Abstract
We calculated an elastase activity index (EAI) by dividing the diameter of the elastin lysis halo by the fungal growth diameter. After 10 days' incubation at 37°C, all strains but one obtained from invasive aspergillosis showed an EAI ≥ 1. Of the 18 strains obtained from colonized patients, only 4 (22.2%) had an EAI ≥ 1, whereas neither of the strains isolated from patients with fungus ball reached this value. Overall, 44 out of the 142 strains obtained from the environment had an EAI ≥ 1 (30.9%).
The diagnosis of invasive aspergillosis (IA) relies on the histologic demonstration of fungal invasion and the isolation of Aspergillus from normally sterile clinical samples. Different compounds have been implicated in the pathogenicity of Aspergillus fumigatus (1, 2, 3, 6, 7, 10, 13, 14, 19), but the presence of elastase activity has been considered particularly relevant because elastin constitutes a significant proportion of total lung protein (18).
In this study, we evaluated the in vitro elastase activity of A. fumigatus strains obtained from clinical and environmental samples and the utility of this characteristic for predicting invasiveness with clinical isolates.
We used 198 strains of A. fumigatus from different sources: 32 from transplant patients with IA, 6 from patients with pulmonary aspergilloma, 18 from different colonized patients, and 142 from environmental samples.
To classify a patient as having invasive disease, fungus ball, or simple colonization by Aspergillus spp., we followed diagnostic criteria and considerations according to a well-recognized medical reference (4). The identification of an isolate of A. fumigatus was made according to standard criteria (17). Environmental samples were obtained from the air outside and inside the hospital.
Elastase activity was studied in a solid medium described by Kothary et al. (12), containing the following: 0.05% elastin (Sigma, St. Louis, Mo.), 0.05% yeast carbon base (Difco Laboratories, Detroit, Mich.), 0.01% rose bengal (Sigma, St. Louis, Mo.), and 1.5% agar (Merck, Darmstadt, Germany) in 0.05 M borate buffer, pH 7.6.
Plates were inoculated in a central spot with a loopful of Aspergillus spore suspension (106/ml) and were incubated for 15 days at 37°C. We measured the diameter of colony growth and the diameter of the halo of elastin lysis (elastase activity) on days 7, 10, and 15 of incubation. We calculated an elastase activity index (EAI) by dividing the elastase diameter by the growth diameter.
To avoid bias, elastase activity was determined without knowing the clinical significance or environmental origin of every isolate. We expressed the EAI as the mean and standard deviation and used the Wilcoxon test for unpaired data to compare it among groups because EAI was not normally distributed.
We determined the sensitivity and specificity of the EAI to predict the IA for every cutoff point. To do this, we calculated the receiver operating characteristic (ROC) curve representing every possible cutoff point of the equation with its sensitivity and the complement of its specificity. The nearer the area below the ROC curve is to unity, the better is the validation, or fit, of the model. For areas near 0.5 the validity is poor or nil (16).
Fifty-four of the 198 strains did not grow on elastin medium and consequently did not show elastase activity; two of these strains were isolated from patients with aspergilloma, six from colonizing processes, and 46 from the environment. None of the strains without elastase activity corresponded to a patient with IA.
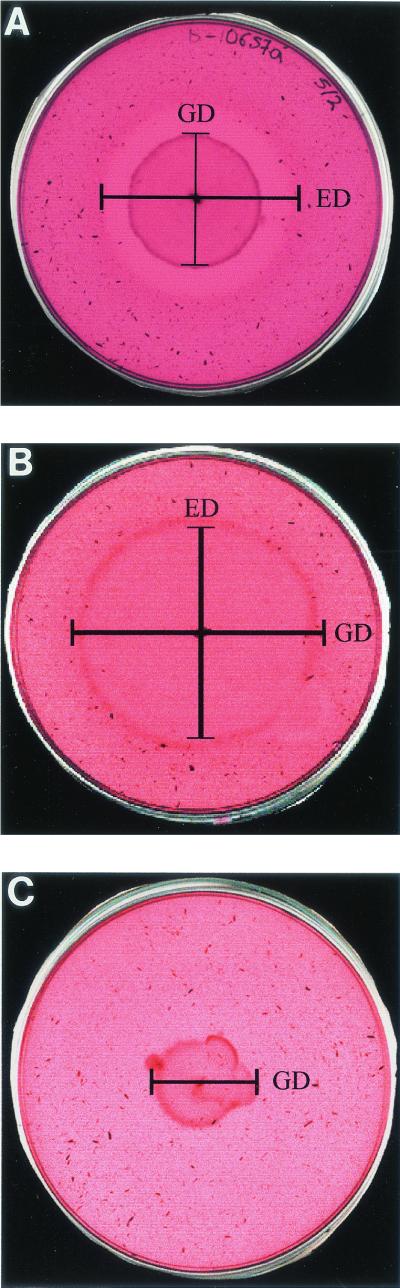
When comparing EAIs on days 7, 10, and 15 of incubation, the optimum reading conditions were obtained on day 10, and these indexes will be the ones referred to from now on. Figure 1 shows the differential growth characteristics of the different groups of strains with regard to their elastase activity. In all three situations the growth temperature was 37°C and the period of incubation was 10 days.
FIG. 1.
(A) Strain producing invasive aspergillosis, with a wide halo of elastase activity. (B) Colonizing strain isolated from an individual with a bacterial respiratory process, in which strain growth is ahead of elastin degradation. (C) Environmental strain which can grow in the medium, albeit slowly and without elastase activity. The nondegraded elastin granules can clearly be seen up to the colony margins. GD, growth diameter; ED, elastase activity diameter.
The EAI of strains causing pulmonary invasion, fungus balls, colonizer strains, and strains from environmental sources are summarized in Table 1. All strains but one obtained from invasive aspergillosis showed an EAI ≥ 1 (range, 0.88 to 1.66). Of the 18 strains obtained from colonized patients, only 4 (22.2%) had an EAI ≥ 1, while neither strain isolated from patients with fungus ball reached this value. Overall, 44 out of the 142 strains obtained from the environment had an EAI ≥ 1 (30.9%).
TABLE 1.
EAI in A. fumigatus strains isolated from different sources
| Source | No. of strains | No. (%) with EAI ≥ 1 | EAI
|
||
|---|---|---|---|---|---|
| Mean | SD | Range | |||
| Invasive pulmonary aspergillosis | 32 | 31 (96.9) | 1.17 | 0.18 | 0.88-1.66 |
| Fungus balls | 6 | 0 (0) | 0.48 | 0.37 | 0-0.77 |
| Colonizers | 18 | 4 (22.2) | 0.56 | 0.48 | 0-1.3 |
| Environment | 142 | 44 (30.9) | 0.67 | 0.58 | 0-2.33 |
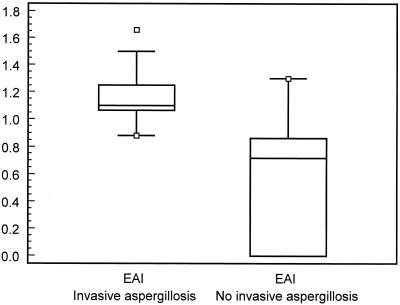
The average EAI in patients with pulmonary invasive aspergillosis was 1.17 (standard deviation [SD], 0.17) versus 0.54 (SD, 0.45) in patients colonized or suffering from aspergilloma (Fig. 2). The difference of 0.63 in the EAI between both groups was significant (95% confidence interval [CI] for the difference, 0.46 to 0.80; Z = −5.26; P < 0.001).
FIG. 2.
Box plot comparing the EAI between patients with and without invasive aspergillosis.
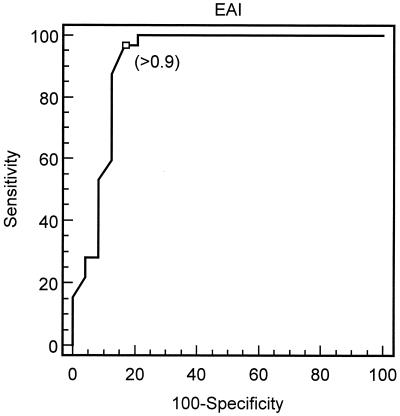
We studied the ability of EAI to detect pulmonary invasive aspergillosis by means of a ROC curve and by comparing the test in patients with and without invasive aspergillosis. The best cutoff point for EAI was 0.9, which corresponded to an area under the ROC curve of 0.913 (95% CI, 0.807 to 0.972) (Fig. 3). Sensitivity and specificity for detection of invasive aspergillosis at this cutoff point were 97% (95% CI, 83.7 to 99.5) and 83.3% (95% CI, 62.6 to 95.2), respectively, with a positive likelihood ratio of 5.81.
FIG. 3.
ROC curve showing the best cutoff point for EAI to detect invasive aspergillosis.
A test capable of differentiating between pathogenic and nonpathogenic Aspergillus strains could be very useful. Reports of different determinants of virulence have not been clinically tested or have failed to show a good correlation with clinical settings (1, 2, 3, 10, 13, 14, 19). Elastase activity is well known for A. fumigatus and other species and occurs in at least 36 to 94% of all strains (5, 8, 9, 12, 15). Despite two previous reports showing occasional correlation between elastase activity in Aspergillus strains obtained from invasive mycoses (8, 11), so far elastase activity has not been clearly linked to pathogenicity in A. fumigatus.
Our study is the first to demonstrate a clear link between elastase activity and pathogenicity. Higher EAIs are related to invasive aspergillosis, whereas if the EAI is <1 the probability of suffering from invasive disease is very low. If we assume a prevalence of between 5 and 10%, the positive predictive value of the test (i.e., the probability that a positive result actually reflects an IA) would be 23.4 to 39.2%. However, in the same situation the negative predictive value (i.e., the probability that a negative corresponds to a true negative) would be 99.8 to 99.6%. In this case, the EAI is very useful to rule out suspected cases. On the other hand, when the prior probability of pulmonary IA is high (i.e., bone marrow transplant patient with new pulmonary nodular infiltrates that are not responding to broad-spectrum antibiotics), the test could be useful in helping clinicians to become aware of the potential pathogenicity of any given isolate of Aspergillus spp.
The significance of the EAI in environmental samples is still unknown. If there is an epidemiological link between strains with higher EAI in the hospital air and cases of pulmonary invasive aspergillosis, it has yet to be determined. However, if hospital isolates tend to have a higher EAI than outdoor environmental isolates, that might help to explain the reason that invasive aspergillosis is such a nosocomial problem.
Results presented in this study confirm those reported by others (8, 11), that the determination of elastase activity in clinical and environmental isolates of Aspergillus spp. may lead to the development of faster laboratory procedures for diagnosing IA and helping clinicians make faster and more informed decisions as to the welfare of their immunocompetent patients.
Acknowledgments
This research was supported by grants 08.2/0027/1997 and 08.2/0018/99 from Proyectos Coordinados de Investigación en Ciencias de la Salud of the COMUNIDAD DE MADRID.
REFERENCES
- 1.Amitani, R., G. Taylor, E. N. Elezis, C. Llewellyn-Jones, J. Mitchell, F. Kuze, P. J. Cole, and R. Wilson. 1995. Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect. Immun. 63:3266-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer, J., M. Gareis, A. Bot, and B. Gedek. 1989. Isolation of a mycotoxin (gliotoxin) from a bovine udder infected with Aspergillus fumigatus. J. Med. Vet. Mycol. 27:45-50. [DOI] [PubMed] [Google Scholar]
- 3.Bouchara, J. P., G. Tronchin, G. Larcher, and D. Chabasse. 1995. The search for virulence determinants in Aspergillus fumigatus. Trends Microbiol. 3:327-330. [DOI] [PubMed] [Google Scholar]
- 4.Denning, D. W. 2000. Aspergillus species. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas and Bennett's principles and practice of infectious diseases, 5th ed. Churchill-Livingston, Philadelphia, Pa.
- 5.Denning, D. W., J. Elliot, and M. Keaney. 1993. Temperature-dependent expression of elastase in Aspergillus species. J. Med. Vet. Mycol. 31:455-458. [Google Scholar]
- 6.Drouhet, E. 1993. Rapid tests for immunodiagnosis of invasive opportunistic mycoses. Rev. Iberoam. Micol. 10:S53-S67. [Google Scholar]
- 7.Fratamico, P. M., W. K. Long, and H. R. Buckley. 1991. Production and characterization of monoclonal antibodies to a 58-kilodalton antigen of Aspergillus fumigatus. Infect. Immun. 59:316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guglielminetti, M., P. D. Piccioni, P. Iadarola, M. Luisetti, and G. Caretta. 1993. Production of serine chymotrypsin-like elastase by Aspergillus fumigatus strains. Bol. Micol. 8:27-33. [Google Scholar]
- 9.Hasegawa, Y., T. Nikai, Y. Yoshikawa, H. Sugimara, K. Ogawa, and K. Takagi. 1994. Elastase activity from Aspergillus species and inhibition by urinastatin. Jpn. J. Med. Mycol. 35:293-298. [Google Scholar]
- 10.Holden, D. W., C. M. Tang, and J. M. Smith. 1994. Molecular genetics of Aspergillus pathogenicity. Antonie Leeuwenhoek 65:251-255. [DOI] [PubMed] [Google Scholar]
- 11.Kolattukudy, P. E., J. D. Lee, L. M. Rogers, P. Zimmerman, S. Ceselski, B. Fox, B. Stein, and E. A. Copelan. 1993. Evidence for possible involvement of an elastolytic serine protease in aspergillosis. Infect. Immun. 61:2357-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kothary, M. H., T. Chase, and J. D. MacMillan. 1984. Correlation of elastase production by some strains of Aspergillus fumigatus with ability to cause pulmonary invasive aspergillosis in mice. Infect. Immun. 43:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar, A., L. V. Reddy, A. Sochanik, and V. P. Kurup. 1993. Isolation and characterization of a recombinant heat shock protein of Aspergillus fumigatus. J. Allergy Clin. Immunol. 91:1024-1030. [DOI] [PubMed] [Google Scholar]
- 14.Moser, M., R. Crameri, G. Menz, T. Schneider, T. Dudler, C. Virchow, M. Gmachl, and K. Blaser. 1992. Cloning and expression of recombinant Aspergillus fumigatus allergen I/a (r Asp f I/a) with IgE binding and type I skin test activity. J. Immunol. 149:454-460. [PubMed] [Google Scholar]
- 15.Rhodes, J. C., R. B. Bode, and C. M. McCuan-Kirsch. 1988. Elastase production in clinical isolates of Aspergillus. Diagn. Microbiol. Infect. Dis. 10:165-170. [DOI] [PubMed] [Google Scholar]
- 16.Sackett, D. L., R. B. Haynes, G. H. Guyatt, and P. Tugwell. 1991. Clinical epidemiology. A basic science for clinical medicine, 2nd ed. Little, Brown and Company, Boston, Mass.
- 17.Sigler, L., and M. J. Kennedy. 1999. Aspergillus, Fusarium, and other opportunistic moniliaceous fungi, p. 1212-1241. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 18.Starcher, B. C. 1986. Elastin and the lung. Thorax 41:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton, P., N. R. Newcombe, P. Waring, and A. Mullbacher. 1994. In vivo immunosuppressive activity of gliotoxin, a metabolite produced by human pathogenic fungi. Infect. Immun. 62:1192-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]