Abstract
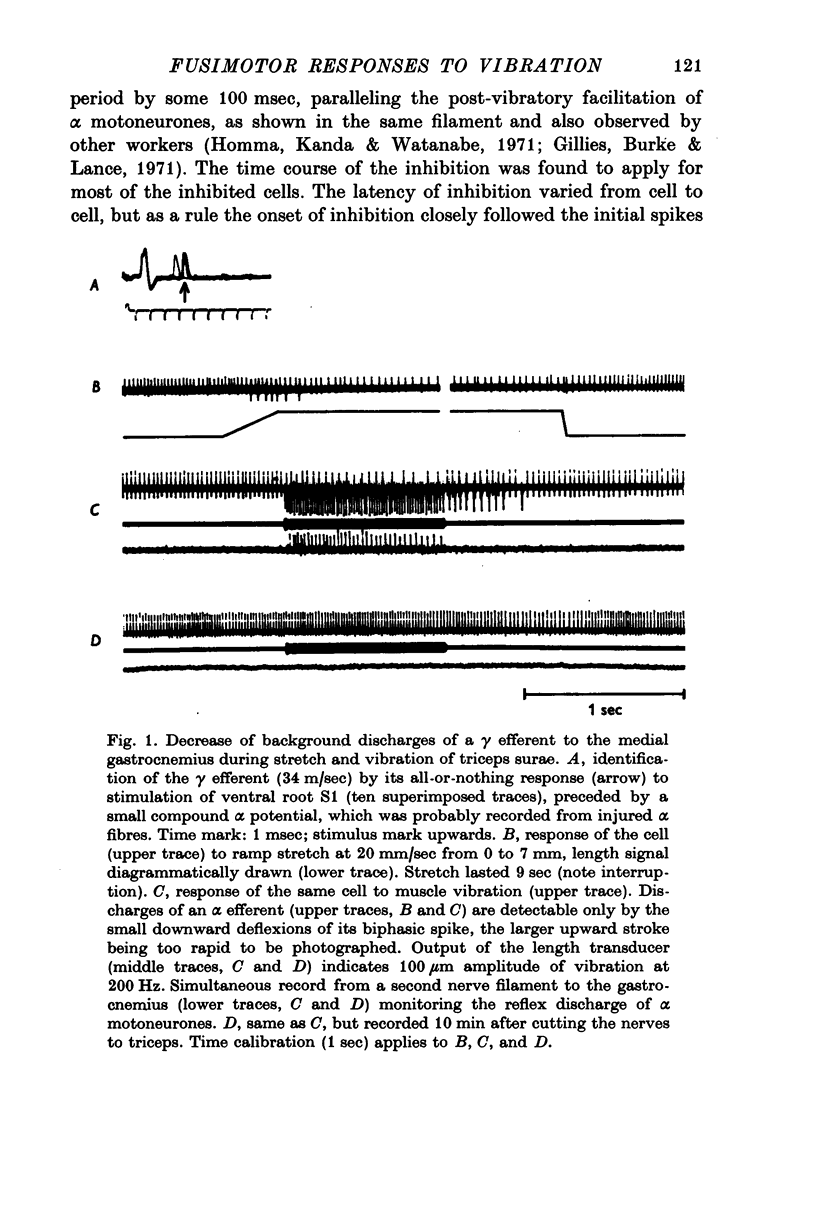
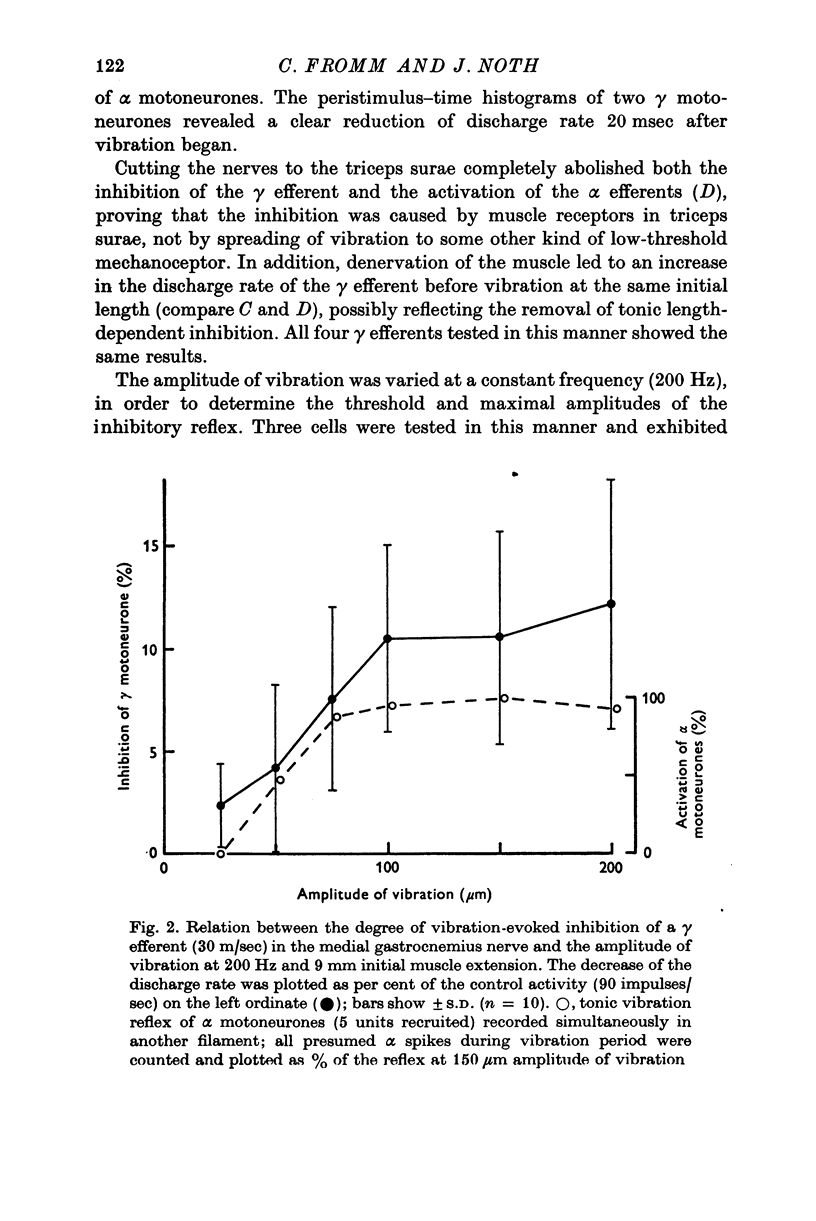
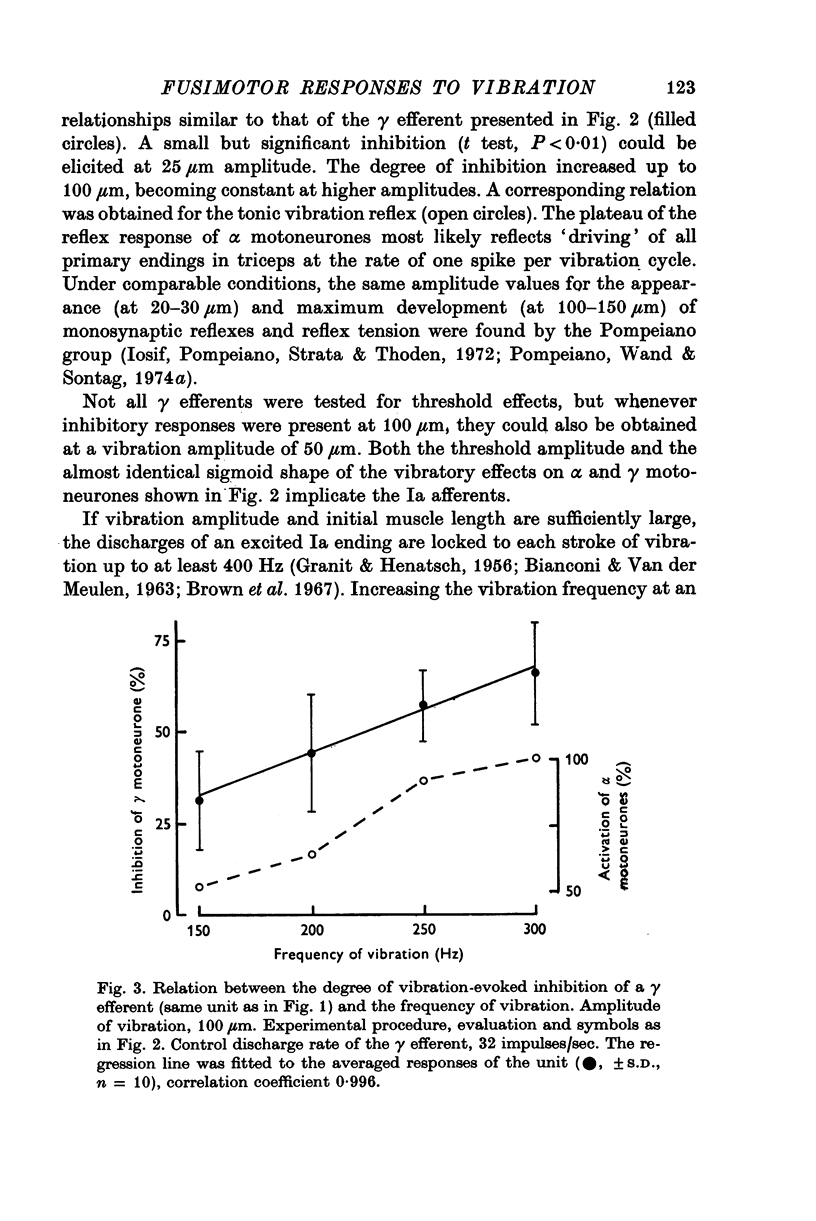
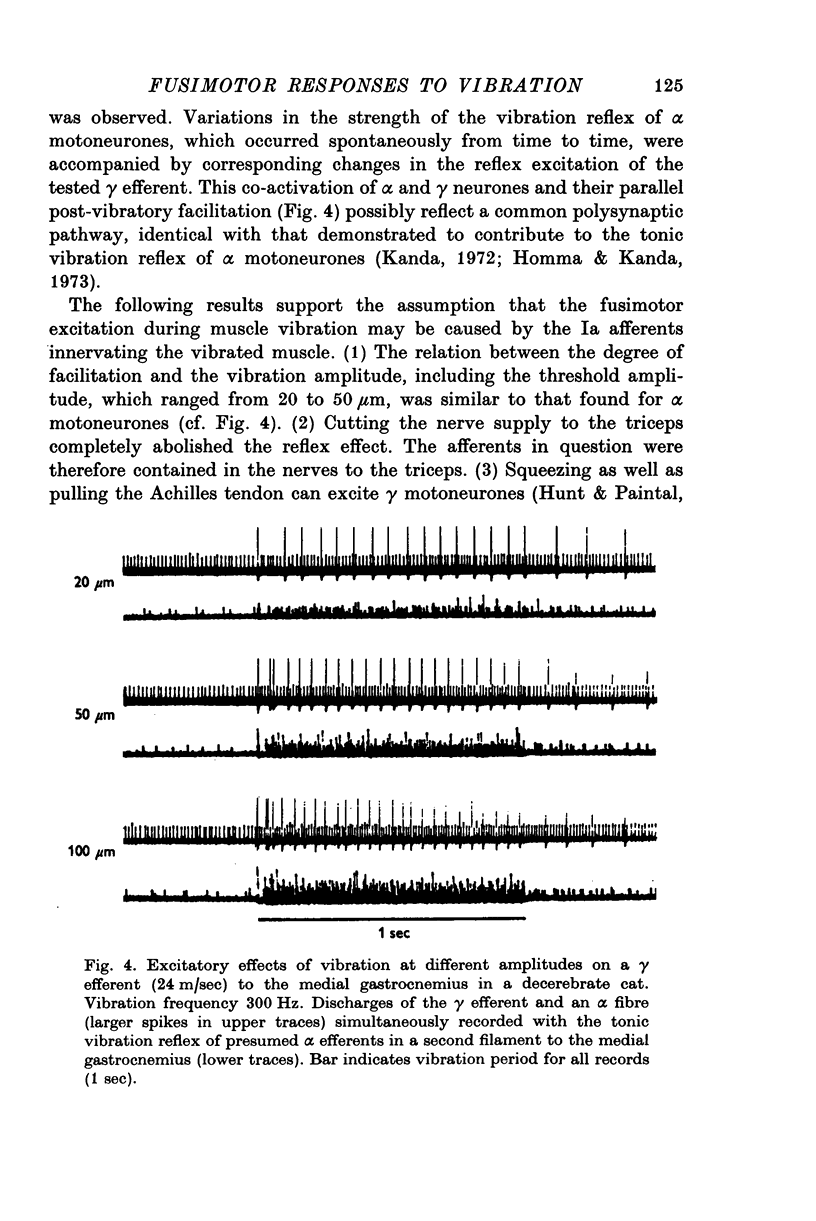
1. High frequency vibration was applied to the tendon of the non-contracting triceps surae muscle while recording the background discharges of single gamma fibres only small nerve bundles were cut, leaving most of the nerve supply to the triceps intact. 2. 22% out of a total of sixty-three gamma efferents were tonically inhibited by vibration. The inhibition appeared between 25 and 50mum peak-to-peak amplitude of vibration and increased to a plateau for amplitudes of about 100mum. The dependence of the tonic vibration reflex of alpha-efferents on the amplitude of vibration was found to be similar. Increasing the frequency of vibration from 150 to 300 Hz increased the degree of inhibition. 3. 33% of the fusimotor neurones investigated responded to muscle vibration with an increase in discharge rate. The threshold amplitudes of this reflex ranged from 20 to 50mum. Some features of the reflex, in particular the parallel post-vibratory facilitation found in alpha and gamma efferents, pointed to a polysynaptic pathway organized in an alpha-gamma linkage. 4. All gamma efferents inhibited by vibration showed inhibitory responses to antidromic stimulation of the parent ventral root, and most of them were inhibited by ramp stretch of the triceps. The gamma motoneurones facilitated by vibration, however, were excited by muscle stretch and were less susceptible to antidromic inhibition, some lacking it completely. 5. Cutting the nerves to triceps abolished the inhibitory as well as the excitatory responses of gamma efferents to muscle vibration. Both fusimotor reflexes were preserved after spinal section and subsequent administration of L-DOPA. 6. It is concluded that both of the fusimotor reflex effects of vibration are caused by excitation of primary spindle endings within the triceps. The inhibition of fusimotor neurones is thought to be mediated by Renshaw cells activated during vibration. The significance of positive feed-back on to gamma motoneurones as a result of autogenetic facilitation by Ia afferents is discussed in connexion with stability in the stretch reflex loop.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALNAES E., JANSEN J. K., RUDJORD T. FUSIMOTOR ACTIVITY IN THE SPINAL CAT. Acta Physiol Scand. 1965 Mar;63:197–212. doi: 10.1111/j.1748-1716.1965.tb04060.x. [DOI] [PubMed] [Google Scholar]
- Andén N. E., Jukes M. G., Lundberg A., Vyklický L. The effect of DOPA on the spinal cord. 1. Influence on transmission from primary afferents. Acta Physiol Scand. 1966 Jul-Aug;67(3):373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- BIANCONI R., van der MEULEN J. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol. 1963 Jan;26:177–190. doi: 10.1152/jn.1963.26.1.177. [DOI] [PubMed] [Google Scholar]
- Bergmans J., Grillner S. Reciprocal control of spontaneous activity and reflex effects in static and dynamic flexor gamma-motoneurones revealed by an injection of DOPA. Acta Physiol Scand. 1969 Sep-Oct;77(1):106–124. doi: 10.1111/j.1748-1716.1969.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm C., Haase J., Noth J. Length-dependent autogenetic inhibition of extensor gamma-motoneurones in the decerebrate cat. Pflugers Arch. 1974;346(3):251–262. doi: 10.1007/BF00595711. [DOI] [PubMed] [Google Scholar]
- Fromm C., Noth J. Autogenetic inhibition of gamma-motoneurons in the spinal cat uncovered by Dopa injection. Pflugers Arch. 1974 Jul 9;349(3):247–256. doi: 10.1007/BF00592452. [DOI] [PubMed] [Google Scholar]
- Fromm C., Noth J. Vibration-induced autogenetic inhibition of gamma motoneurons. Brain Res. 1975 Jan 17;83(3):495–497. doi: 10.1016/0006-8993(75)90842-2. [DOI] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D. Gamma control of dynamic properties of muscle spindles. J Neurophysiol. 1956 Jul;19(4):356–366. doi: 10.1152/jn.1956.19.4.356. [DOI] [PubMed] [Google Scholar]
- GRANIT R. Reflex rebound by post-tetanic potentiation; temporal summation-spasticity. J Physiol. 1956 Jan 27;131(1):32–51. doi: 10.1113/jphysiol.1956.sp005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies J. D., Burke D. J., Lance J. W. Tonic vibration reflex in the cat. J Neurophysiol. 1971 Mar;34(2):252–262. doi: 10.1152/jn.1971.34.2.252. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., McGrath G. J., Matthews P. B. The tonic vibration reflex seen in the acute spinal cat after treatment with DOPA. Brain Res. 1973 Jan 30;49(2):463–466. doi: 10.1016/0006-8993(73)90443-5. [DOI] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Reinking R. M., Stuart D. G. Physiological extent, range and rate of muscle stretch for soleus, medial gastrocnemius and tibialis anterior in the cat. Pflugers Arch. 1973;341(1):77–86. doi: 10.1007/BF00587332. [DOI] [PubMed] [Google Scholar]
- Grillner S. The influence of DOPA on the static and the dynamic fusimotor activity to the triceps surae of the spinal cat. Acta Physiol Scand. 1969 Dec;77(4):490–509. doi: 10.1111/j.1748-1716.1969.tb04592.x. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., PAINTAL A. S. Spinal reflex regulation of fusimotor neurones. J Physiol. 1958 Sep 23;143(2):195–212. doi: 10.1113/jphysiol.1958.sp006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. The reflex activity of mammalian small-nerve fibres. J Physiol. 1951 Dec 28;115(4):456–469. doi: 10.1113/jphysiol.1951.sp004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase J., Cleveland S., Ross H. G. Problems of postsynaptic autogenous and recurrent inhibition in the mammalian spinal cord. Rev Physiol Biochem Pharmacol. 1975;73:73–129. doi: 10.1007/BFb0034660. [DOI] [PubMed] [Google Scholar]
- Hellweg C., Meyer-Lohmann J., Benecke R., Windhorst U. Responses of Renshaw cells to muscle ramp stretch. Exp Brain Res. 1974;21(4):353–360. doi: 10.1007/BF00237898. [DOI] [PubMed] [Google Scholar]
- Homma S., Kanda K., Watanabe S. Monosynaptic coding of group Ia afferent discharges during vibratory stimulation of muscles. Jpn J Physiol. 1971 Aug;21(4):405–417. doi: 10.2170/jjphysiol.21.405. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Convergence on interneurones in the reciprocal Ia inhibitory pathway to motoneurones. Acta Physiol Scand Suppl. 1972;375:1–42. doi: 10.1111/j.1748-1716.1972.tb05298.x. [DOI] [PubMed] [Google Scholar]
- Kanda K. Contribution of polysynaptic pathways to the tonic vibration reflex. Jpn J Physiol. 1972 Aug;22(4):367–377. doi: 10.2170/jjphysiol.22.367. [DOI] [PubMed] [Google Scholar]
- Lennerstrand G., Thoden U. Position and velocity sensitivity of muscle spindles in the cat. II. Dynamic fusimotor single-fibre activation of primary endings. Acta Physiol Scand. 1968 Sep-Oct;74(1):16–29. doi: 10.1111/j.1748-1716.1968.tb04211.x. [DOI] [PubMed] [Google Scholar]
- Nothe J. Recurrente Hemmung der Extensor-Fusimotoneurone. Pflugers Arch. 1971;329(1):23–33. doi: 10.1007/BF00586898. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. Participation by pressure-pain receptors of mammalian muscles in the flexion reflex. J Physiol. 1961 May;156:498–514. doi: 10.1113/jphysiol.1961.sp006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano O., Wand P., Sontag K. H. Excitation of Renshaw cells by orthodromic group Ia volleys following vibration of extensor muscles. Pflugers Arch. 1974 Jan 11;347(2):137–144. doi: 10.1007/BF00592395. [DOI] [PubMed] [Google Scholar]
- Stein R. B. Peripheral control of movement. Physiol Rev. 1974 Jan;54(1):215–243. doi: 10.1152/physrev.1974.54.1.215. [DOI] [PubMed] [Google Scholar]
- VOORHOEVE P. E., VERHEY B. A. PRE- AND POST-SYNAPTIC EFFECTS ON FUSIMOTOR AND ALPHA MOTONEURONES OF THE CAT UPON ACTIVATION OF MUSCLE SPINDLE AFFERENTS BY SUCCINYLCHOLINE. Acta Physiol Pharmacol Neerl. 1963 Apr;12:12–22. [PubMed] [Google Scholar]
- VOORHOEVE P. E., van KANTEN R. Reflex behaviour of fusimotor neurones of the cat upon electrical stimulation of various afferent fibers. Acta Physiol Pharmacol Neerl. 1962;10:391–407. [PubMed] [Google Scholar]


