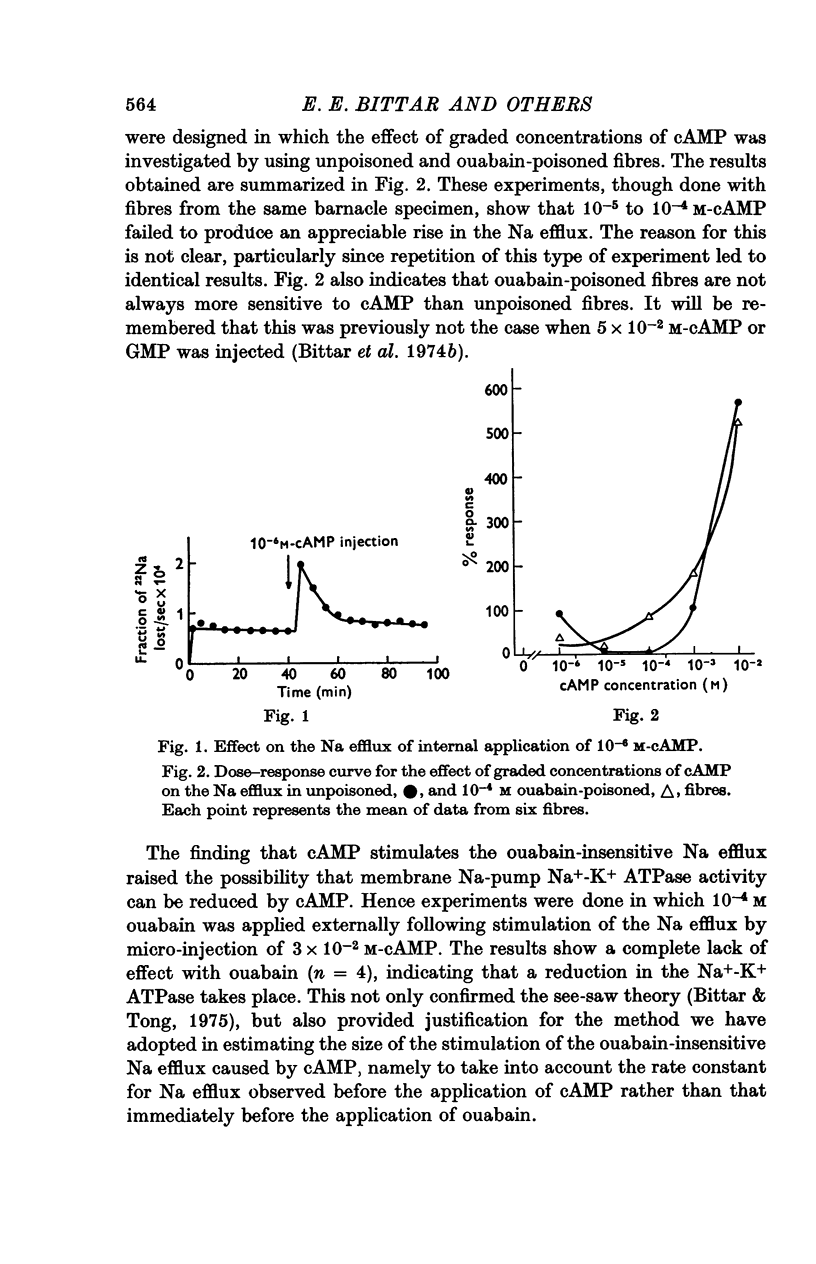
Abstract
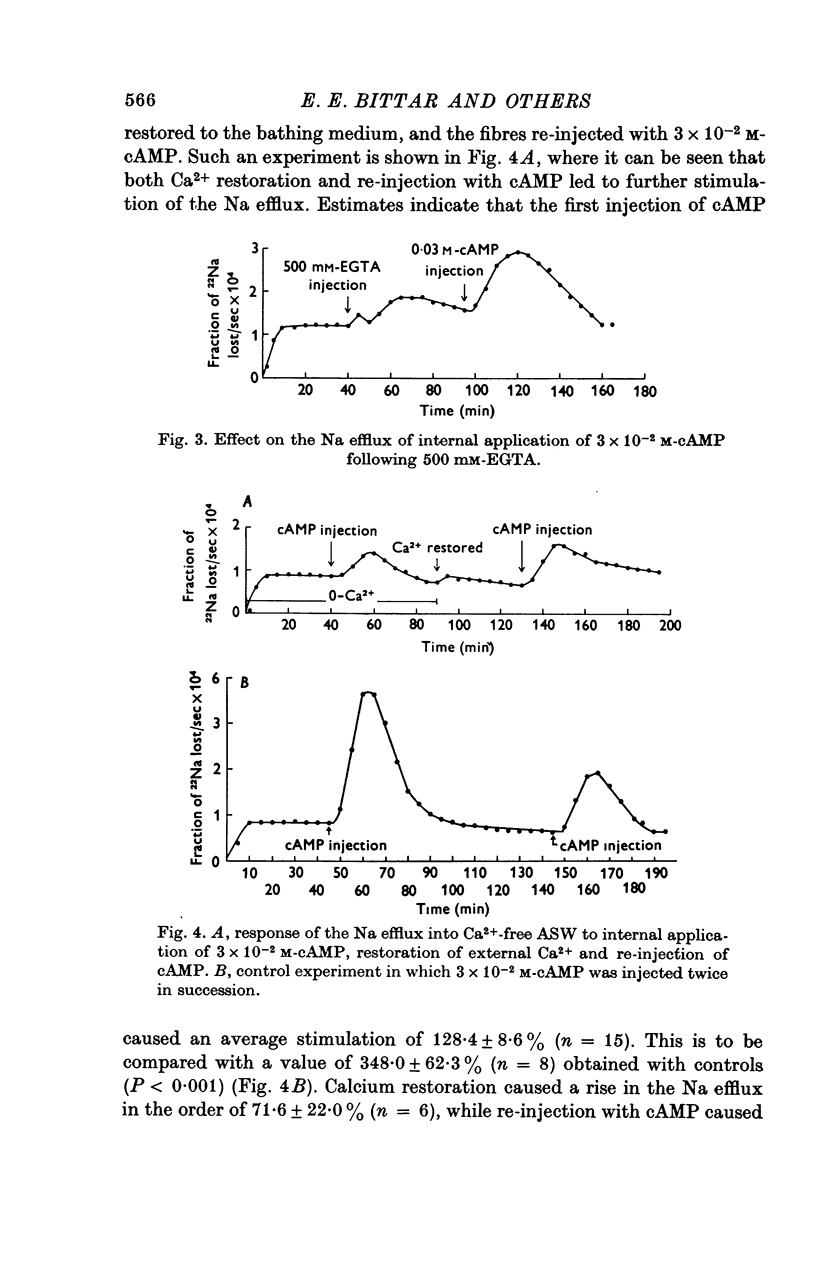
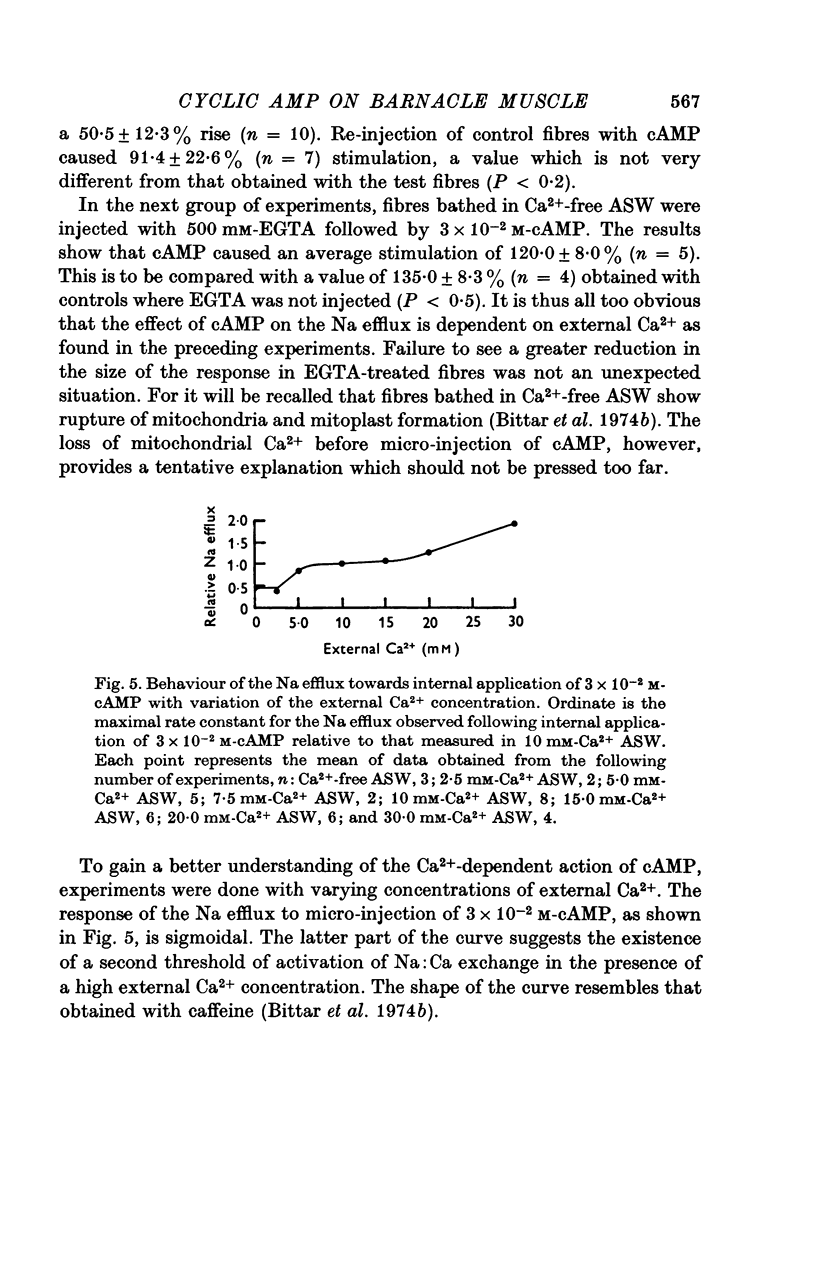
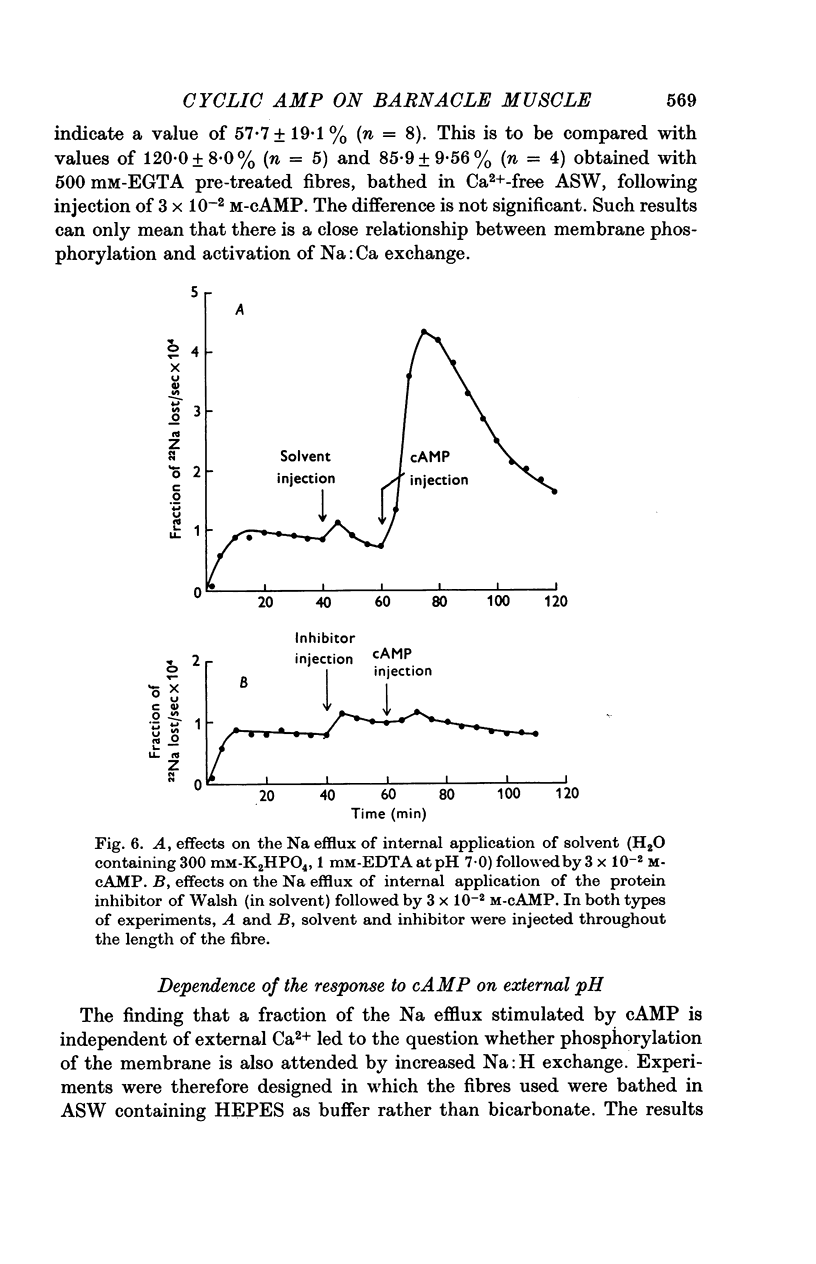
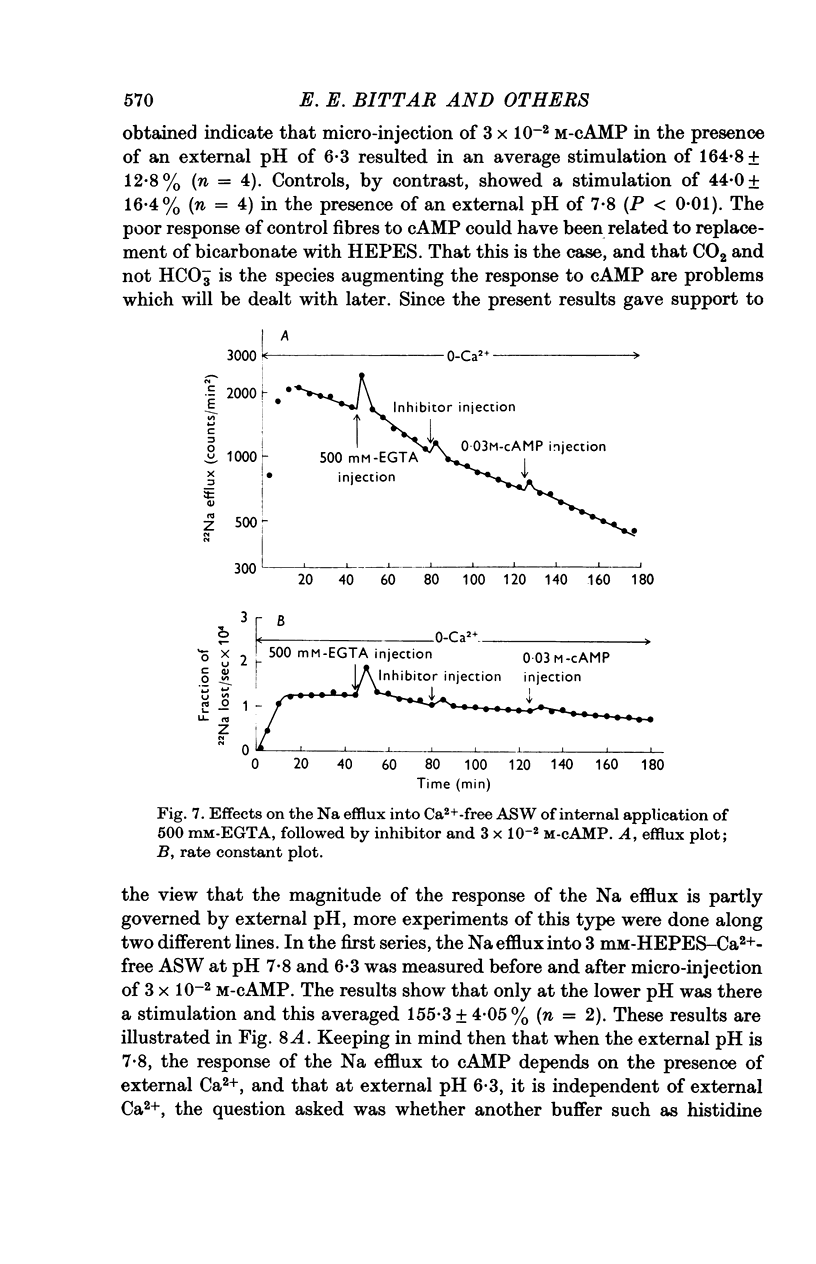
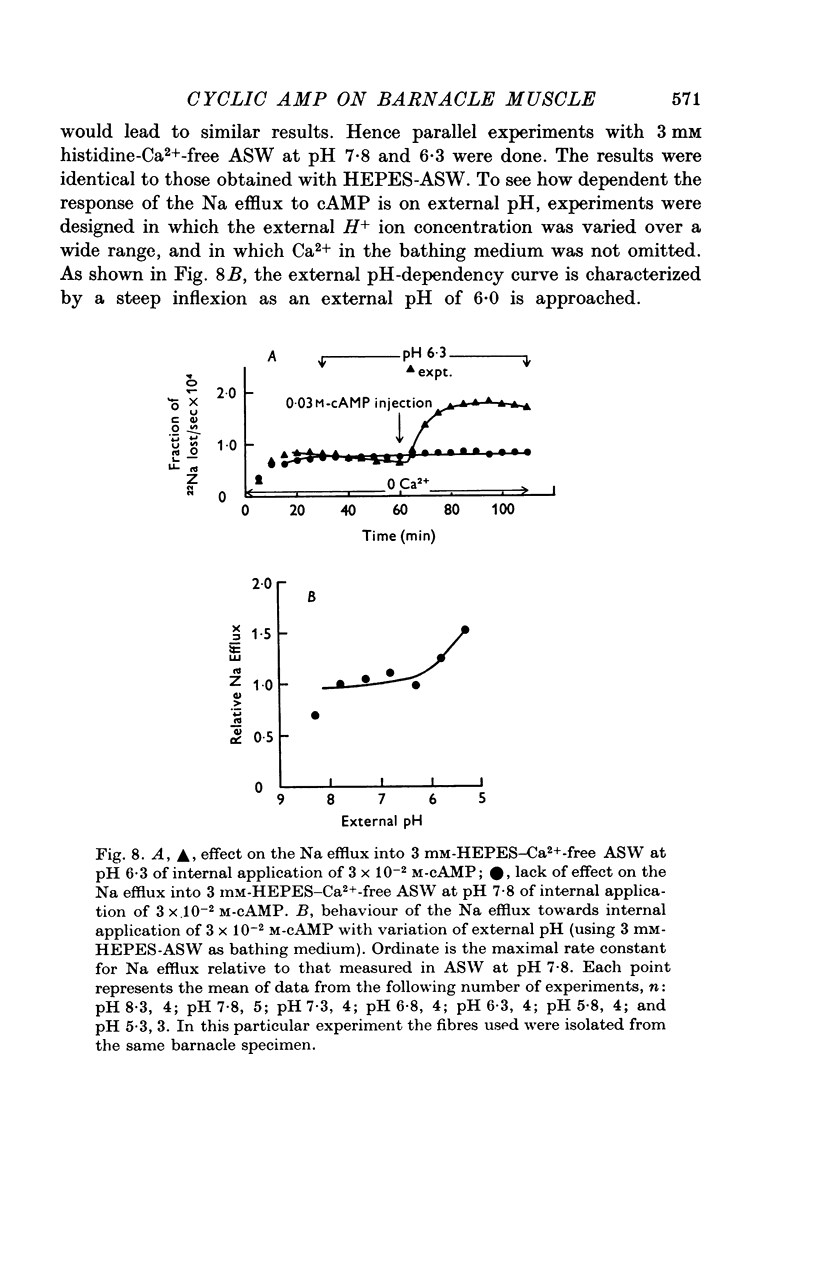
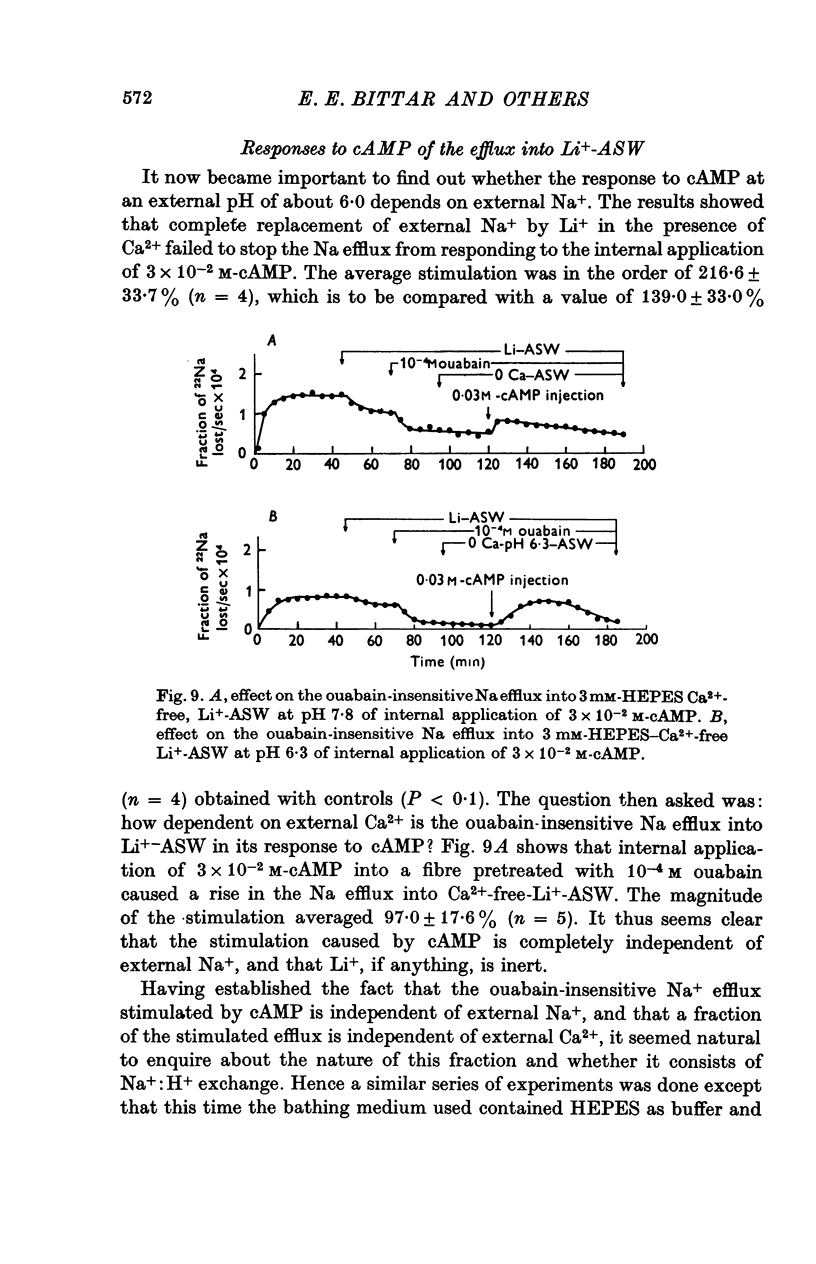
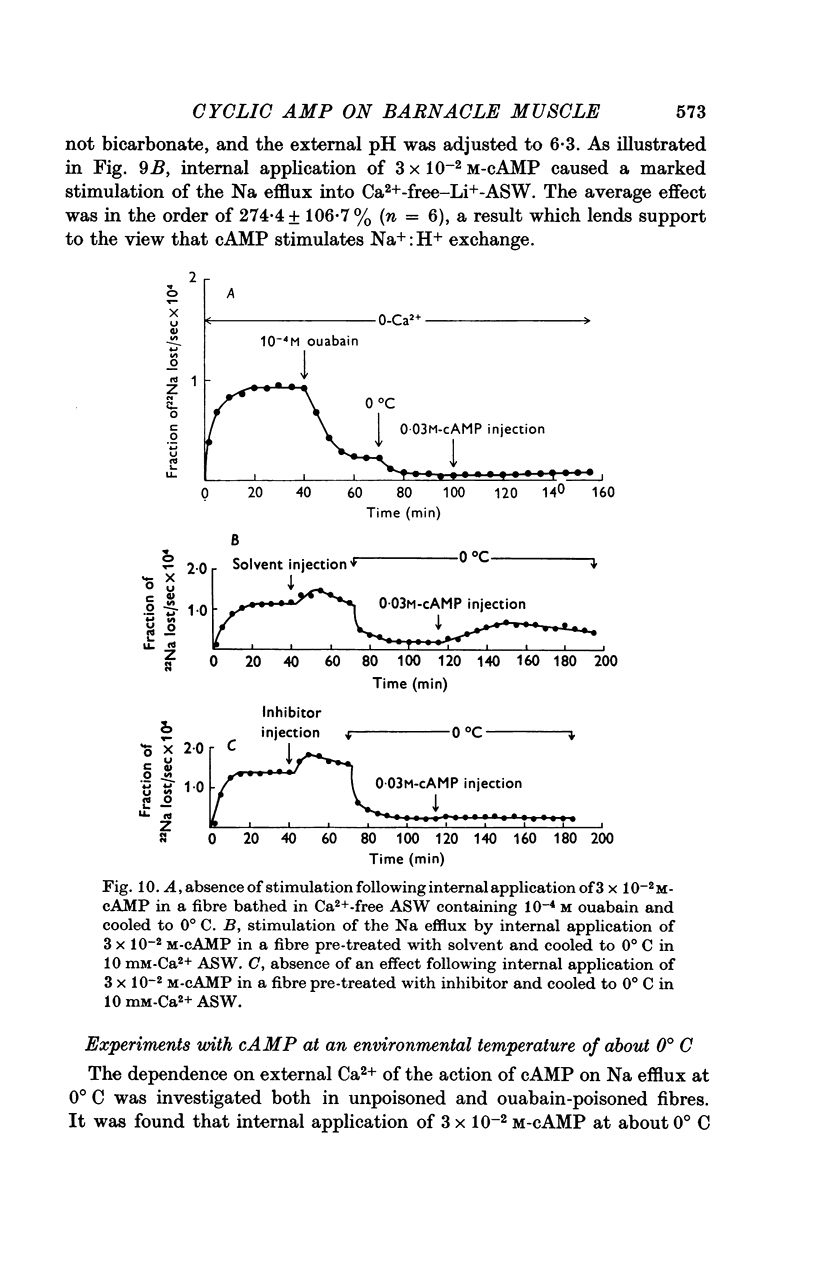
1. Giant fibres of the barnacle Balanus nubilus have been used as a preparation for studying the mode of action of cAMP on sodium transport. 2. It is shown that a concentration of cAMP as low as 10(-6)M, when micro-injected, causes a sharp rise in the radio-Na efflux. Ouabain fails to reverse the cAMP effect. 3. The magnitude of the response of the Na efflux to cAMP is markedly reduced by pre-injecting 100 or 500 mM-EGTA solutions or by omitting Ca2+ from the bathing medium. Both together fail to bring about a greater reduction in the response. 4. The response to cAMP is greatly reduced by pre-injecting the protein inhibitor of Walsh and practically abolished by pre-injecting 500 mM-EGTA and soaking in Ca-free artificial sea water, ASW. 5. The Ca2+-independent component of the Na efflux which is also stimulated by cAMP is shown to involve Na for H exchange. The magnitude of this exchange is governed by external pH. 6. The Na efflux into Ca2+-free, Li+-ASW is shown to be markedly stimulated by injecting cAMP, an effect which is enhanced by reducing external pH. 7. The Na efflux at 0 degrees C is stimulated by injecting cAMP. This is shown to be related to activation of the protein kinase by cAMP and to depend on the presence of external Ca2+. 8 (i) Ethacrynic acid when injected reduces the ouabain-insensitive Na efflux into HEPES-Ca2+-free ASW at pH 6-3. These same fibres show a marked response to cAMP. (II) The ouabain-insensitive Na efflux into HCO3-, Ca2+-free ASW from fibres pre-treated with ethacrynic acid fails to respond to external acidification. This is interpreted as indicating that ethacrynic acid inactivates the CO2-sensitive adenyl cyclase system. These same fibres when injected with cAMP show a marked response. (iii) Stimulation of the ouabain-insensitive Na efflux into HCO-3, Ca2+-free ASW by external acidification is reversed by injecting ethacrynic acid. These fibres when injected with cAMP show a reduced response. 9. It is concluded that: (i) stimulation of the Na efflux by injected cAMP is mainly due to activation of cAMP-dependent protein kinase; (ii) the underlying exchange mechanism consists of Na:Ca and Na:H exchange. Interaction of Ca2+ with a phosphorylated membrane, thereby modifying permeability remains as a real possibility; (iii) the site of action of CO2 and ethacrynic acid is the adenyl cyclase system. 10. The implications of activation of the adenyl cyclase system by CO2 and Na:H exchange are briefly touched upon.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby C. D., Walsh D. A. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. I. Interaction with the catalytic subunit of the protein kinase. J Biol Chem. 1972 Oct 25;247(20):6637–6642. [PubMed] [Google Scholar]
- Ashby C. D., Walsh D. A. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. II. Mechanism of action with the holoenzyme. J Biol Chem. 1973 Feb 25;248(4):1255–1261. [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Activation of protein kinase by physiological concentrations of cyclic AMP. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3580–3583. doi: 10.1073/pnas.71.9.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Mechanisms of control for cAMP-dependent protein kinase from skeletal muscle. Adv Cyclic Nucleotide Res. 1975;5:241–251. [PubMed] [Google Scholar]
- Bittar E. E., Chen S., Danielson B. G., Hartmann H. A., Tong E. Y. An investigation of sodium transport in barnacle muscle fibres by means of the microsyringe technique. J Physiol. 1972 Mar;221(2):389–414. doi: 10.1113/jphysiol.1972.sp009757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E. Effect of inhibitors and uncouplers on the Na pump of the Maia muscle fibre. J Physiol. 1966 Nov;187(1):81–103. doi: 10.1113/jphysiol.1966.sp008077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Hift H., Huddart H., Tong E. The effects of caffeine on sodium transport, membrane potential, mechanical tension and ultrastructure in barnacle muscle fibres. J Physiol. 1974 Oct;242(1):1–34. doi: 10.1113/jphysiol.1974.sp010691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Tallitsch R. B. Mode of stimulation by aldosterone of the sodium efflux in barnacle muscle fibres: effects of ouabain, ethacrynic acid, diphenylhydantoin, (ATPMg)(2-), adenine translocase inhibitors, pyruvate and oxythiamine. J Physiol. 1976 Feb;255(1):29–56. doi: 10.1113/jphysiol.1976.sp011268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Tong E. Sensitivity of the sodium efflux in barnacle muscle fibers to the microinjection of ATP. Life Sci. 1975 Jan 15;16(2):289–296. doi: 10.1016/0024-3205(75)90027-2. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Cyclic AMP stimulation of calcium efflux from kidney, liver and heart mitochondria. J Membr Biol. 1974;16(3):221–236. doi: 10.1007/BF01872416. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C., WALSTER G. STUDIES ON THE MICRO-INJECTION OF VARIOUS SUBSTANCES INTO CRAB MUSCLE FIBRES. J Physiol. 1963 Nov;169:353–372. doi: 10.1113/jphysiol.1963.sp007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson B. G., Bittar E. E., Chen S. S., Tong E. Y. Inhibition of the CO 2 -sensitive Na efflux in barnacle muscle fibers by micro-injection of ethacrynic acid. Experientia. 1972 Nov 15;28(11):1304–1304. doi: 10.1007/BF01965309. [DOI] [PubMed] [Google Scholar]
- Danielson B. G., Bittar E. E., Chen S. S., Tong E. Y. On the mode of action of ethacrynic acid, using the barnacle muscle fiber as a model. Life Sci I. 1972 Jan 1;11(1):13–21. doi: 10.1016/0024-3205(72)90237-8. [DOI] [PubMed] [Google Scholar]
- Danielson B. G., Bittar E. E., Chen S., Tong E. The influence of low pH, high K and microinjected CaCl 2 on the ouabain-insensitive component of sodium efflux in barnacle muscle fibers. Life Sci I. 1971 Jul 15;10(14):833–839. doi: 10.1016/0024-3205(71)90038-5. [DOI] [PubMed] [Google Scholar]
- Ebel H. Effect of diuretics on renal NaK-ATPase and adenyl cyclase. Naunyn Schmiedebergs Arch Pharmacol. 1974;281(3):301–314. doi: 10.1007/BF00500599. [DOI] [PubMed] [Google Scholar]
- Entman M. L. The role of cyclic AMP in the modulation of cardiac contractility. Adv Cyclic Nucleotide Res. 1974;4(0):163–193. [PubMed] [Google Scholar]
- Gordon E. E., de Hartog M. The relationship between cell membrane potassium ion transport and glycolysis. The effect of ethacrynic acid. J Gen Physiol. 1969 Nov;54(5):650–663. doi: 10.1085/jgp.54.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddox M. K., Newton N. E., Hartle D. K., Goldberg N. D. ATP(Mg 2+ ) induced inhibition of cyclic AMP reactivity with a skeletal muscle protein kinase. Biochem Biophys Res Commun. 1972 May 26;47(4):653–661. doi: 10.1016/0006-291x(72)90542-6. [DOI] [PubMed] [Google Scholar]
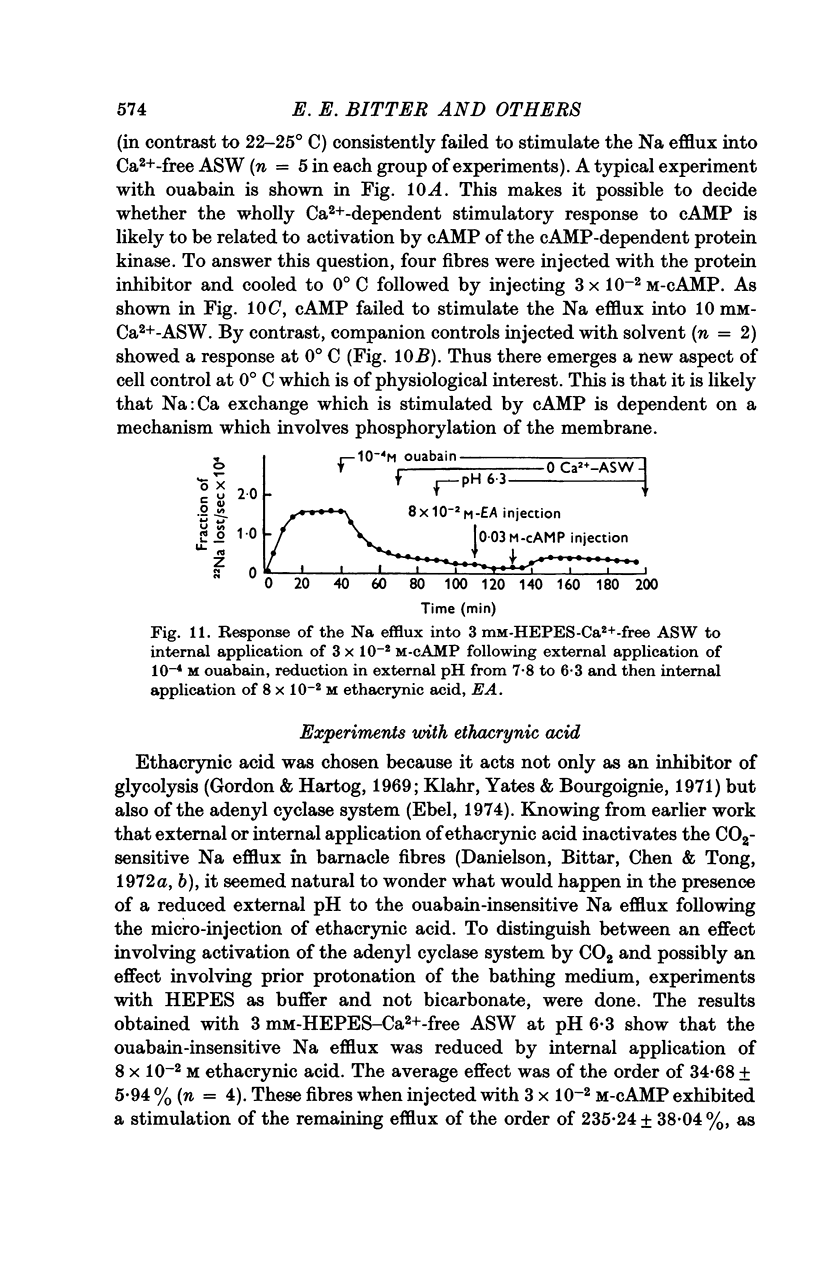
- Heilmeyer L. M., Jr, Meyer F., Haschke R. H., Fischer E. H. Control of phosphorylase activity in a muscle glycogen particle. II. Activation by calcium. J Biol Chem. 1970 Dec 25;245(24):6649–6656. [PubMed] [Google Scholar]
- Klahr S., Yates J., Bourgoignie J. Inhibition of glycolysis by ethacrynic acid and furosemide. Am J Physiol. 1971 Oct;221(4):1038–1043. doi: 10.1152/ajplegacy.1971.221.4.1038. [DOI] [PubMed] [Google Scholar]


