Abstract
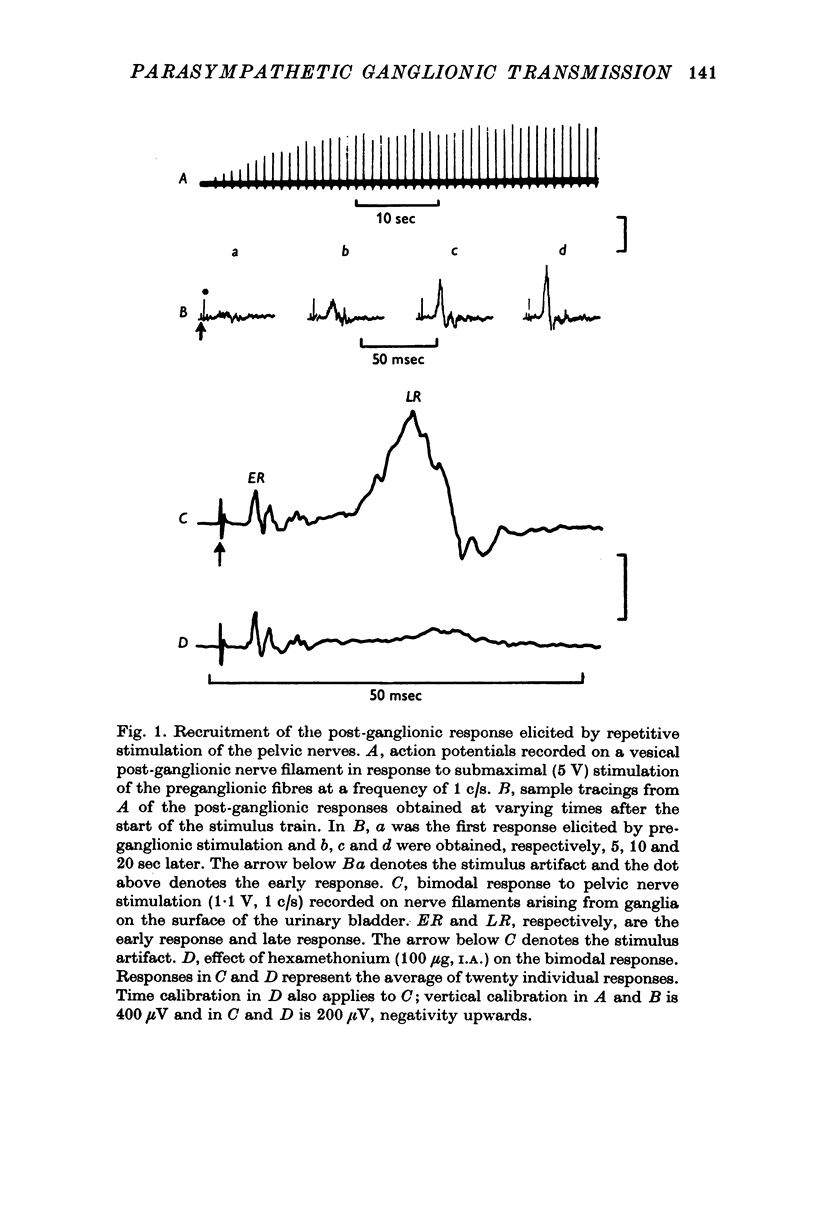
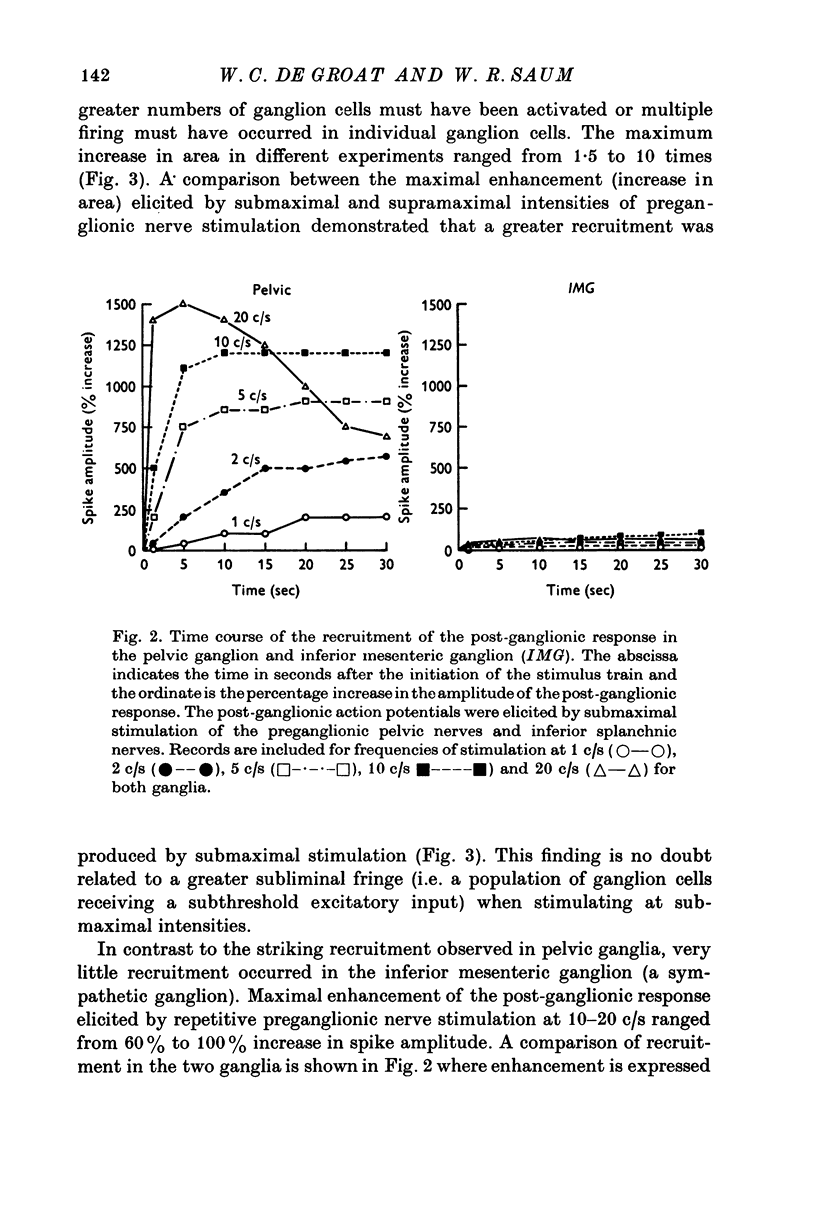
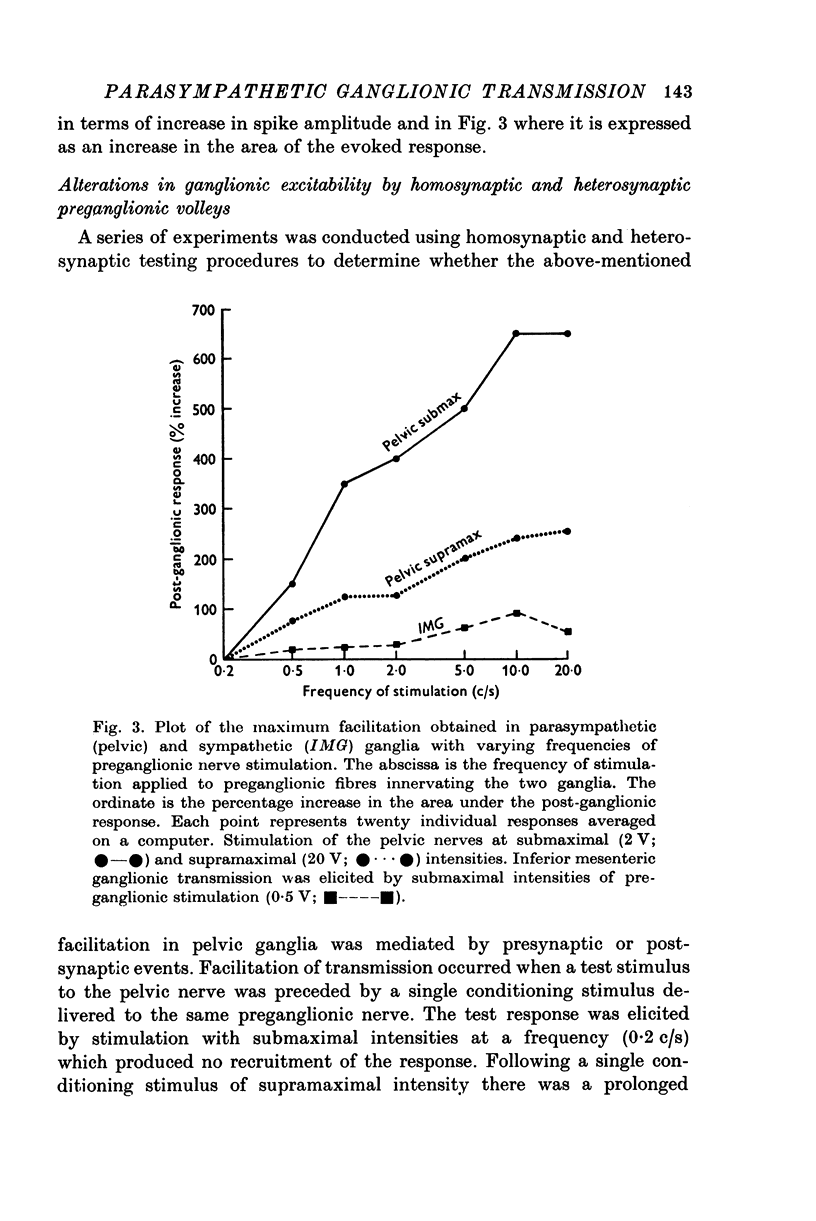
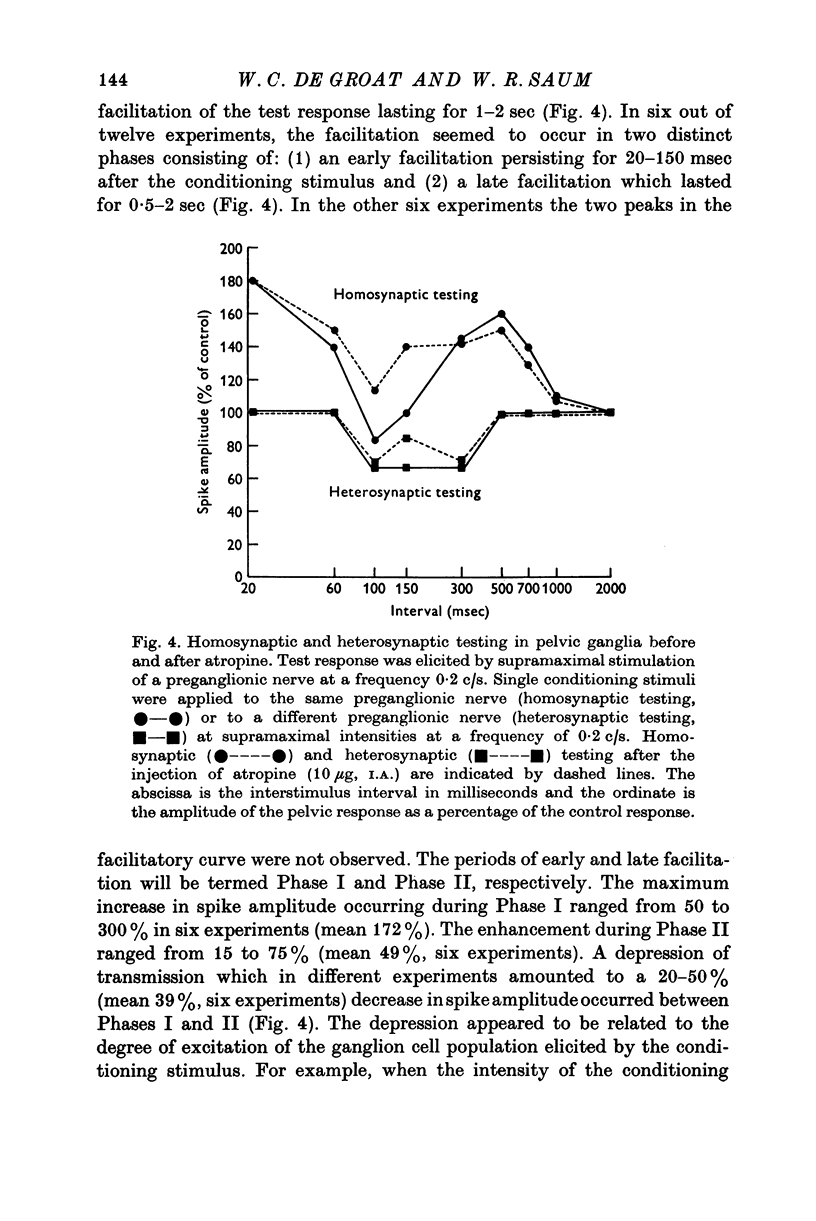
1. Electrophysiological techniques were used to study the sacral para-sympathetic input to pelvic ganglia located on the surface of the urinary bladder of the cat. 2. Synaptic transmission in pelvic ganglia was mediated primarily via nicotinic receptors although muscarinic excitatory receptors were present. 3. The most prominent characteristic of transmission in pelvic ganglia was the marked recruitment elicited by increasing frequencies of preganglionic nerve stimulation. Post-ganglionic action potentials were of low amplitude at low frequencies of stimulation (0-1-0-5c/s), but commonly increased to five to twenty times control amplitudes during continuous stimulation at frequencies between 5 and 10c/s. Thus, it is proposed that vesical ganglia may act as "filters" in the micturition pathway; blocking the excitatory input to the bladder when intravesical pressure and parasympathetic firing is low and facilitating the neural input to the bladder during micturition when preganglionic activity is high. 4. Information was also obtained about the characteristics of the parasympathetic post-ganglionic neurones innervating the bladder. Stimulation of the preganglionic fibres in the pelvic nerve elicited a bimodal contraction consisting of an initial phasic response, which was atropine-resistant and a tonic response which was blocked by atropine. This suggests that two types of neurones, cholinergic and non-cholinergic, may mediate the sacral input to the vesical smooth muscle.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N. The use and limitations of atropine for pharmacological studies on autonomic effectors. Pharmacol Rev. 1955 Dec;7(4):467–494. [PubMed] [Google Scholar]
- Brimble M. J., Wallis D. I., Woodward B. Facilitation and inhibition of cell groups within the superior cervical ganglion of the rabbit. J Physiol. 1972 Nov;226(3):629–652. doi: 10.1113/jphysiol.1972.sp010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M. Cardiac sympathetic adrenergic pathways in which synaptic transmission is blocked by atropine sulfate. J Physiol. 1967 Jul;191(2):271–288. doi: 10.1113/jphysiol.1967.sp008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Dumsday B., Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972 Mar;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C., Lalley P. M. Reflex firing in the lumbar sympathetic outflow to activation of vesical afferent fibres. J Physiol. 1972 Oct;226(2):289–309. doi: 10.1113/jphysiol.1972.sp009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C. Nervous control of the urinary bladder of the cat. Brain Res. 1975 Apr 11;87(2-3):201–211. doi: 10.1016/0006-8993(75)90417-5. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Saum W. R. Adrenergic inhibition in mammalian parasympathetic ganglia. Nat New Biol. 1971 Jun 9;231(23):188–189. doi: 10.1038/newbio231188a0. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Saum W. R. Sympathetic inhibition of the urinary bladder and of pelvic ganglionic transmission in the cat. J Physiol. 1972 Jan;220(2):297–314. doi: 10.1113/jphysiol.1972.sp009708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LIBET B. Origin and blockade of the synaptic responses of curarized sympathetic ganglia. J Physiol. 1961 Aug;157:484–503. doi: 10.1113/jphysiol.1961.sp006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsen P. Nervous control of urinary bladder in cats. I. The collecting phase. Acta Physiol Scand. 1968 Jan-Feb;72(1):157–171. doi: 10.1111/j.1748-1716.1968.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Edvardsen P. Nervous control of urinary bladder in cats. IV. Effects of autonomic blocking agents on responses to peripheral nerve stimulation. Acta Physiol Scand. 1968 Jan-Feb;72(1):234–247. doi: 10.1111/j.1748-1716.1968.tb03845.x. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B., STEINER E. C. The cerebral distributions of a tertiary and a quaternary anticholinesterase agent following intravenous and intraventricular injection. J Pharmacol Exp Ther. 1956 Dec;118(4):420–434. [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIBET B. SLOW SYNAPTIC RESPONSES AND EXCITATORY CHANGES IN SYMPATHETIC GANGLIA. J Physiol. 1964 Oct;174:1–25. doi: 10.1113/jphysiol.1964.sp007471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau E. M., Smolinsky A., Lass Y. Post-tetanic potentiation and facilitation do not share a common calcium-dependent mechanism. Nat New Biol. 1973 Aug 1;244(135):155–157. doi: 10.1038/newbio244155a0. [DOI] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The Innervation of the Pelvic and adjoining Viscera: Part II. The Bladder. Part III. The External Generative Organs. Part IV. The Internal Generative Organs. Part V. Position of the Nerve Cells on the Course of the Efferent Nerve Fibres. J Physiol. 1895 Dec 30;19(1-2):71–139. doi: 10.1113/jphysiol.1895.sp000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. The effect of various poisons upon the response to nervous stimuli chiefly in relation to the bladder. J Physiol. 1911 Oct 20;43(2):125–181. doi: 10.1113/jphysiol.1911.sp001463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- Magleby K. L. The effect of repetitive stimulation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):327–352. doi: 10.1113/jphysiol.1973.sp010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A dual effect of repetitive stimulation on post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975 Feb;245(1):163–182. doi: 10.1113/jphysiol.1975.sp010839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McISAAC R. J., KOELLE G. B. Comparison of the effects of inhibition of external, internal and total acetylcholinesterase upon ganglionic transmission. J Pharmacol Exp Ther. 1959 May;126(1):9–20. [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. Post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1969 Jul;203(1):121–133. doi: 10.1113/jphysiol.1969.sp008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saum W. R., De Groat W. C. Parasympathetic ganglia: activation of an adrenergic inhibitory mechanism by cholinomimetic agents. Science. 1972 Feb 11;175(4022):659–661. doi: 10.1126/science.175.4022.659. [DOI] [PubMed] [Google Scholar]
- TAKESHIGE C., VOLLE R. L. Bimodal response of sympathetic ganglia to acetylcholine following eserine or repetitive preganglionic stimulation. J Pharmacol Exp Ther. 1962 Oct;138:66–73. [PubMed] [Google Scholar]
- Taira N. The autonomic pharmacology of the bladder. Annu Rev Pharmacol. 1972;12:197–208. doi: 10.1146/annurev.pa.12.040172.001213. [DOI] [PubMed] [Google Scholar]
- Tauc L. Transmission in invertebrate and vertebrate ganglia. Physiol Rev. 1967 Jul;47(3):521–593. doi: 10.1152/physrev.1967.47.3.521. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U. Transmission of preganglionic impulses through the muscarinic receptors of the superior cervical ganglion of the cat. J Pharmacol Exp Ther. 1966 Dec;154(3):426–440. [PubMed] [Google Scholar]
- Weinreich D. Ionic mechanism of post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1971 Jan;212(2):431–446. doi: 10.1113/jphysiol.1971.sp009333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat W. C., Ryall R. W. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol. 1969 Jan;200(1):87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat W. C. The actions of gamma-aminobutyric acid and related amino acids on mammalian autonomic ganglia. J Pharmacol Exp Ther. 1970 Apr;172(2):384–396. [PubMed] [Google Scholar]


