Abstract
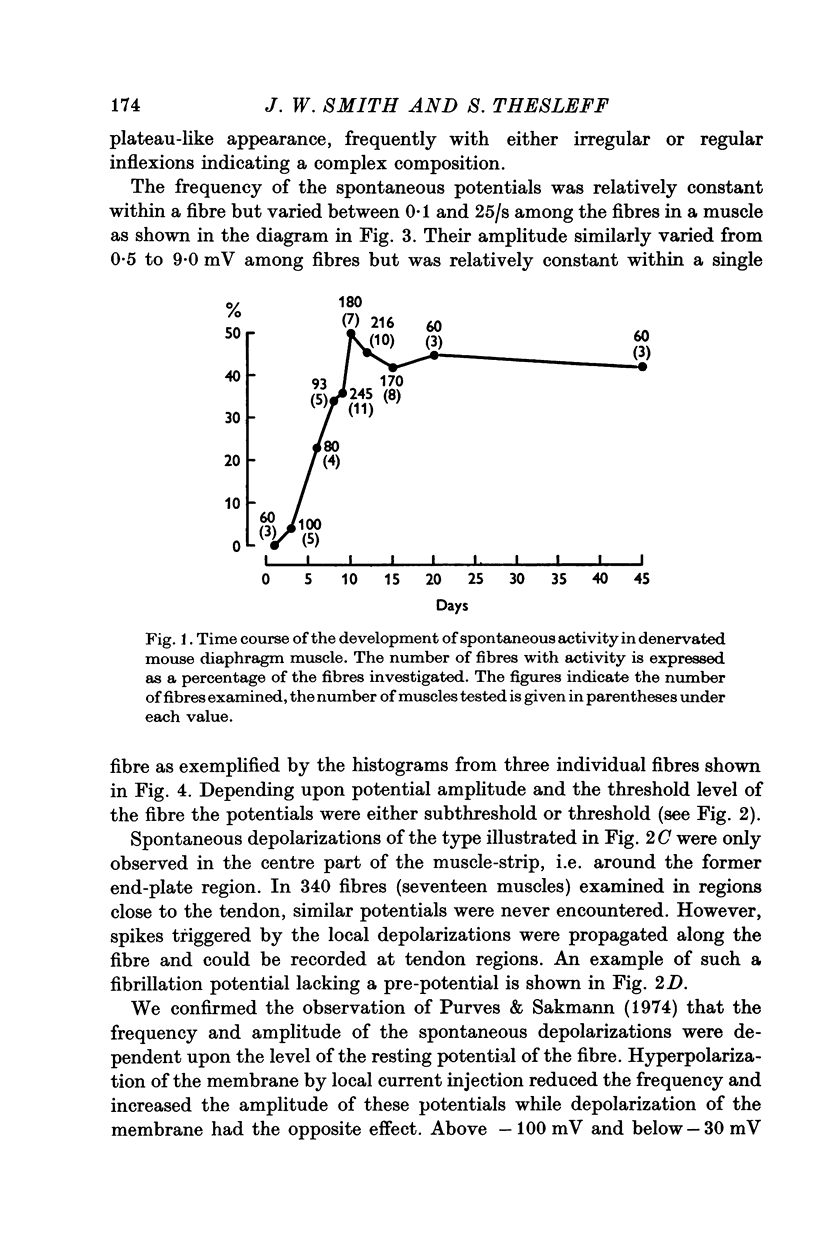
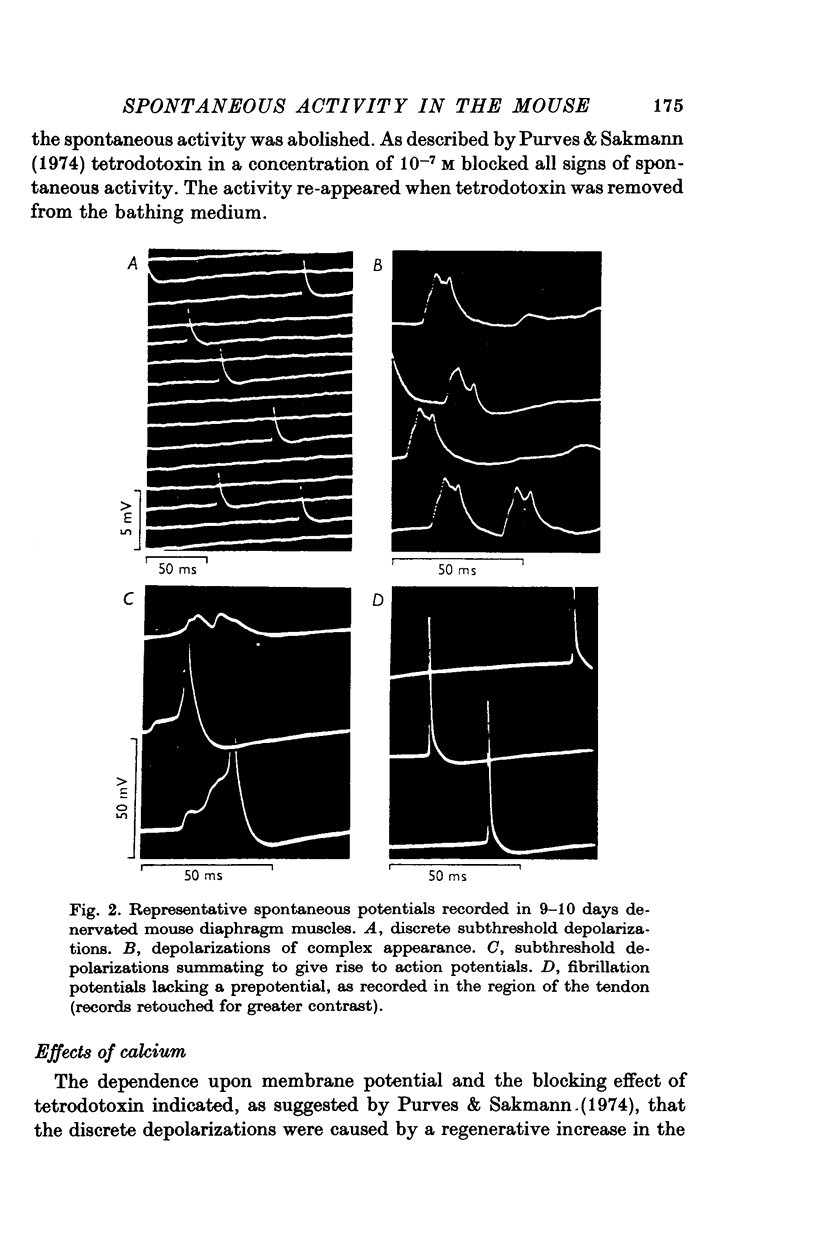
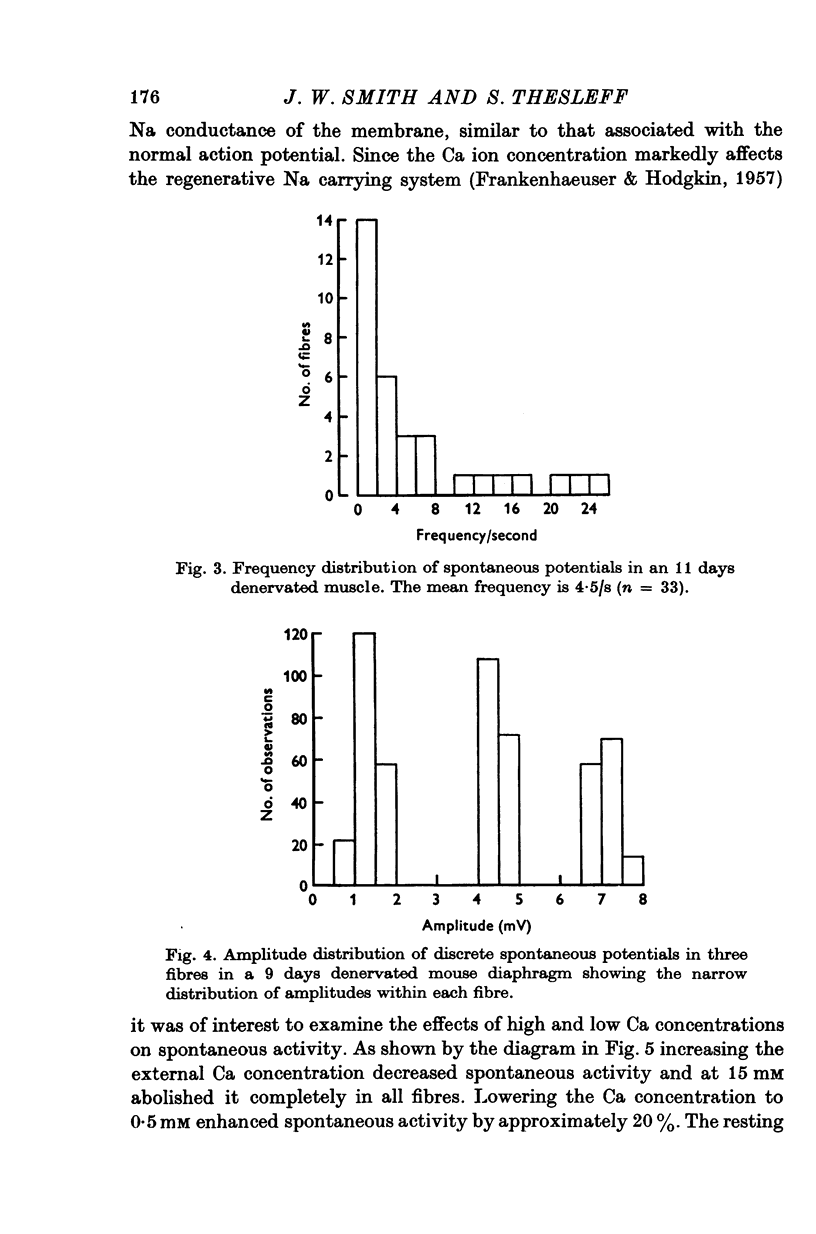
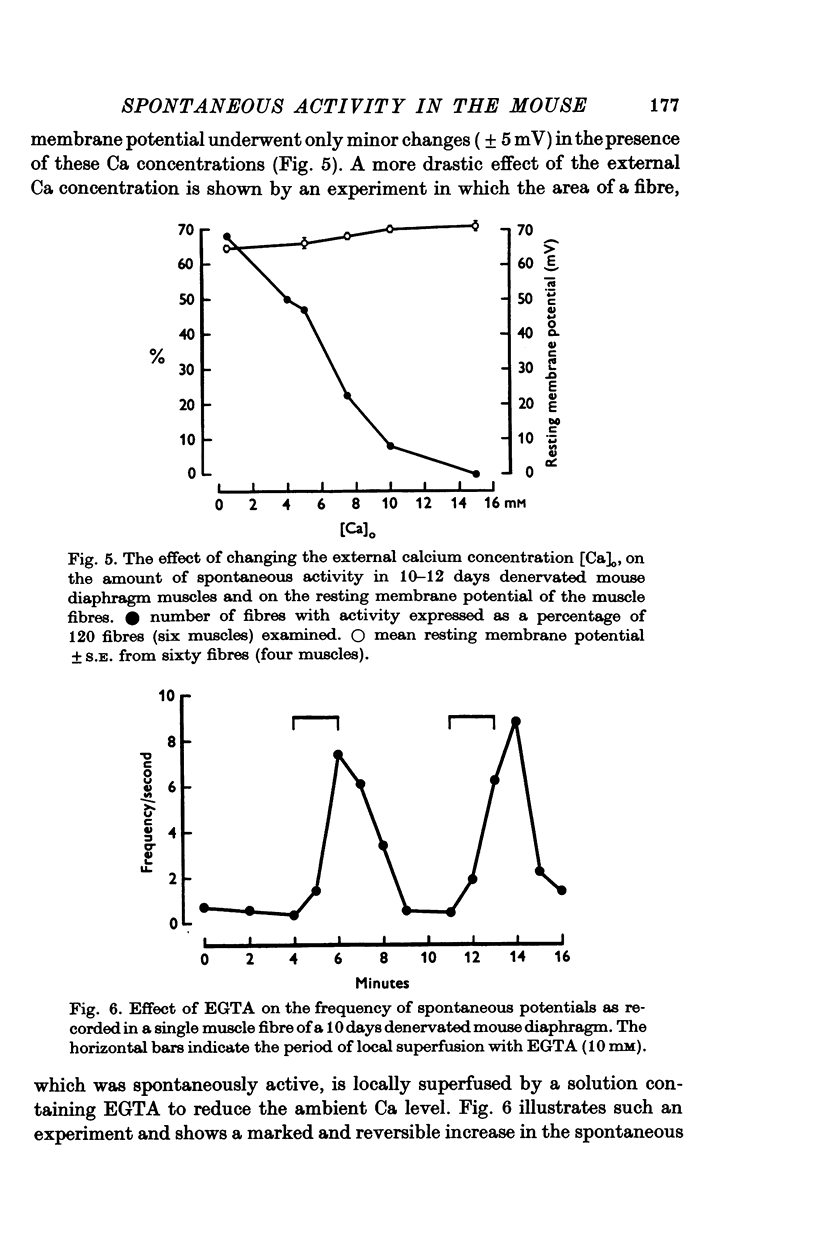
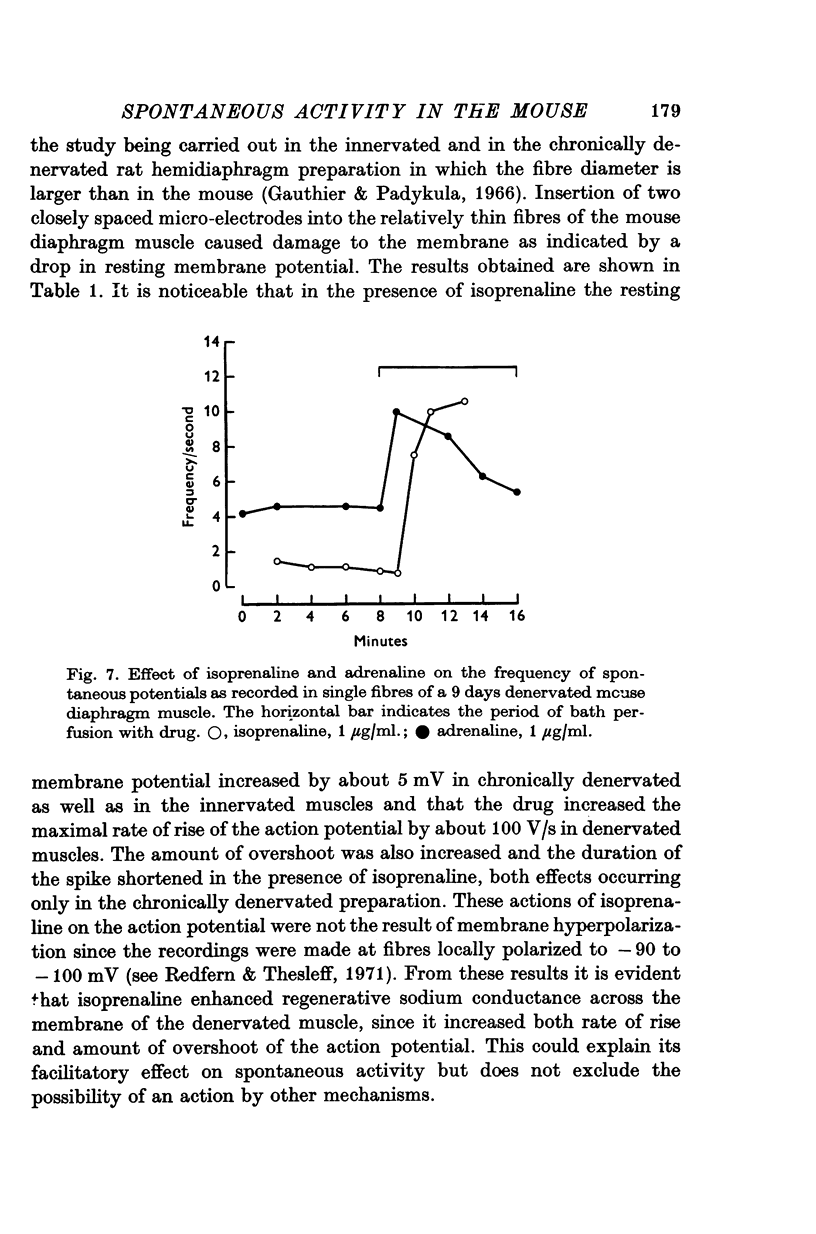
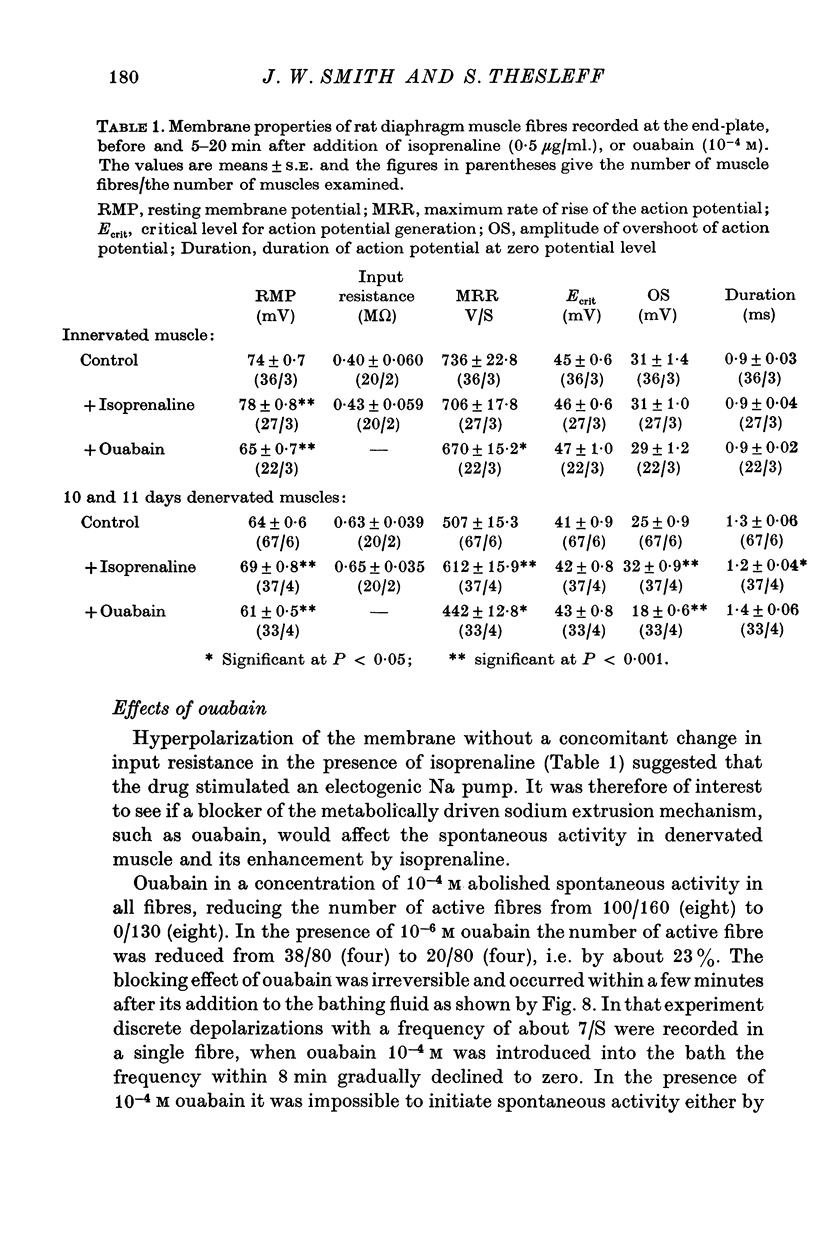
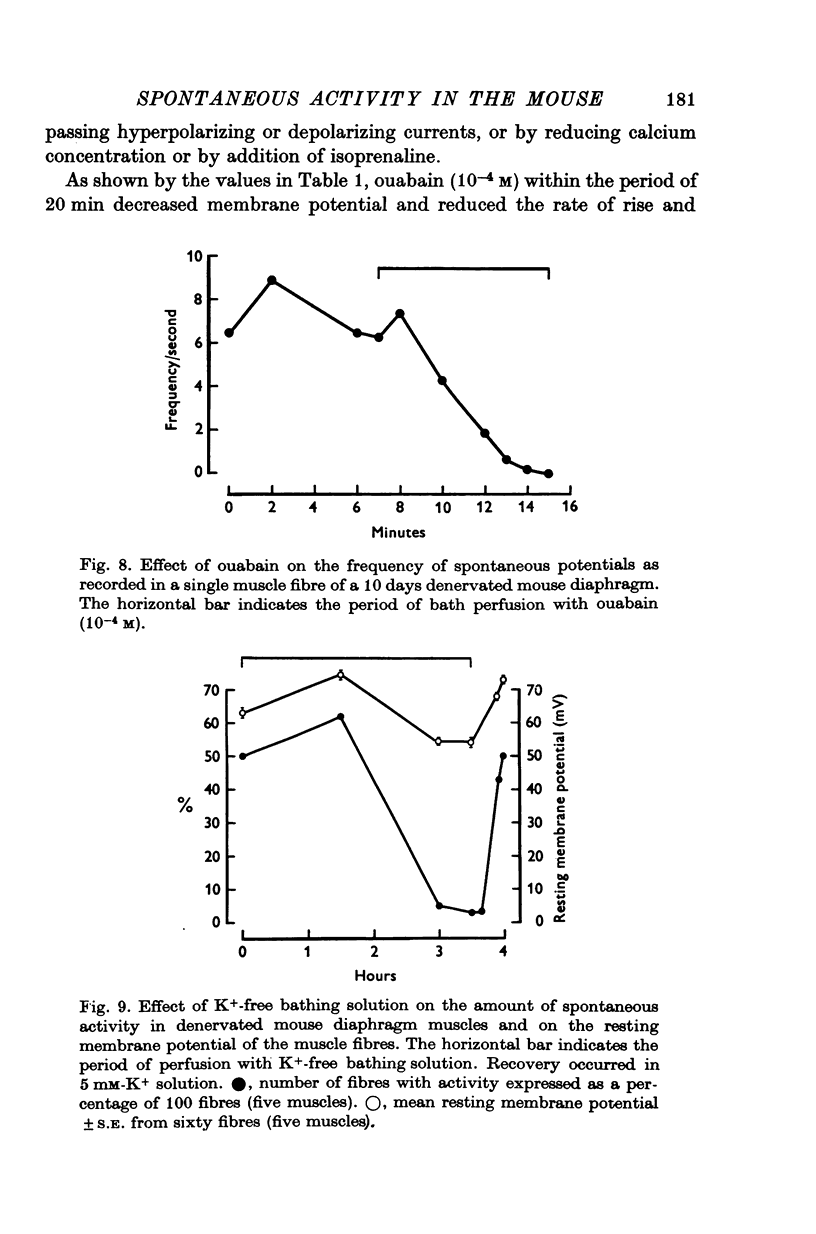
Intracellular electrodes were used to study the discrete depolarizations which trigger fibrillation potentials in chronically denervated mouse diaphragm muscles. Provided that the muscles were perfused on both sides spontaneous activity was maintained in vitro. 2. Discrete spontaneous depolarizations, present only in the centre of the muscle, were recorded from the third day of denervation reaching a maximum in prevalence 9-12 days after sectioning the nerve. These potentials had random occurrence and nearly constant amplitude and frequency within a fibre, dependence of amplitude and frequency on membrane potential, and low temperature dependence. 3. The spontaneous activity was enhanced and could be initiated in previously quiescent fibres by lowering the external Ca concentration. The activity was reduced by increasing external Ca and was abolished at 15mM-[Ca] 0. Tetrodotoxin (10-(7)M) blocked spontaneous activity. 4. The spontaneous activity was enhanced by the catecholamines isoprenaline and adrenaline (0.5-10 mug/ml.). This effect of isoprenaline was accompanied by an increase in the rate of rise and the amount of overshoot of the action potential. 5. Ouabain (10-(6)-10-(4)M) of K+-free solutions reversibly blocked spontaneous activity. Ouabain (10-(4)M) reduced the rate of rise and the amount of overshoot of the action potential. 6. Detubulation of muscle fibres with glycerol of the presence of hypertonic solutions abolished spontaneous activity which could not be restarted by reducing Ca or by the addition of isoprenaline. 7. The results support the suggestion that the spontaneous discrete depolarizations which give rise to fibrillation potentials in denervated muscle result from regenerative sodium conductance increases within the transverse tubular system of the muscle fibres. Catecholamines and ouabain could affect this activity either directly, through an action on membrane excitability, or indirectly via the Na+-K+ pump.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWMAN W. C., RAPER C. THE EFFECTS OF SYMPATHOMIMETIC AMINES ON CHRONICALLY DENERVATED SKELETAL MUSCLES. Br J Pharmacol Chemother. 1965 Feb;24:98–109. doi: 10.1111/j.1476-5381.1965.tb02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoola K. D., Evans R. H., Smith J. W. Catecholamine induced contractures in denervated muscle. Br J Pharmacol. 1972 Nov;46(3):531P–532P. [PMC free article] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockry M., Kernan R. P., Tangney A. Active transport of sodium and potassium in mammalian skeletal muscle and its modification by nerve and by cholinergic and adrenergic agents. J Physiol. 1966 Sep;186(1):187–200. doi: 10.1113/jphysiol.1966.sp008028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B., Eisenberg R. S. Selective disruption of the sarcotubular system in frog sartorius muscle. A quantitative study with exogenous peroxidase as a marker. J Cell Biol. 1968 Nov;39(2):451–467. doi: 10.1083/jcb.39.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K. O., Bryant S. H. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):107–109. doi: 10.1007/BF00501011. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freygang W. H., Jr, Rapoport S. I., Peachey L. D. Some relations between changes in the linear electrical properties of striated muscle fibers and changes in ultrastructure. J Gen Physiol. 1967 Nov;50(10):2437–2458. doi: 10.1085/jgp.50.10.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Padykula H. A. Cytological studies of fiber types in skeletal muscle. A comparative study of the mammalian diaphragm. J Cell Biol. 1966 Feb;28(2):333–354. doi: 10.1083/jcb.28.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luco C. F., Luco J. V. Sympathetic effects on fibrillary activity of denervated striated muscles. J Neurophysiol. 1971 Nov;34(6):1066–1071. doi: 10.1152/jn.1971.34.6.1066. [DOI] [PubMed] [Google Scholar]
- MONTAGU K. A. On the mechanism of action of adrenaline in skeletal nerve-muscle. J Physiol. 1955 Jun 28;128(3):619–628. doi: 10.1113/jphysiol.1955.sp005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Sakmann B. Membrane properties underlying spontaneous activity of denervated muscle fibres. J Physiol. 1974 May;239(1):125–153. doi: 10.1113/jphysiol.1974.sp010559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. I. Quantitative aspects. Acta Physiol Scand. 1971 Apr;81(4):557–564. doi: 10.1111/j.1748-1716.1971.tb04932.x. [DOI] [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Muscle fibre growth in five different muscles in both sexes of mice. J Anat. 1969 May;104(Pt 3):519–530. [PMC free article] [PubMed] [Google Scholar]
- Thesleff S., Vyskocil F., Ward M. R. The action potential in end-plate and extrajunctional regions of rat skeletal muscle. Acta Physiol Scand. 1974 Jun;91(2):196–202. doi: 10.1111/j.1748-1716.1974.tb05676.x. [DOI] [PubMed] [Google Scholar]
- Thesleff S., Ward M. R. Studies on the mechanism of fibrillation potentials in denervated muscle. J Physiol. 1975 Jan;244(2):313–323. doi: 10.1113/jphysiol.1975.sp010800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachar J., Zacharova D., Adrian R. H. Observations on "detubulated" muscle fibres. Nat New Biol. 1972 Oct 4;239(92):153–155. doi: 10.1038/newbio239153a0. [DOI] [PubMed] [Google Scholar]



