Abstract
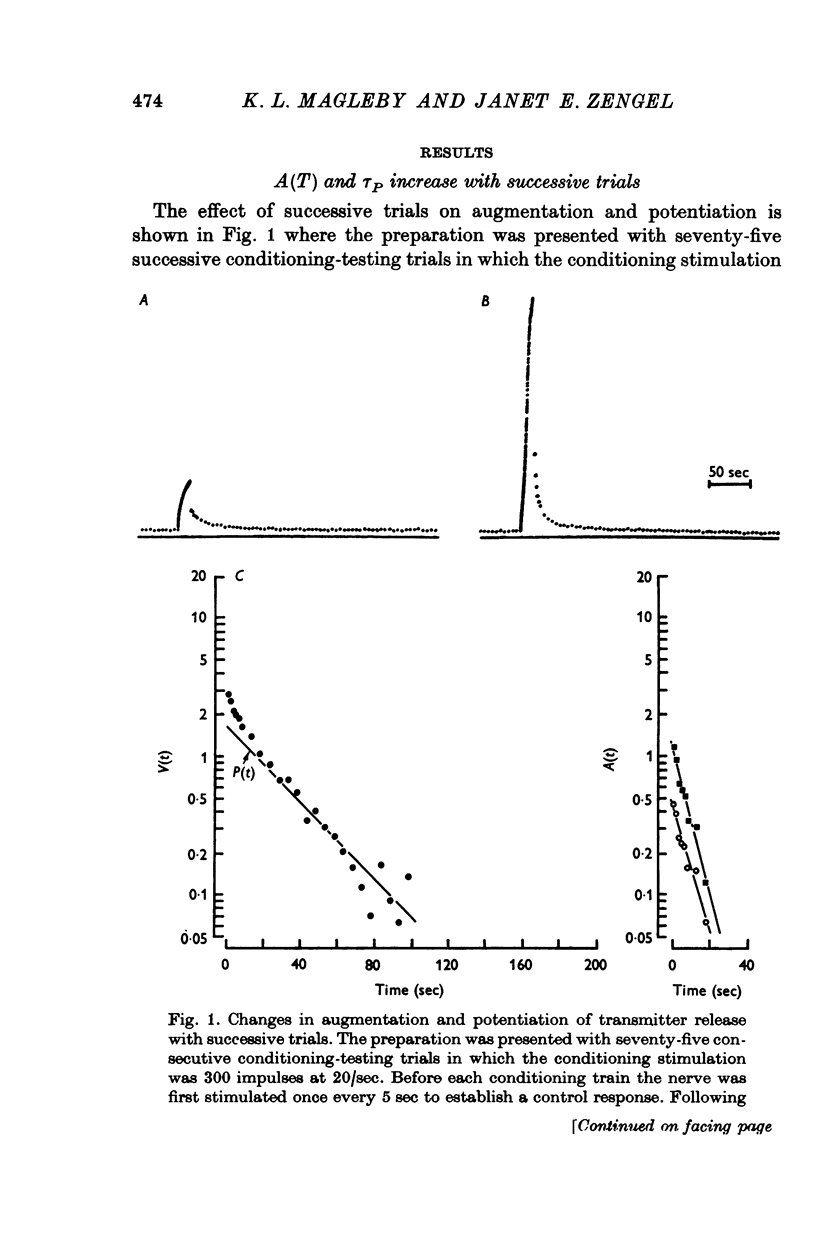
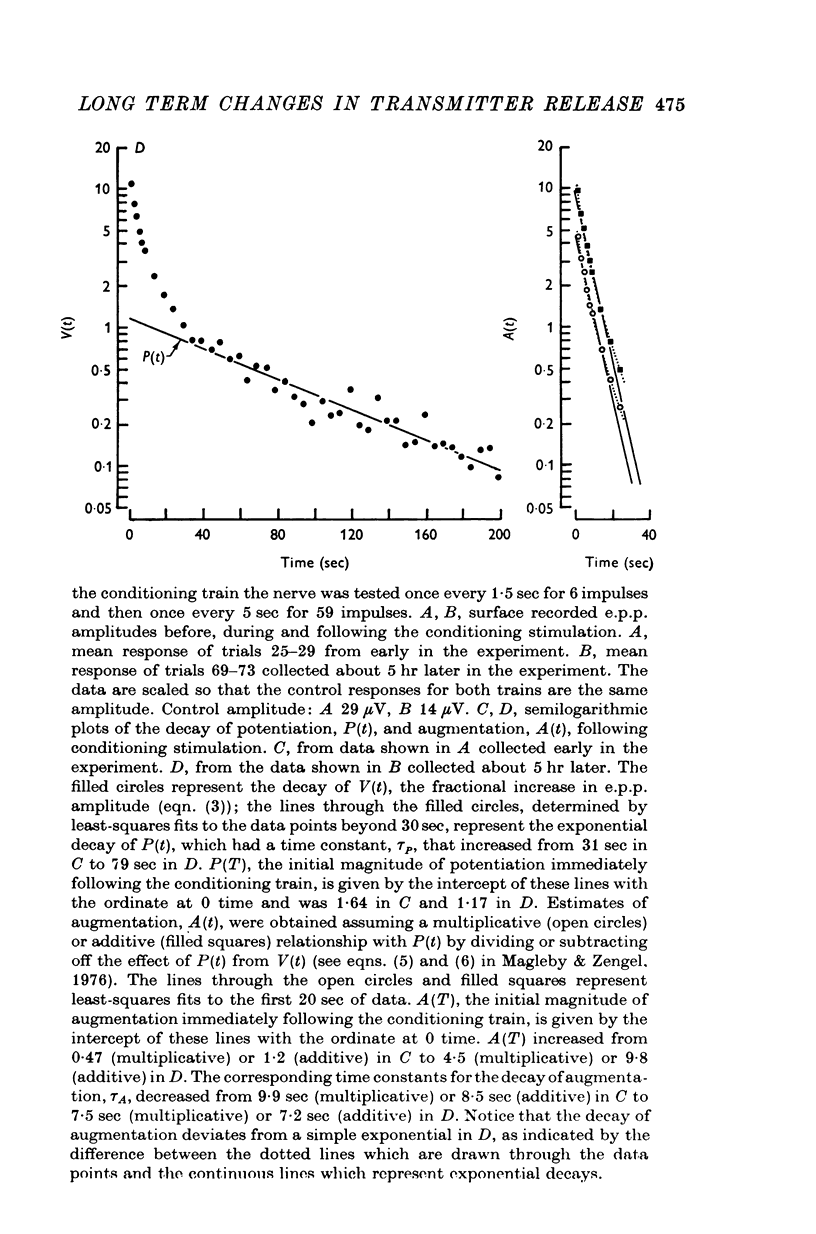
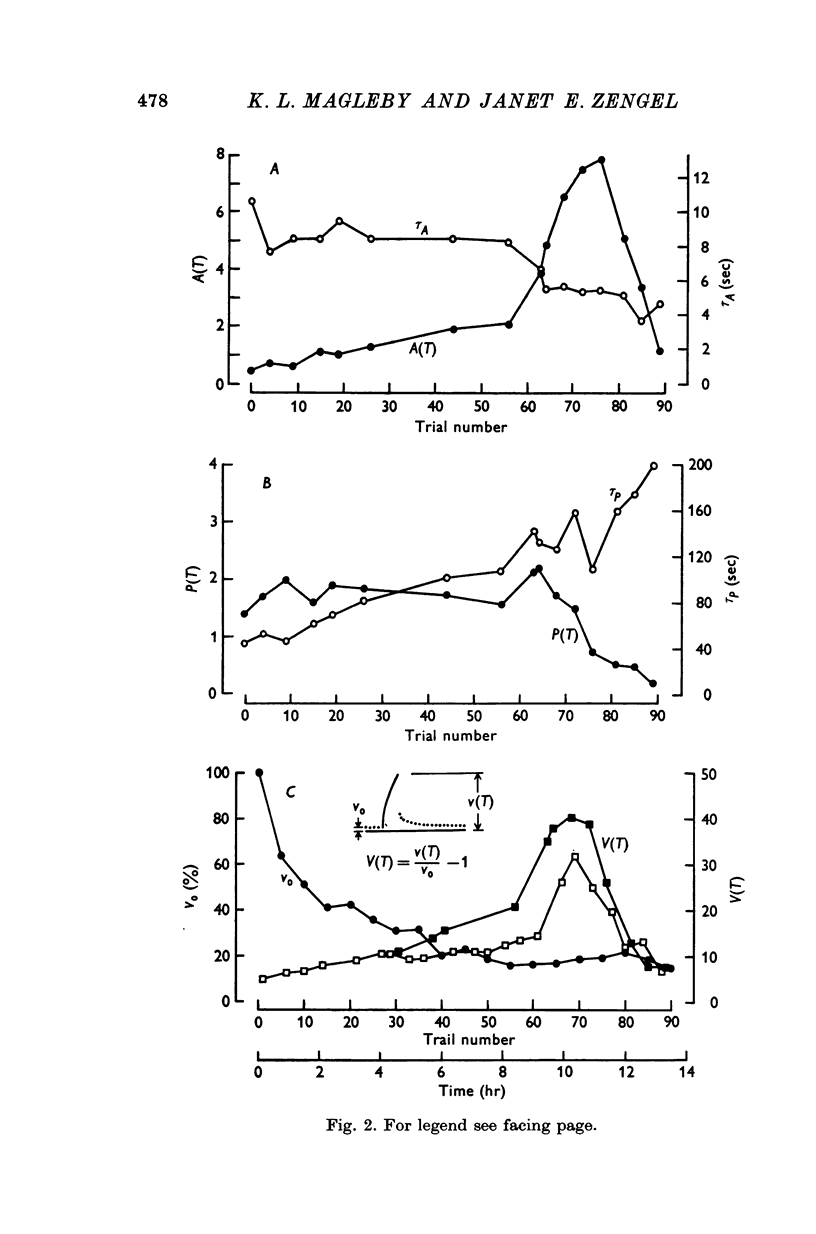
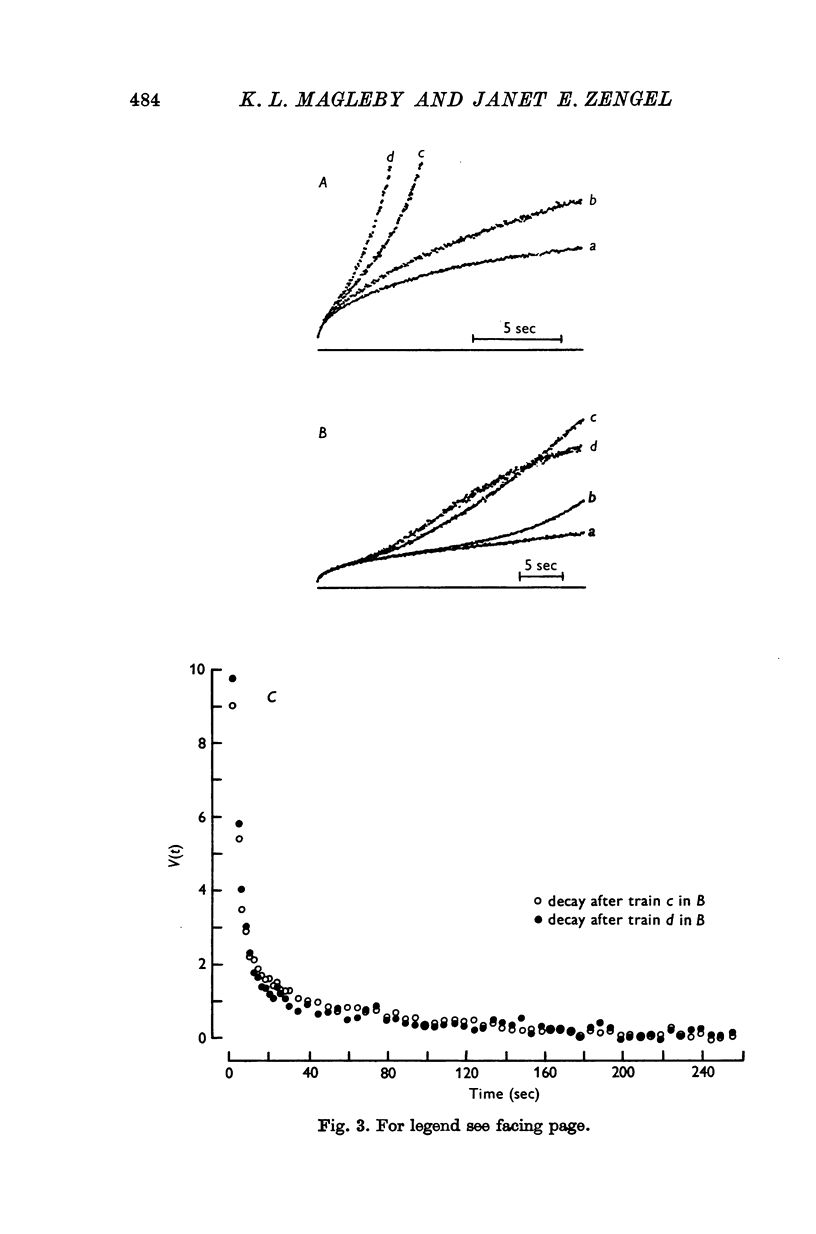
1. End-plate potentials (e.p.p.s) were recorded from frog neuromuscular junctions under conditions of low quantal content to study the long-term effects of repeated synaptic activity on transmitter release. 2. The nerve terminal was presented with 30-100 successive conditioning-testing trials applied once every 7-10 min over a 4-16 hr perod. Each conditioning-testing trial consisted of a 200-600 impulse conditioning train followed by a series of testing impulses. The magnitudes and time constants of decay of augmentation and potentiation following each successive conditioning train were determined by measuring the e.p.p. amplitudes resulting from the testing impulses. 3. The magnitude of augmentation immediately following the conditioning trains increased an average of 3-4 times (range 1-20) with sucessive trials. 4. As the magnitude of augmentation increased with successive trials the decay of augmentation deviated from a simple exponential, decaying faster immediately after the conditioning train. This faster decay led to a 20% decrease with successive trials in estimates of the time constant obtained from the first 10 or 20 sec of the decay of augmentation. The deviation of the decay of augmentation from a simple exponential could be accounted for if augmentation is related to the 4th power of some substance which decays with a simple exponential time course. Some alternative explantations for the non-exponential decay of augmentation are also discussed. 5. The magnitude of potentiation increased or decreased about 25% with successive trials. 6. The time constant characterizing the decay of potentiation inceased an average of 1-5 times (range 0-8-5 times) with successive trials. 7. The increase in the magnitude of augmentation with successive trials was accompanied by a similar increase in the magnitude of the e.p.p. amplitudes during the conditioning trains, suggesting that augmentation develops during the conditioning train. In some preparatons augmentation appeared to be the major factor acting to increase e.p.p. amplitudes during the conditioning train, having a greater effect than facilitation or potentiation. 8. If a sufficiently large number of successive trials were applied, a depression of e.p.p. amplitudes developed during the conditioning trains and estimates of the magnitude of potentiation following the depressed conditioning trains were reduced...
Full text
PDF










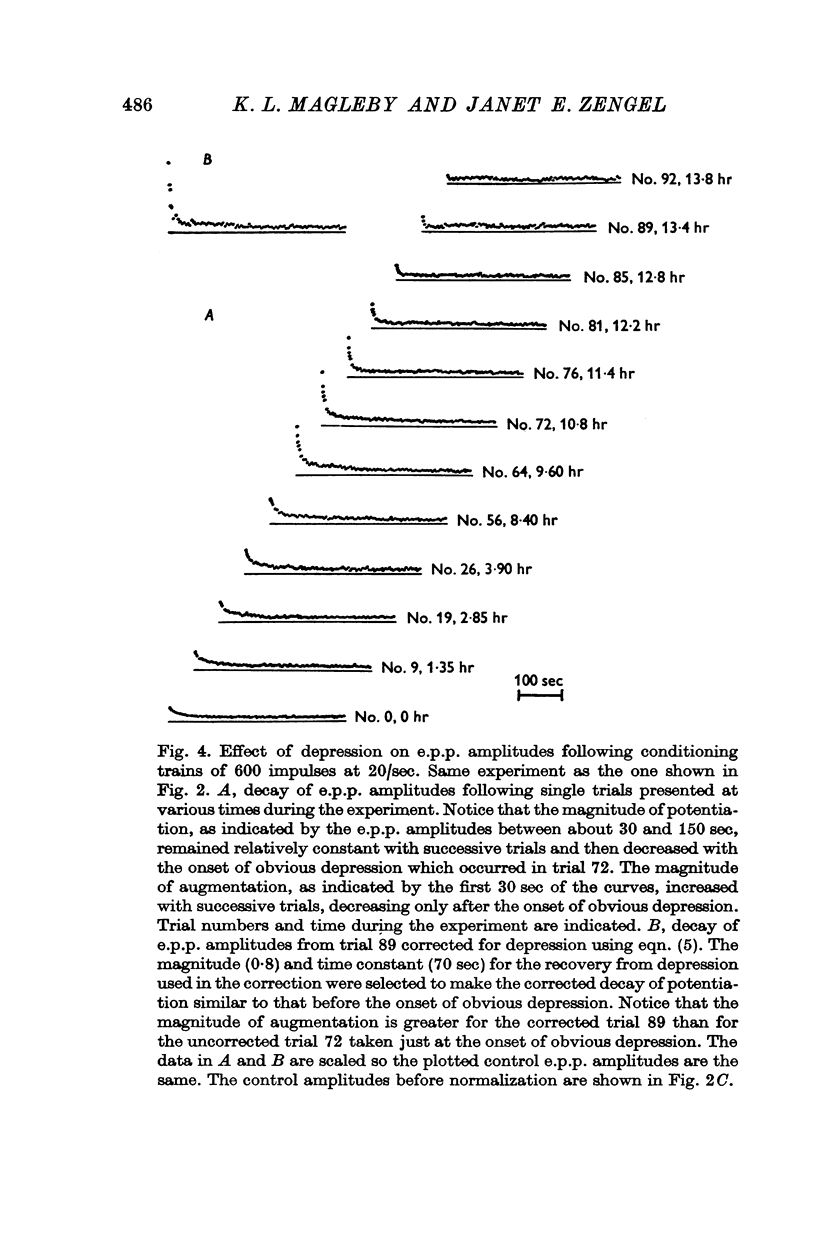



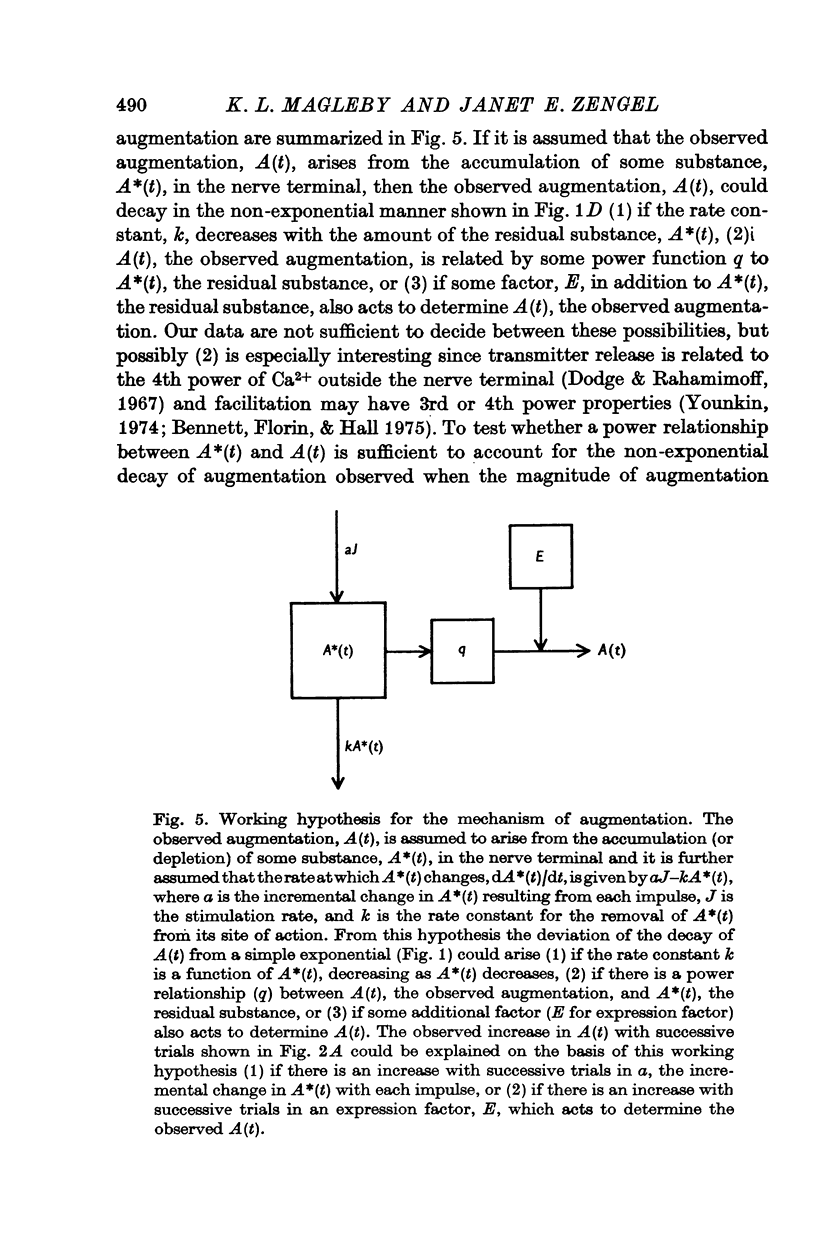
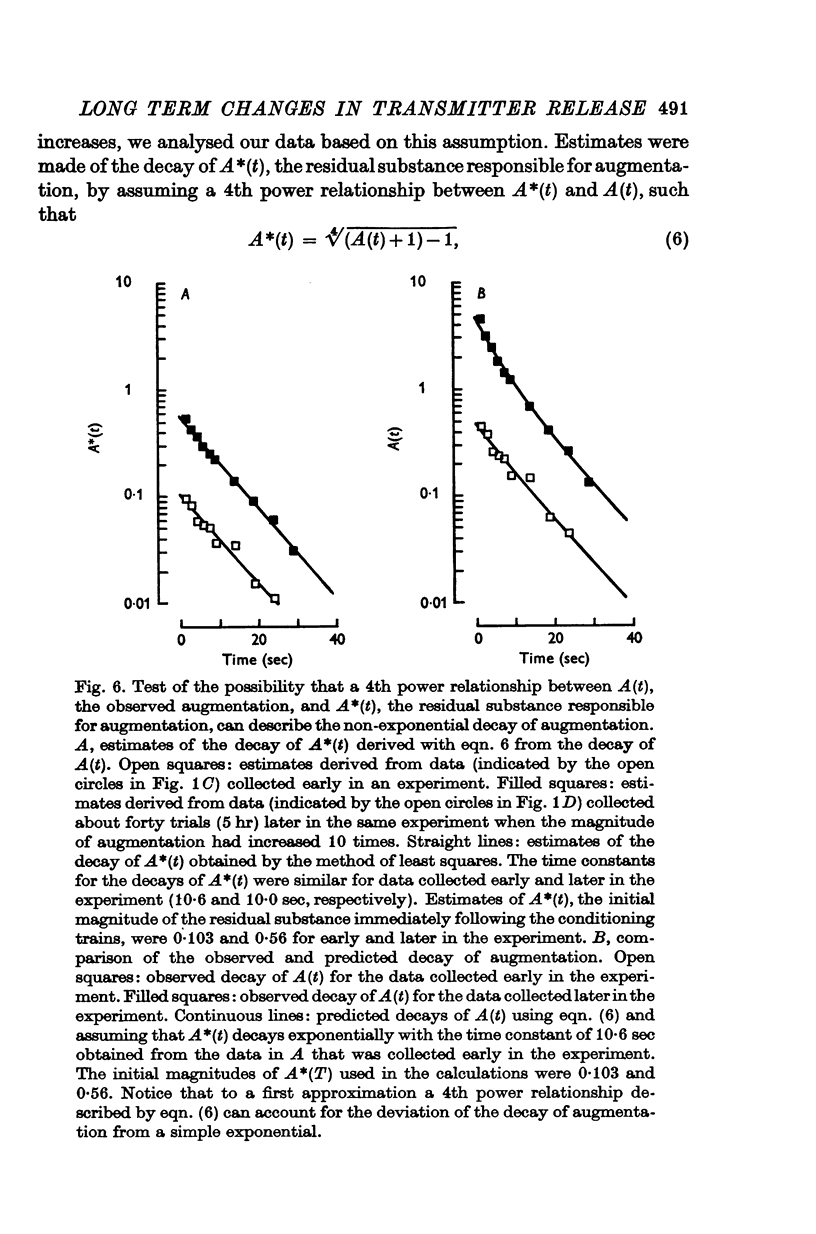



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Hall R. The effect of calcium ions on the binomial statistic parameters which control acetylcholine release at synapses in striated muscle. J Physiol. 1975 May;247(2):429–446. doi: 10.1113/jphysiol.1975.sp010939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I. Microphysiology of vertebrate neuromuscular transmission. Physiol Rev. 1973 Jul;53(3):674–723. doi: 10.1152/physrev.1973.53.3.674. [DOI] [PubMed] [Google Scholar]
- Lass Y., Halevi Y., Landau E. M., Gitter S. A new model for transmitter mobilization in the frog neuromuscular junction. Pflugers Arch. 1973 Oct 17;343(2):157–163. doi: 10.1007/BF00585711. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L. The effect of repetitive stimulation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):327–352. doi: 10.1113/jphysiol.1973.sp010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L. The effect of tetanic and post-tetanic potentiation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):353–371. doi: 10.1113/jphysiol.1973.sp010349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A dual effect of repetitive stimulation on post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975 Feb;245(1):163–182. doi: 10.1113/jphysiol.1975.sp010839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A quantitative description of tetanic and post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975 Feb;245(1):183–208. doi: 10.1113/jphysiol.1975.sp010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Augmentation: A process that acts to increase transmitter release at the frog neuromuscular junction. J Physiol. 1976 May;257(2):449–470. doi: 10.1113/jphysiol.1976.sp011378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. Post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1969 Jul;203(1):121–133. doi: 10.1113/jphysiol.1969.sp008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A. The long-lasting depression in neuromuscular transmission of frog. Jpn J Physiol. 1958 Jun 15;8(2):102–113. doi: 10.2170/jjphysiol.8.102. [DOI] [PubMed] [Google Scholar]
- Wernig A. Estimates of statistical release parameters from crayfish and frog neuromuscular junctions. J Physiol. 1975 Jan;244(1):207–221. doi: 10.1113/jphysiol.1975.sp010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younkin S. G. An analysis of the role of calcium in facilitation at the frog neuromuscular junction. J Physiol. 1974 Feb;237(1):1–14. doi: 10.1113/jphysiol.1974.sp010466. [DOI] [PMC free article] [PubMed] [Google Scholar]



