Abstract
1. Three mutations which eliminate specific types of photoreceptors in Drosophila were characterized.
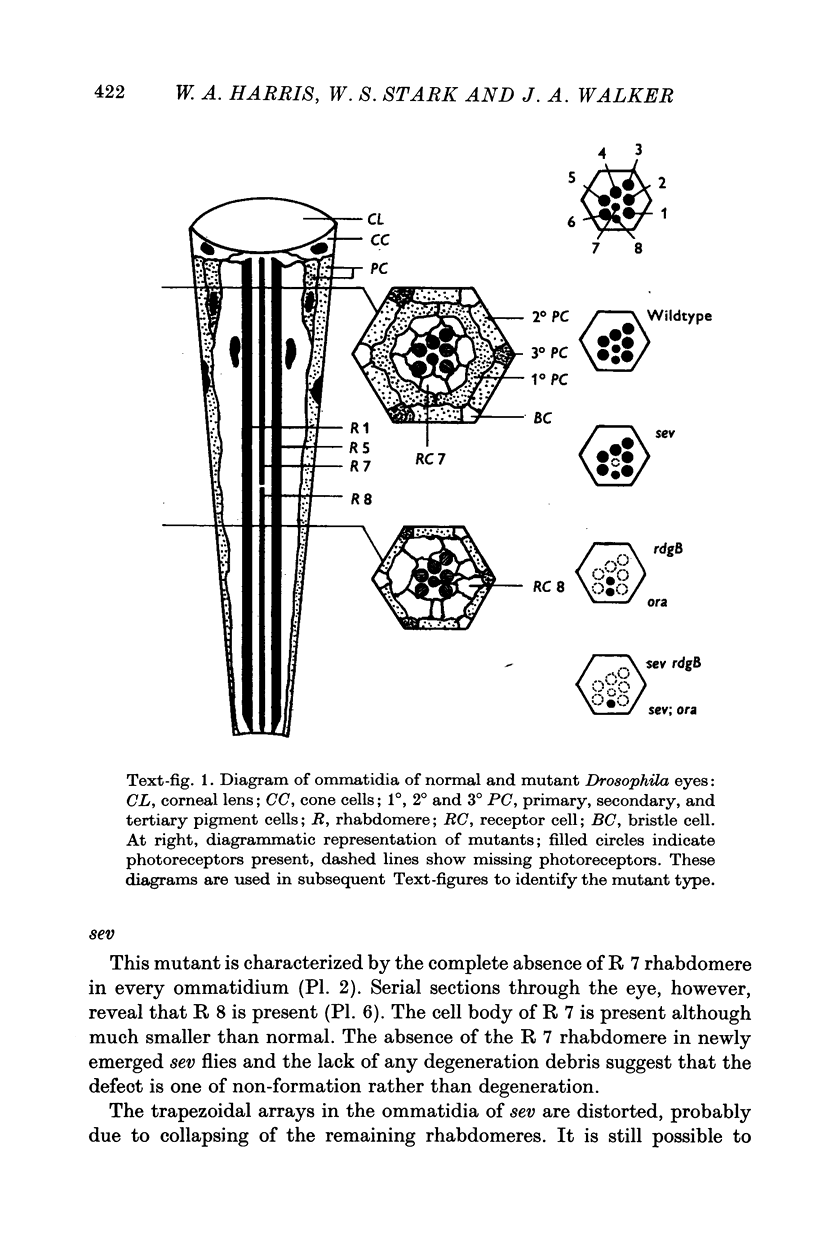
2. Of the eight photoreceptors in each facet, two mutations delete the outer six (R 1-6). The third eliminates R 7, one of the two central photoreceptors. Double mutants can be constructed in which only photoreceptor R 8 is present.
3. The spectral sensitivities, photopigments, and behavioural properties of these mutants were investigated.
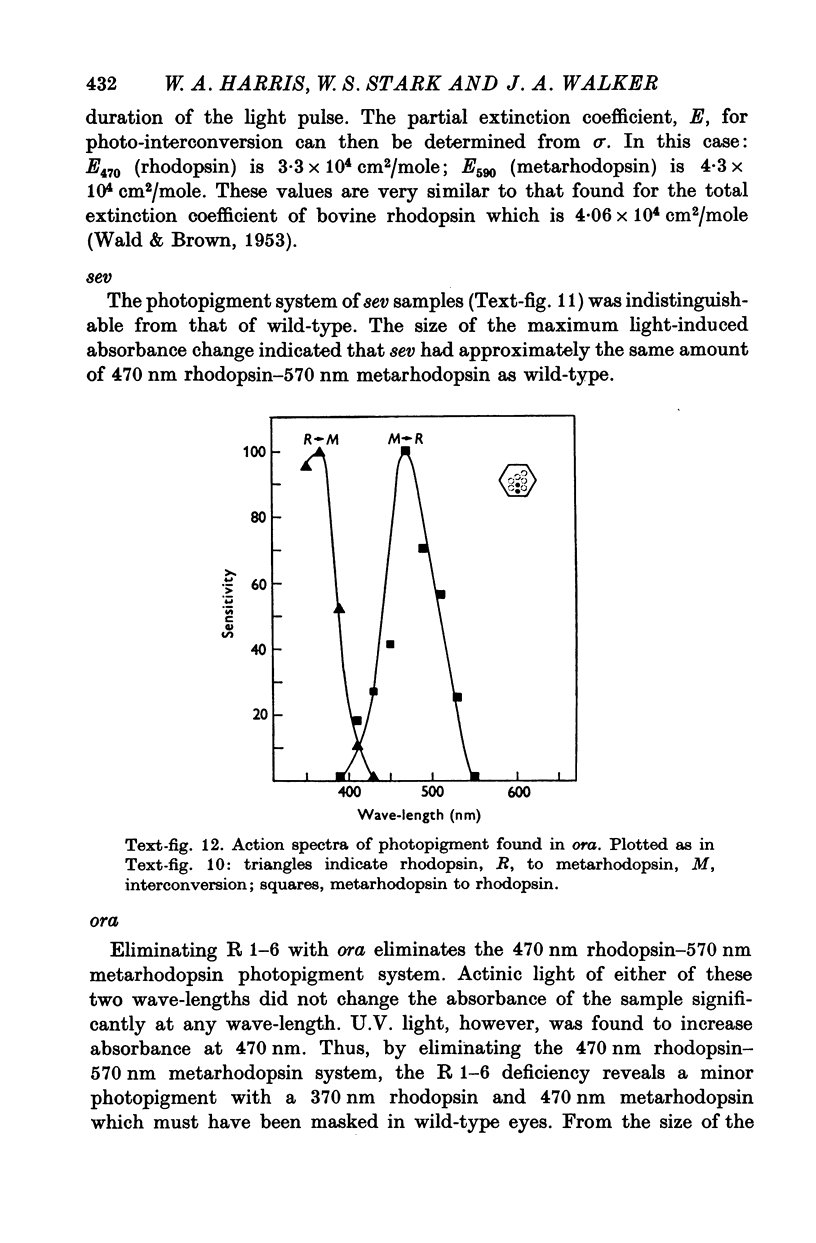
4. R 1-6 have two sensitivity peaks, near 350 and 470 nm. These receptors contain a rhodopsin with these absorption peaks. It interconverts with a metarhodopsin that absorbs around 570 nm.
5. R 7 is a U.V.-receptor, containing rhodopsin that absorbs around 370 nm and interconverts with a metarhodopsin which absorbs around 470 nm.
6. R 8 is a non-adapting blue-receptor with a third type of rhodopsin.
7. The properties of these photopigments explain the different sensitivities and spectral adaptation phenomena of the various photoreceptors.
8. All the photoreceptors have input into phototaxis. Spectral analysis of this behaviour provides evidence for integration of the input from the different photoreceptors.
Full text
PDF



























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alawi A. A., Jennings V., Grossfield J., Pak W. L. Phototransduction mutants of Drosophila melanogaster. Adv Exp Med Biol. 1972;24(0):1–21. doi: 10.1007/978-1-4684-8231-7_1. [DOI] [PubMed] [Google Scholar]
- Alawi A. A., Pak W. L. On-transient of insect electroretinogram: its cellular origin. Science. 1971 Jun 4;172(3987):1055–1057. doi: 10.1126/science.172.3987.1055. [DOI] [PubMed] [Google Scholar]
- Benzer S. BEHAVIORAL MUTANTS OF Drosophila ISOLATED BY COUNTERCURRENT DISTRIBUTION. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitenberg V. Patterns of projection in the visual system of the fly. I. Retina-lamina projections. Exp Brain Res. 1967;3(3):271–298. doi: 10.1007/BF00235589. [DOI] [PubMed] [Google Scholar]
- Eckert H. Die spektrale Empfindlichkeit des Komplexauges von Musca (Bestimmung aus Messungen der optomotorischen Reaktion. Kybernetik. 1971 Oct;9(4):145–156. doi: 10.1007/BF00290480. [DOI] [PubMed] [Google Scholar]
- Franceschini N., Kirschfeld K. Etude optique in vivo des éléments photorécepteurs dans l'oeil composé de Drosophila. Kybernetik. 1971 Jan;8(1):1–13. doi: 10.1007/BF00270828. [DOI] [PubMed] [Google Scholar]
- Goldsmith T. H. Do Flies Have A Red Receptor? J Gen Physiol. 1965 Nov 1;49(2):265–287. doi: 10.1085/jgp.49.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdorf K., Schwemer J., Gogala M. Insect visual pigment sensitive to ultraviolet light. Nature. 1971 Jun 18;231(5303):458–459. doi: 10.1038/231458a0. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Benzer S. Genetic dissection of the Drosophila nervous system by means of mosaics. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1156–1163. doi: 10.1073/pnas.67.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschfeld K., Franceschini N. Optische Eigenschaften der Ommatidien im Komplexauge von Musca. Kybernetik. 1968 Aug;5(2):47–52. doi: 10.1007/BF00272694. [DOI] [PubMed] [Google Scholar]
- McCann G. D., Arnett D. W. Spectral and polarization sensitivity of the dipteran visual system. J Gen Physiol. 1972 May;59(5):534–558. doi: 10.1085/jgp.59.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B., Hochstein S., Hillman P. Letter: Antagonistic process as source of visible-light suppression of afterpotential in Limulus UV photoreceptors. J Gen Physiol. 1973 Dec;62(6):787–791. doi: 10.1085/jgp.62.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroy S. E., Wilson M., Pak W. L. Drosophila rhodopsin: photochemistry, extraction and differences in the norp AP12 phototransduction mutant. Biochem Biophys Res Commun. 1974 Aug 5;59(3):960–966. doi: 10.1016/s0006-291x(74)80073-2. [DOI] [PubMed] [Google Scholar]
- Pak W. L., Lidington K. J. Fast electrical potential from a long-lived, long-wavelength photoproduct of fly visual pigment. J Gen Physiol. 1974 Jun;63(6):740–756. doi: 10.1085/jgp.63.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn W. G., Harris W. A., Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974 Mar;71(3):708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALD G., BROWN P. K. The molar extinction of rhodopsin. J Gen Physiol. 1953 Nov 20;37(2):189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]








