Abstract
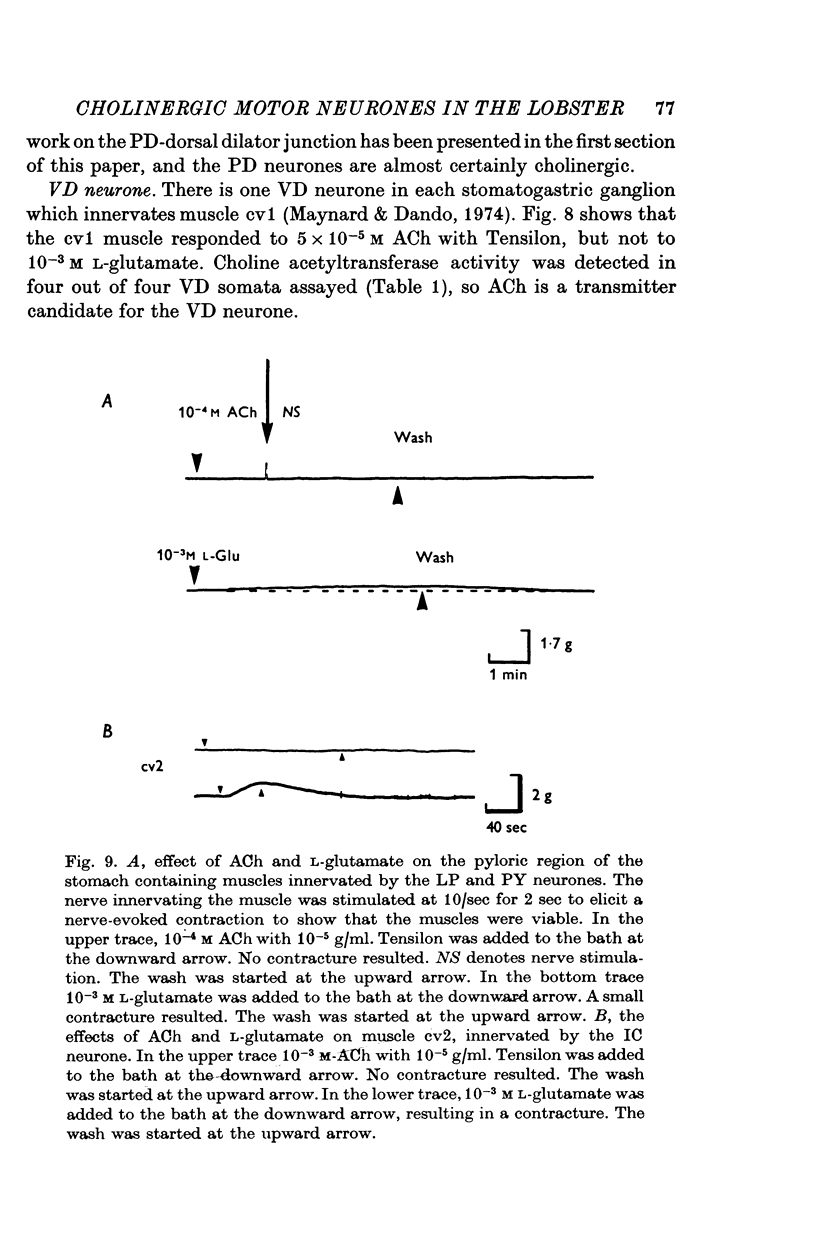
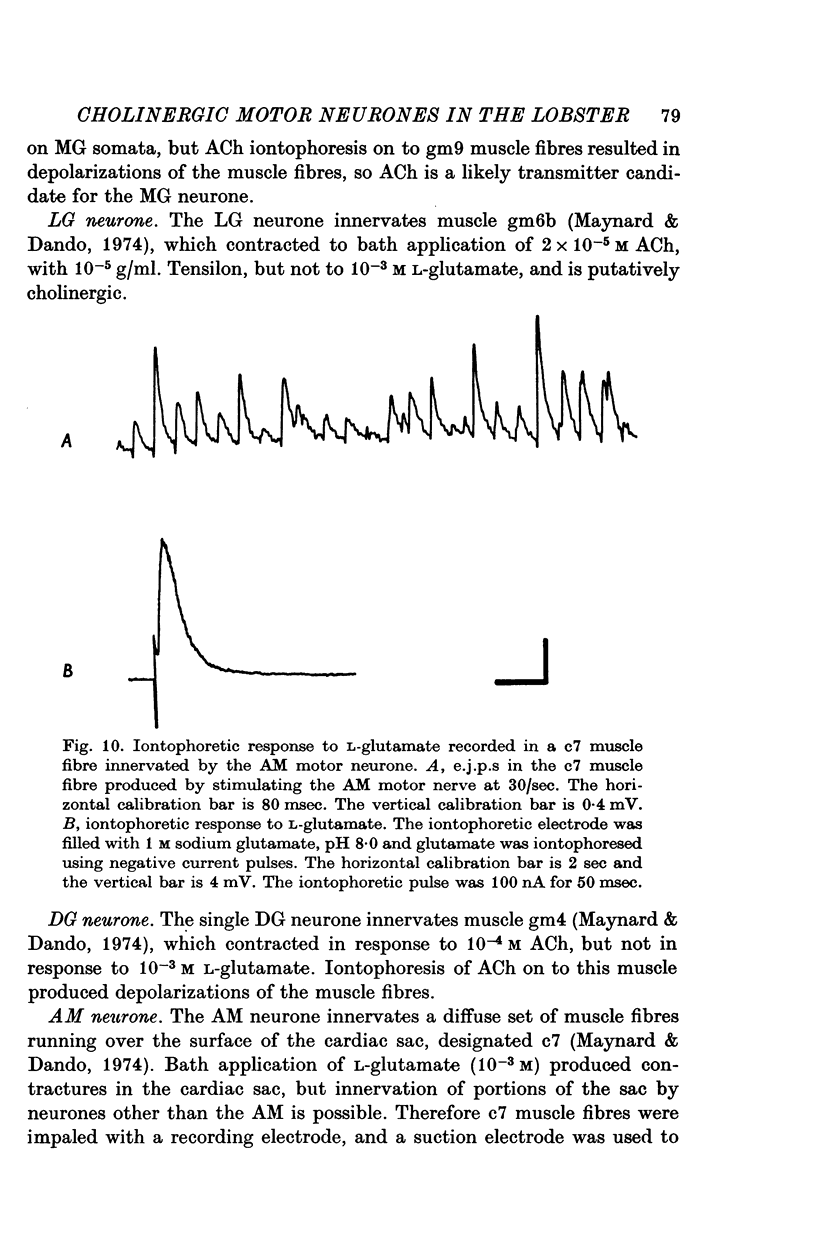
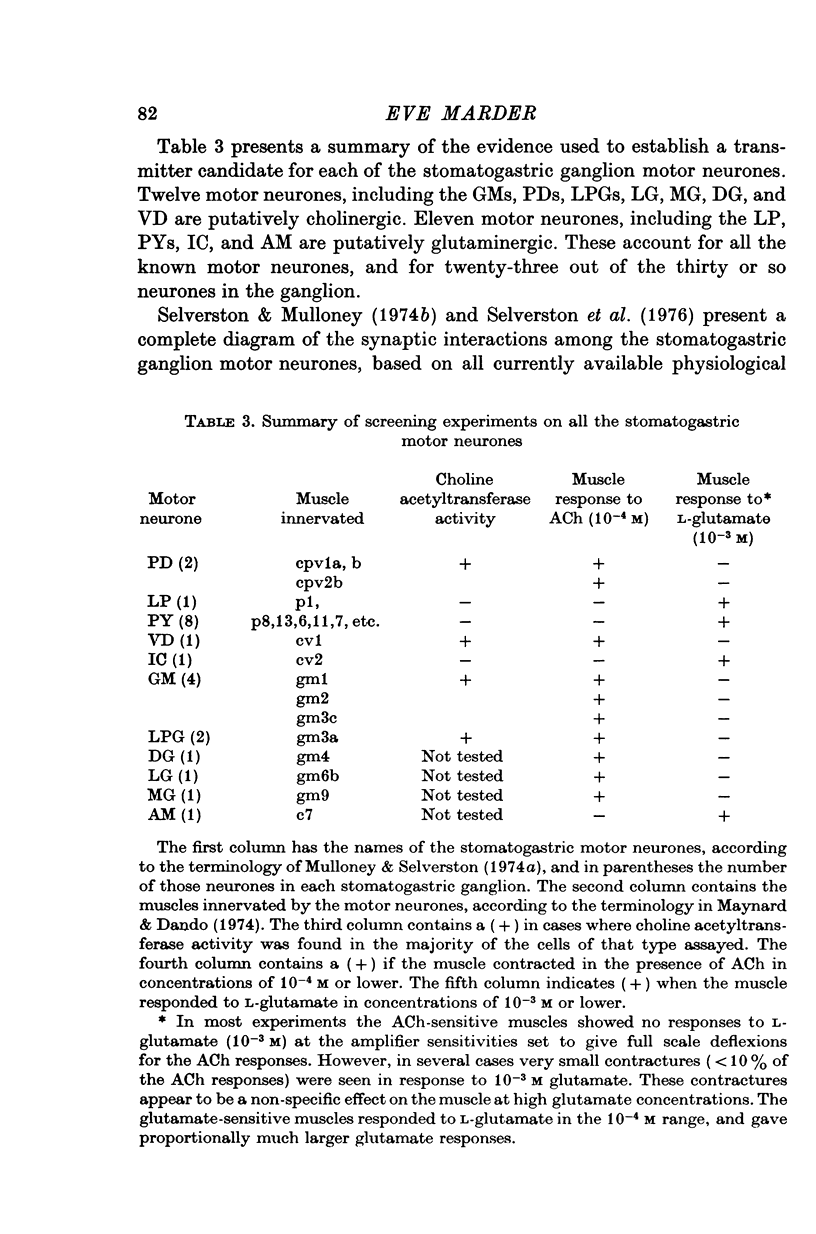
1. A study of the neurotransmitters used by each of the eleven types of excitatory motor neurones (identified according to the muscle innervated) of the lobster stomatogastric ganglion was undertaken. 2. The dorsal dilator muscle is innervated by the two motor neurones designated 'PD'. Bath and iontophoretic applications of acetylcholine (ACh) produce contractures and depolarizations respectively in the dorsal dilator muscle. 3. Pharmacological experiments support the cholinergic nature of the excitatory junctional potentials (e.j.p.s) recorded in the dorsal dilator muscle when the PD motor nerve is stimulated. 4. The apparent reversal potentials for the e.j.p.s and the iontophoretic ACh response in the dorsal dilator muscle are the same. 5. On the basis of choline acetyltransferase assays on identified stomatogastric ganglion motor neurone somata and tension measurements on the muscles innervated by each type of stomatogastric ganglion motor neurone, a transmitter candidate was established for each type of motor neurone. Motor neurones named VD, LPG, GM, MG, LG, and DG are putatively cholinergic. L-Glutamate is a transmitter candidate for the motor neurones called LP, PY, IC, and AM. 6. Potential correlations between the distribution of putatively cholinergic and glutaminergic motor neurones and the electrical coupling among the stomatogastric ganglion motor neurones are discussed.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE W., GINSBORG B. L. The action of the neuromuscular transmitter on the slow fibre membrane. J Physiol. 1956 Jun 28;132(3):599–610. doi: 10.1113/jphysiol.1956.sp005552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R. E., Dewhurst S. A., Weinreich D., McCaman R. E. Aromatic acid decarboxylase and choline acetylase activities in a single identified 5-HT containing cell of the leech. J Neurobiol. 1972;3(3):259–265. doi: 10.1002/neu.480030308. [DOI] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Mechanism of facilitation at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:530–542. doi: 10.1113/jphysiol.1961.sp006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:514–529. doi: 10.1113/jphysiol.1961.sp006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Evoy W. H., Beránek R. Pharmacological localization of excitatory and inhibitory synaptic regions in crayfish slow abdominal flexor muscle-fibres. Comp Gen Pharmacol. 1972 Jun;3(10):178–186. doi: 10.1016/0010-4035(72)90024-9. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Giller E., Jr, Schwartz J. H. Choline acetyltransferase in identified neurons of abdominal ganglion of Aplysia californica. J Neurophysiol. 1971 Jan;34(1):93–107. doi: 10.1152/jn.1971.34.1.93. [DOI] [PubMed] [Google Scholar]
- Hartline D. K., Maynard D. M. Motor patterns in the stomatogastric ganglion of the lobster Panulirus argus. J Exp Biol. 1975 Apr;62(2):405–420. doi: 10.1242/jeb.62.2.405. [DOI] [PubMed] [Google Scholar]
- Hebb C. Biosynthesis of acetylcholine in nervous tissue. Physiol Rev. 1972 Oct;52(4):918–957. doi: 10.1152/physrev.1972.52.4.918. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. The interaction between edrophonium (tensilon) and acetylcholine at the motor end-plate. Br J Pharmacol Chemother. 1957 Jun;12(2):260–264. doi: 10.1111/j.1476-5381.1957.tb00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowagie C., Gerschenfeld H. M. Glutamate antagonists at a crayfish neuromuscular junction. Nature. 1974 Apr 5;248(448):533–535. doi: 10.1038/248533a0. [DOI] [PubMed] [Google Scholar]
- Marder E. Acetylcholine as an excitatory neuromuscular transmitter in the stomatogastric system of the lobster. Nature. 1974 Oct 25;251(5477):730–731. doi: 10.1038/251730a0. [DOI] [PubMed] [Google Scholar]
- Maynard D. M., Dando M. R. The structure of the stomatogastric neuromuscular system in Callinectes sapidus, Homarus americanus and Panulirus argus (Decapoda Crustacea). Philos Trans R Soc Lond B Biol Sci. 1974 Aug 1;268(892):161–220. doi: 10.1098/rstb.1974.0024. [DOI] [PubMed] [Google Scholar]
- Maynard D. M. Simpler networks. Ann N Y Acad Sci. 1972 Aug 25;193:59–72. doi: 10.1111/j.1749-6632.1972.tb27823.x. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Kravitz E. A., Potter D. D. Physiological and chemical architecture of a lobster ganglion with particular reference to gamma-aminobutyrate and glutamate. J Neurophysiol. 1967 Jul;30(4):725–752. doi: 10.1152/jn.1967.30.4.725. [DOI] [PubMed] [Google Scholar]
- Ozeki M., Freeman A. R., Grundfest H. The membrane components of crustacean neuromuscular systems. II. Analysis of interactions among the electrogenic components. J Gen Physiol. 1966 Jul;49(6):1335–1349. doi: 10.1085/jgp.0491335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REUBEN J. P., GAINER H. Membrance conductance during depolarizing postsynaptic potentials of crayfish muscle fibres. Nature. 1962 Jan 13;193:142–143. doi: 10.1038/193142a0. [DOI] [PubMed] [Google Scholar]
- ROBBINS J. The excitation and inhibition of crustacean muscle by amino acids. J Physiol. 1959 Oct;148:39–50. doi: 10.1113/jphysiol.1959.sp006272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. LOCALIZED ACTION OF GAMMA-AMINOBUTYRIC ACID ON THE CRAYFISH MUSCLE. J Physiol. 1965 Mar;177:225–238. doi: 10.1113/jphysiol.1965.sp007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraskevich P. S. Reversal potentials of L-glutamate and the excitatory transmitter at the neuromuscular junction of the crayfish. Biochim Biophys Acta. 1971 Aug 13;241(2):700–703. doi: 10.1016/0005-2736(71)90071-x. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A., MENDELSON M. Glutamate-induced contractions in crustacean muscle. J Cell Comp Physiol. 1959 Aug;54:85–94. doi: 10.1002/jcp.1030540109. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish neuromuscular facilitation activated by constant presynaptic action potentials and depolarizing pulses. J Physiol. 1974 Aug;241(1):69–89. doi: 10.1113/jphysiol.1974.sp010641. [DOI] [PMC free article] [PubMed] [Google Scholar]



