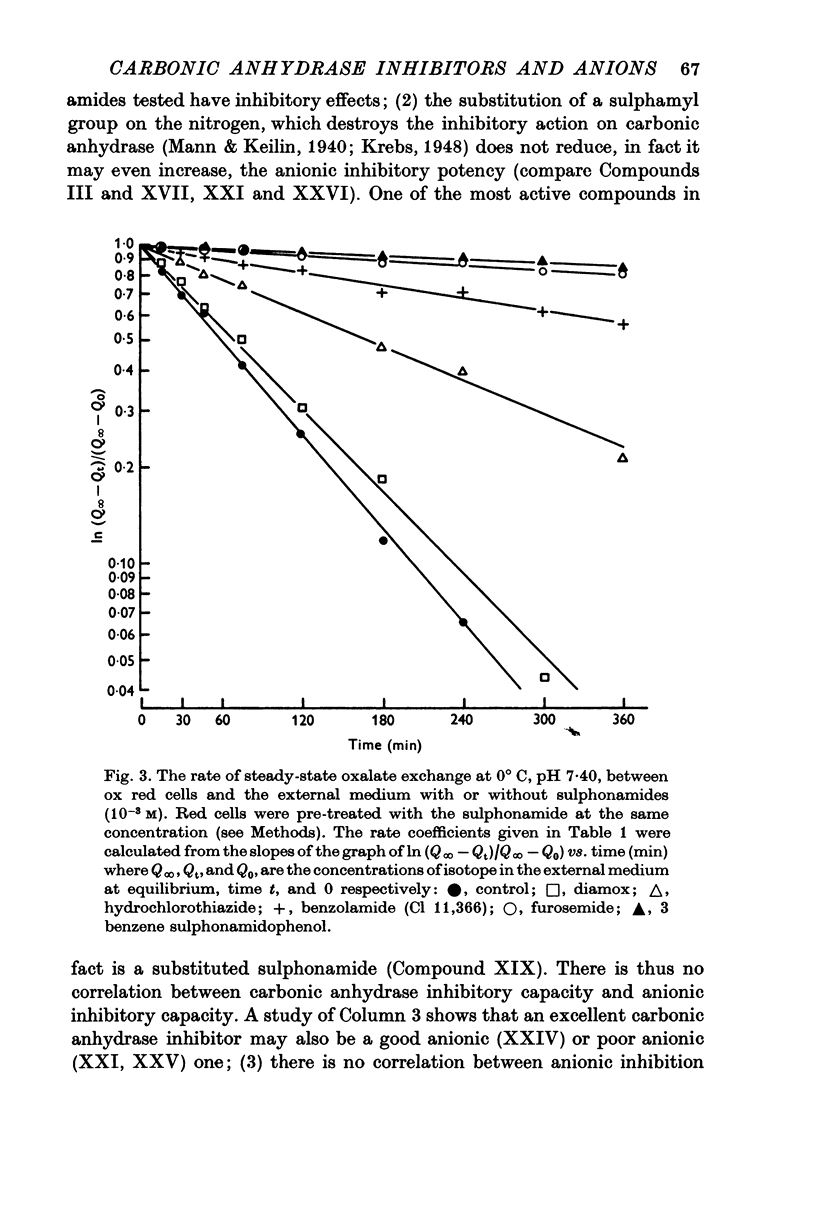
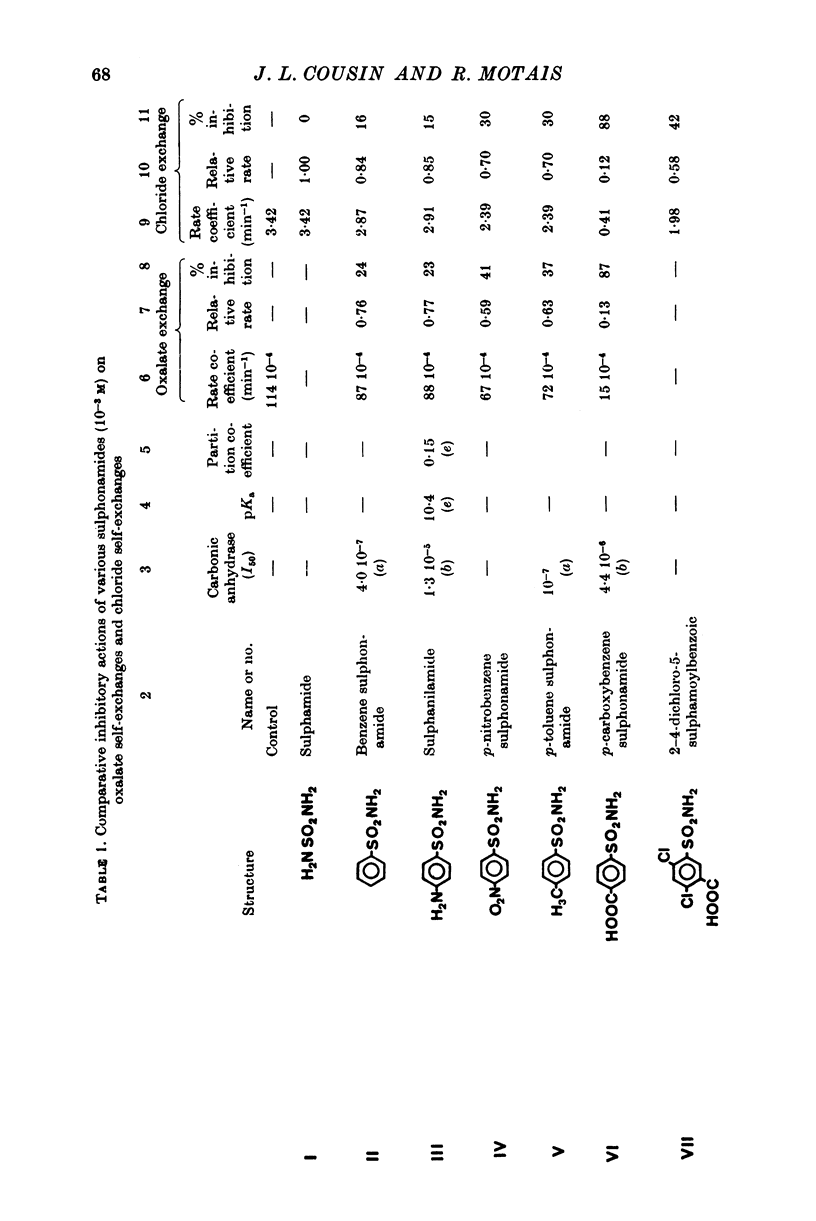





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubert L., Motais R. Molecular features of organic anion permeablity in ox red blood cell. J Physiol. 1975 Mar;246(1):159–179. doi: 10.1113/jphysiol.1975.sp010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEYER K. H., BAER J. E. Physiological basis for the action of newer diuretic agents. Pharmacol Rev. 1961 Dec;13:517–562. [PubMed] [Google Scholar]
- Burg M., Stoner L., Cardinal J., Green N. Furosemide effect on isolated perfused tubules. Am J Physiol. 1973 Jul;225(1):119–124. doi: 10.1152/ajplegacy.1973.225.1.119. [DOI] [PubMed] [Google Scholar]
- Candia O. A. Short-circuit current related to active transport of chloride in frog cornea: effects of furosemide and ethacrynic acid. Biochim Biophys Acta. 1973 Apr 16;298(4):1011–1014. doi: 10.1016/0005-2736(73)90407-0. [DOI] [PubMed] [Google Scholar]
- Cousin J. L., Motais R., Sola F. Transmembrane exchange of chloride with bicarbonate ion in mammalian red blood cells: evidence for a sulphonamide-sensitive "carrier". J Physiol. 1975 Dec;253(2):385–399. doi: 10.1113/jphysiol.1975.sp011195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmark M., Wieth J. O. Temperature dependence of chloride, bromide, iodide, thiocyanate and salicylate transport in human red cells. J Physiol. 1972 Aug;224(3):583–610. doi: 10.1113/jphysiol.1972.sp009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara S., Fox K. R., Hogben C. A. Depression of chloride transport by carbonic anhydrase inhibitors in the absence of carbonic anhydrase. Nature. 1967 May 20;214(5090):836–837. doi: 10.1038/214836a0. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Inhibition of carbonic anhydrase by sulphonamides. Biochem J. 1948;43(4):525–528. doi: 10.1042/bj0430525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAREN T. H. Carbonic anhydrase kinetics and inhibition at 37 degrees: an approach to reaction rates in vivo. J Pharmacol Exp Ther. 1963 Feb;139:129–139. [PubMed] [Google Scholar]
- MAREN T. H., ROBINSON B., PALMER R. F., GRIFFITH M. E. The binding of aromatic sulfonamides to erythrocytes. Biochem Pharmacol. 1961 Apr;6:21–46. doi: 10.1016/0006-2952(61)90066-1. [DOI] [PubMed] [Google Scholar]
- MAREN T. H., WILEY C. E. RENAL ACTIVITY AND PHARMACOLOGY OF N-ACYL AND RELATED SULFONAMIDES. J Pharmacol Exp Ther. 1964 Feb;143:230–242. [PubMed] [Google Scholar]
- Maren T. H., Wiley C. W. Kinetics of carbonic anhydrase in whole red cells as measured by transfer of carbon dioxide and ammonia. Mol Pharmacol. 1970 Jul;6(4):430–440. [PubMed] [Google Scholar]
- Motais R., Cousin J. L., Sola F. Les échanges transmembranaires de C1- et de HCO3- dans le globule rouge: mise en évidence d'une action directe des inhibiteurs de l'anhydrase carbonique sur le mécanisme de transfert. C R Acad Sci Hebd Seances Acad Sci D. 1975 Mar 3;280(9):1119–1122. [PubMed] [Google Scholar]
- Motais R., Sola F. Characteristics of a sulphydryl group essential for sodium exchange diffusion in beef erythrocytes. J Physiol. 1973 Sep;233(2):423–438. doi: 10.1113/jphysiol.1973.sp010315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISTRAND P. J., RAWLS J. A., Jr, MAREN T. H. Sulphonamide carbonic anhydrase inhibitors and intra-ocular pressure in rabbits. A comparison between in vitro and in vivo activities based on tissue distributions and physical and chemical properties of nine compounds. Acta Pharmacol Toxicol (Copenh) 1961;17:337–355. doi: 10.1111/j.1600-0773.1961.tb01653.x. [DOI] [PubMed] [Google Scholar]


