Abstract
A study set of 180 Mycobacterium tuberculosis and Mycobacterium bovis isolates having low copy numbers of IS6110 were genotyped using the recently introduced method based on the variable-number tandem repeats of mycobacterial interspersed repetitive units (MIRU-VNTR). The results were compared with results of the more commonly used methods, IS6110 restriction fragment length polymorphism (RFLP) and spoligotyping. The isolates were collected in Michigan from 1996 to 1999 as part of a project to genotype all isolates from new cases of tuberculosis in the state. Twelve MIRU loci were amplified, and the amplicons were analyzed by agarose gel electrophoresis to determine the copy number at each MIRU locus. MIRU-VNTR produced more distinct patterns (80 patterns) than did IS6110 RFLP (58 patterns), as would be expected in this study set. Spoligotyping identified 59 patterns. No single method defined all unique isolates, and the combination of all three typing methods generated 112 distinct patterns identifying 90 unique isolates and 90 isolates in 22 clusters. The results confirm the potential utility of MIRU-VNTR typing and show that typing with multiple methods is required to attain maximum specificity.
DNA fingerprinting of Mycobacterium tuberculosis isolates is useful for determining the extent of recent transmission in a community and the potential risk factors for recent transmission, for identifying previously unsuspected transmission, for monitoring the transmission of drug-resistant strains, and for confirming laboratory cross contamination (reviewed in reference 19). Although a large number of DNA-fingerprinting methods for typing M. tuberculosis isolates have been developed in recent years, all have significant drawbacks, and only a few have been adopted for widespread use. In the most widely used method, IS6110 restriction fragment length polymorphism (RFLP) (18), both the number of copies and the location of the IS6110 insertion element generate variation in the RFLP pattern. The molecular clock of IS6110 RFLP pattern variation is slow enough to be useful for outbreak investigations and yet fast enough for this to be the most discriminatory of the typing techniques (8). Unfortunately, it is technically demanding and requires culturing of the organism to obtain the necessary quantity of DNA. In contrast, spacer oligonucleotide typing (spoligotyping) (12), a PCR-based technique which detects the presence or absence of 43 spacers in the direct-repeat locus, is easy to perform and requires only a limited number of organisms. Spoligotyping, however, is less useful than IS6110 RFLP in discriminating between strains and provides more conserved genetic information, allowing the grouping of isolates into families, such as the Beijing and Haarlem genotype families (8). Spoligotyping has been useful both as a prescreening method to reduce the number of isolates to be typed by IS6110 RFLP (3) and as a secondary typing method for isolates with a low copy number for IS6110, whose RFLP patterns are often more stable than their spoligopatterns (1, 6, 15, 21).
A new PCR-based typing method, introduced recently by Mazars et al. (11), is based on the variable-number tandem repeats of mycobacterial interspersed repetitive units (MIRU-VNTR) (17). Each isolate is typed by the number of copies of repeated units at 12 independent loci scattered throughout the genome. The repeated units are 52 to 77 nucleotides in length, and the number of repeated units can be determined by the size of the fragment produced by amplification of the entire locus. In a study of 44 isolates from Paris with no known epidemiological links, it was reported that MIRU-VNTR provided a resolution comparable to that of IS6110 RFLP (11). The high resolution, the fast turnaround time, the ability to easily compare digital results between laboratories, and the possibility of high-throughput analysis make MIRU-VNTR an attractive method for fingerprinting large numbers of M. tuberculosis isolates.
The purpose of the present study was to carry out a direct comparison of MIRU-VNTR, IS6110 RFLP, and spoligotyping on a large set (n = 180) of M. tuberculosis IS6110 low-copy-number isolates (isolates which contain zero to six copies of IS6110) collected in Michigan from 1996 to 1999. These isolates had been typed previously by both IS6110 RFLP and spoligotyping, allowing us to compare rates of clustering using each of the three methods independently and in combination. Typing of low-copy-number isolates presents a significant challenge due to their high rate of clustering by IS6110 RFLP. Here, we demonstrate that MIRU-VNTR can be used to discriminate low-copy-number isolates of M. tuberculosis with a resolution surpassing both IS6110 RFLP and spoligotyping.
MATERIALS AND METHODS
Study sample.
One hundred eighty isolates of M. tuberculosis were included in this study. The isolates were from individual cases of tuberculosis diagnosed in the state of Michigan during the period 1996 to 1999 and were collected as part of the Centers for Disease Control and Prevention (CDC)-sponsored National Tuberculosis Genotyping and Surveillance Network (NTGSN) project.
IS6110 RFLP typing.
IS6110 fingerprinting was performed at the Michigan Department of Community Health following the international standard protocol (18), and images were analyzed using the BioImage Whole Band Analyzer software package version 4.3 (Genomic Solutions, Ann Arbor, Mich.). All distinct IS6110 RFLP patterns in the Michigan database were submitted to the CDC, where they were assigned an NTGSN pattern number.
Spoligotyping.
Spoligotyping was performed using a commercial kit (Isogen Bioscience BV, Maarssen, The Netherlands) following the method of Molhuizen et al. (12). The results were recorded as an octal code, as described previously (2). Briefly, the 43-digit binary result representing the 43 spacers (where 1 is positive and 0 is negative) was divided into 14 sets of three digits (spacers 1 to 42) plus one additional digit (spacer 43). Each three-digit set was converted to octal code (000 = 0, 001 = 1, 010 = 2, 011 = 3, 100 = 4, 101 = 5, 110 = 6, and 111 = 7), with the final digit remaining either 1 or 0. This yields a 15-digit octal designation.
MIRU-VNTR typing.
Each MIRU locus (17) was amplified individually with primers specific for sequences flanking the MIRU units (Table 1). The reaction mixture for all loci except MIRU 24 contained a 1-μl DNA sample, 1× Taq PCR buffer, 1 U of AmpliTaq DNA polymerase (Perkin-Elmer Applied Biosystems), deoxynucleoside triphosphates (0.2 mM each; Amersham Pharmacia Biotech, Piscataway, N.J.), and a 0.5 μM concentrarion of the primer pair in a final volume of 20 μl. The amplification profile consisted of 1 min at 94°C followed by 40 cycles of 30 s at 94°C, 30 s at 65°C, and 1 min at 72°C, using a GeneAmp 9700PCR system (Perkin-Elmer Applied Biosystems). For MIRU 24, the reaction mixture contained in addition 1× Q solution (Qiagen, Valencia, Calif.), and an annealing temperature of 55°C was used. The PCR products were analyzed on a 2.5% agarose (Gibco-BRL Products, Grand Island, N.Y.) gel in 1× Tris-borate-EDTA containing 1 μg of ethidium bromide/ml using the Sub-cell Model 192 apparatus (Bio-Rad, Hercules, Calif.), a 25- by 25-cm gel tray, and two rows of 51 wells (well width, 0.75 mm). The number of MIRU repeats at each locus was determined by the size of the amplicon, using the convention described in Table 1. Selected MIRU-VNTR amplicons were sequenced using the corresponding primer pair for forward and reverse sequencing primers and the Dye Terminator Cycle Sequencing kit and CEQ2000 capillary sequencer (Beckman Coulter, Fullerton, Calif.).
TABLE 1.
Sequences of oligonucleotides used in this study
| Oligonucleotide | Sequence | Positiona | Predicted size of amplicon containing 1 MIRU copy + size of additional copies (bp) |
|---|---|---|---|
| MIRU-VNTR | |||
| miru 2a | 5′CATCGAATTGGACTTGCAGCAAT | 153941 | 580 + 53 |
| miru 2b | 5′CGACGTCGTAGAGAGCATCGAAT | 154521 | |
| miru 4a | 5′GTCAAACAGGTCACAACGAGAGGAA | 580540 | 191 + 77 |
| miru 4b | 5′CCTCCACAATCAACACACTGGTCAT | 580831 | |
| miru 10a | 5′ACCGTCTTATCGGACTGCACTATCAA | 960130 | 273 + 53 |
| miru 10c | 5′CACCTTGGTGATCAGCTACCTCGAT | 960508 | |
| miru 16a | 5′CGGGTCCAGTCCAAGTACCTCAAT | 1644034 | 422 + 53 |
| miru 16b | 5′GATCCTCCTGATTGCCCTGACCTA | 1644508 | |
| miru 20a | 5′GCCCTTCGAGTTAGTATCGTCGGTT | 2059297 | 298 + 77 |
| miru 20b | 5′CAATCACCGTTACATCGACGTCATC | 2059671 | |
| miru 23a | 5′CGAATTCTTCGGTGGTCTCGAGT | 2531862 | 130 + 53 |
| miru 23b | 5′ACCGTCTGACTCATGGTGTCCAA | 2532256 | |
| miru 24a | 5′CGACCAAGATGTGCAGGAATACAT | 2686949 | 447 + 52 |
| miru 24b | 5′GGGCGAGTTGAGCTCACAGAA | 2687395 | |
| miru 26a | 5′GCGGATAGGTCTACCGTCGAAATC | 2995975 | 297 + 51 |
| miru 26b | 5′TCCGGGTCATACAGCATGATCA | 2996373 | |
| miru 27a | 5′TCTGCGTGCCAGTAAGAGCCA | 3006884 | 330 + 53 |
| miru 27b | 5′CTGATGGTGACTTCGGTGCCTT | 3007319 | |
| miru 31a | 5′CGTCGAAGAGAGCCTCATCAATCAT | 3192174 | 162 + 53 |
| miru 31b | 5′AACCTGCTGACCGATGGCAATATC | 3192441 | |
| miru 39a | 5′CGGTCAAGTTCAGCACCTTCTACATC | 4348555 | 712 + 53 |
| miru 39b | 5′CTCGGTGTTCCTTGAAGGTGGTTT | 4349319 | |
| miru 40a | 5′GATTCCAACAAGACGCAGATCAAGA | 802236 | 284 + 54 |
| miru 40b | 5′TCAGGTCTTTCTCTCACGCTCTCG | 802519 | |
| Insertion site | |||
| 1551-1 | 5′GCGCTCCTCGCGGATCACCTTGAAC | 483277b | |
| 1551-3 | 5′GCGCCAATGAAGCCAGCAACGCCGT | 3377699b | |
| 1551-4 | 5′GCGCGTGTCCCGATGTTGAGGTGGT | 1985453b | |
| IS6110-outward | 5′GCCGGTCGAACTCGAGGCTGCC | 1258c |
Insertion site detection.
Primers were designed to amplify the 3′ end of IS6110 and its flanking sequence for three of the IS6110 insertion sites in strains CDC1551, insertion site 1 (INS 1), INS 3, and INS 4 (13) (Table 1). INS 1 and INS 4 correspond to DK1 and DK3, respectively (4). The remaining insertion site in strain CDC1551, INS 2, is in the direct-repeat locus and is found in many strains in the M. tuberculosis complex. The reaction mixture contained 1× Taq PCR buffer, 1 U of AmpliTaq DNA polymerase, deoxynucleoside triphosphates (0.2 mM each), and 0.25 μM concentrations of the IS6110 outward primer and either primer 1551-1, 1551-3, or 1551-4 (Table 1). Primer 1551-1 was designed for amplification of the 3′ sequence flanking IS6110 at INS 1, primer 1551-3 was designed for that of INS 3, and primer 1551-4 was designed for that of INS 4 in the M. tuberculosis CDC1551 genome (4, 13; GenBank genome NC002755). The amplification profile consisted of 1 min at 94°C followed by 25 cycles of 30 s at 94°C, 30 s at 55°C, and 1 min at 72°C. The PCR products were analyzed on a 1% agarose gel in 1× Tris-borate-EDTA.
Genotyping analysis.
Isolates were determined to be either clustered or unique depending on the genotype generated by either a single fingerprinting method or a combination of methods. Isolates with the same genotype were identified as a cluster, while those with genotypes not matching those of any other isolates in this study set of 180 isolates were identified as unique.
All primers were synthesized by the Biotechnology Core Facility, CDC, Atlanta, Ga.
RESULTS
The set of isolates for the evaluation of MIRU typing originated from the NTGSN project. The IS6110 RFLP fingerprint was determined for one M. tuberculosis isolate per culture-positive case collected in Michigan from 1996 to 1999. Secondary typing methods for isolates with less than seven copies of IS6110 (low-copy-number isolates) were also employed. All low-copy-number isolates (n = 330) were typed by spoligotyping, and 180 of these 330 isolates were chosen at random for MIRU-VNTR typing.
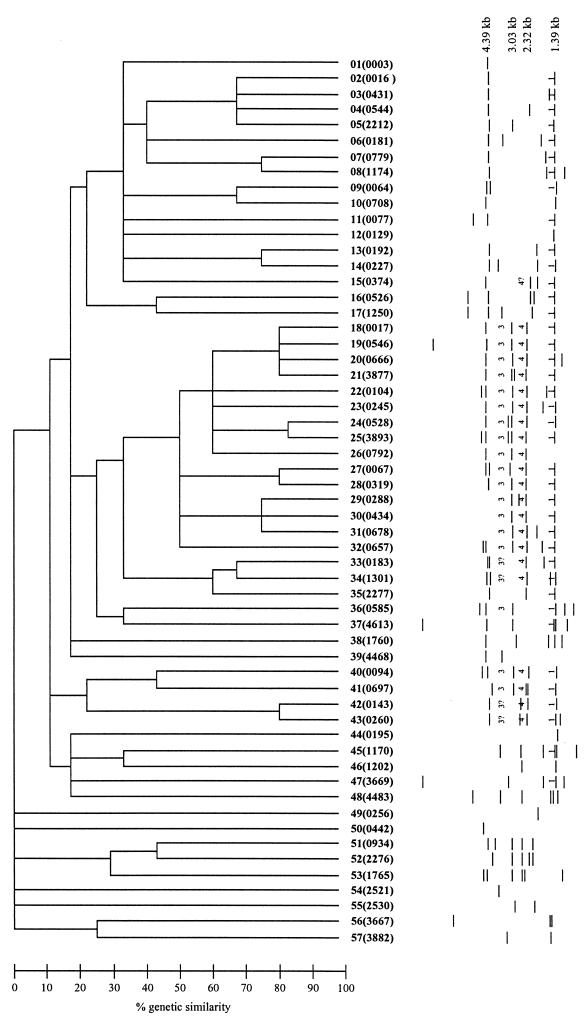
Table 2 summarizes the isolates, sorted by IS6110 RFLP pattern. Thirty-eight of the isolates had unique IS6110 RFLP patterns, and 142 isolates were grouped into 20 clusters (n = 2 to 64 isolates). Fifty-seven of the 58 IS6110 RFLP patterns are shown in Fig. 1; one isolate lacked a copy of IS6110. Because many of the fingerprint patterns resembled that of M. tuberculosis strain CDC1551, we tested one isolate with each fingerprint pattern for three of the four IS6110 insertion sites in strain CDC1551 (INS 1 [DK1], INS 3, and INS 4 [DK3]) by PCR amplification (4, 13). As indicated in Fig. 1, 20 of the 57 RFLP patterns tested contained copies of IS6110 at all three insertion sites (50 isolates). An additional 19 of the 57 RFLP patterns tested were found to contain a copy of IS6110 at INS 1 (95 isolates). We did not test for the copy of IS6110 in the direct-repeat locus (INS 2 [DK2]).
TABLE 2.
Fingerprinting results of all typing methods used
| RFLP no.copy no. | No. of isolates | Spoligotypeb | No. of isolates | Groupc | MIRU-VNTRd | No. of Isolates |
|---|---|---|---|---|---|---|
| 00.0 | 1 | 777777000000011 | 1 | 364225223433 (71) | 1 | |
| 01.1 | 4 | 777777777760731 | 1 | 242325142323 (64) | 1 | |
| 777777777760331 | 1 | 225126113322 (3) | 1 | |||
| 777777757760731 | 1 | 225125113322 (2) | 1 | |||
| 777777777760331 | 1 | 225125113322 (2) | 1 | |||
| 02.2 | 64 | 777777770000000 | 1 | 225325153323 (11) | 1 | |
| 777776777760771 (a) | 4 | 125325143225 (66) | 2 | |||
| 225325053223 (4) | 1 | |||||
| 225325153223 (5) | 1 | |||||
| 777776777760731 | 1 | 225325153214 (33) | 1 | |||
| 777776777760601 (b) | 37 | 224225153323 (47) | 7 | |||
| 224315153323 (24) | 1 | |||||
| 224322153323 (14) | 1 | |||||
| 22432514-323 (45) | 1 | |||||
| 224325143323 (46) | 3 | |||||
| 224325153322 (56) | 2 | |||||
| 224325153323 (10) | 18 | |||||
| 224325153324 (51) | 1 | |||||
| 224326153323 (15) | 1 | |||||
| 244325153322 (59) | 1 | |||||
| 323325151326 (65) | 1 | |||||
| 777776756360711 | 1 | 224325153312 (58) | 1 | |||
| 777776377760601 | 1 | 224325153213 (31) | 1 | |||
| 776000017760471 | 3 | 225325153223 (5) | 3 | |||
| 037776777760601 | 15 | 224325153323 (10) | 4 | |||
| 224325153423 (27) | 11 | |||||
| 001776777760601 | 1 | 224325153321 (50) | 1 | |||
| 03.2 | 1 | 767776777760771 | 1 | 225325162323 (16) | 1 | |
| 04.3 | 1 | 777736777760601 | 1 | 224325153323 (10) | 1 | |
| 05.3 | 1 | 777776777760601 (b) | 1 | 224325143323 (46) | 1 | |
| 06.4 | 1 | 777746777760601 | 1 | 224325153323 (10) | 1 | |
| 07.3 | 1 | 777776777760601 (b) | 1 | 224325143325 (43) | 1 | |
| 08.4 | 2 | 777776777760601 (b) | 2 | 222325143325 (42) | 1 | |
| 224325143324 (38) | 1 | |||||
| 09.3 | 1 | 777776777760771 (a) | 1 | 223325153323 (9) | 1 | |
| 10.2 | 1 | 777776777760771 (a) | 1 | 125325143225 (66) | 1 | |
| 11.3 | 9 | 777776777760601 (b) | 1 | 224325153423 (27) | 1 | |
| 700036777760731 | 7 | 222325143223 (7) | 7 | |||
| 700036777560471 | 1 | 222325153323 (8) | 1 | |||
| 12.1 | 6 | 777777777413731 (c) | 1 | 1 | 254323324512 (79) | 1 |
| 777777777413700 | 1 | 1 | 256326224513 (78) | 1 | ||
| 777777777413071 | 1 | 1 | 264225223522 (69) | 1 | ||
| 765777777413771 | 1 | 1 | 252226223532 (75) | 1 | ||
| 717477777413731 | 1 | 1 | 244326223512 (77) | 1 | ||
| 477777777413171 | 1 | 1 | 254326123334 (80) | 1 | ||
| 13.3 | 2 | 777776777760601 (b) | 2 | 224325163322 (57) | 1 | |
| 2∗3325153323 (13) | 1 | |||||
| 14.4 | 2 | 777776777760601 (b) | 2 | 224325153322 (56) | 2 | |
| 15.4 | 1 | 777776777760771 (a) | 1 | 224225153324 (48) | 1 | |
| 16.5 | 1 | 777776777760601 (b) | 1 | 224325153323 (10) | 1 | |
| 17.5 | 2 | 777776777760601 (b) | 2 | 224325153323 (10) | 2 | |
| 18.4a | 17 | 777776777760771 (a) | 8 | 224225153324 (48) | 4 | |
| 224225163324 (54) | 1 | |||||
| 224325143314 (37) | 2 | |||||
| 224325143322 (55) | 1 | |||||
| 777776777760701 | 1 | 224325143323 (46) | 1 | |||
| 777774077760771 | 1 | 224325172326 (35) | 1 | |||
| 777740777760771 | 5 | 224115153324 (22) | 2 | |||
| 224315153224 (29) | 1 | |||||
| 234315153322 (60) | 1 | |||||
| 224315153324 (23) | 1 | |||||
| 777740617760771 | 1 | 225315153324 (19) | 1 | |||
| 500076777760771 | 1 | 224325133325 (44) | 1 | |||
| 19.5a | 1 | 777776775760771 | 1 | 224326143324 (39) | 1 | |
| 20.5a | 1 | 777776777760771 (a) | 1 | 224225153324 (48) | 1 | |
| 21.5a | 1 | 760000007760731 | 1 | 224325153214 (32) | 1 | |
| 22.6a | 1 | 777776777760771 (a) | 1 | 224325124324 (41) | 1 | |
| 23.5a | 1 | 777776777760771 (a) | 1 | 224225143324 (52) | 1 | |
| 24.5a | 2 | 777776777760771 (a) | 1 | 224225153324 (48) | 1 | |
| 777736777760771 | 1 | 224225153324 (48) | 1 | |||
| 25.6a | 1 | 700076777760771 (d) | 1 | 215325153325 (18) | 1 | |
| 26.3 | 1 | 777776777760771 (a) | 1 | 224225153324 (48) | 1 | |
| 27.5a | 3 | 777776777760771 (a) | 3 | 224225143325 (53) | 2 | |
| 224325123324 (40) | 1 | |||||
| 28.4a | 1 | 700076777760700 (e) | 1 | 224425153324 (49) | 1 | |
| 29.4a | 1 | 700076777760671 (f) | 1 | 224325153324 (51) | 1 | |
| 30.3a | 6 | 700076770000071 | 1 | 224325153324 (51) | 1 | |
| 700076760000011 (g) | 1 | 224325153324 (51) | 1 | |||
| 700076700000071 | 3 | 224325153224 (30) | 3 | |||
| 000076700000031 | 1 | 224225173224 (62) | 1 | |||
| 31.4a | 1 | 700076760000011 (g) | 1 | 224325153322 (56) | 1 | |
| 32.6a | 2 | 700076777760771 (d) | 2 | 225325153324 (20) | 2 | |
| 33.5a | 1 | 777776777720731 | 1 | 224325163326 (34) | 1 | |
| 34.5a | 1 | 777776777760771 (a) | 1 | 224325154327 (36) | 1 | |
| 35.3 | 2 | 777776777760601 (b) | 2 | 224325153323 (10) | 1 | |
| 224325153324 (51) | 1 | |||||
| 36.6 | 1 | 700076777760771 (d) | 1 | 222235153224 (61) | 1 | |
| 37.6 | 1 | 777777707760771 | 1 | 228325163323 (17) | 1 | |
| 38.5 | 1 | 777776777760771 (a) | 1 | 227315153324 (21) | 1 | |
| 39.2 | 1 | 777777777760771 (h) | 1 | 225125113322 (2) | 1 | |
| 40.5a | 1 | 700076777760771 (d) | 1 | 224325153324 (51) | 1 | |
| 41.5a | 1 | 700076777760700 (e) | 1 | 224325153324 (51) | 1 | |
| 42.4a | 2 | 700076777760671 (f) | 2 | 224325153324 (51) | 2 | |
| 43.5a | 6 | 700076777760671 (f) | 6 | 224225153323 (47) | 6 | |
| 44.1 | 5 | 777777774413771 | 2 | 1 | 263225223533 (73) | 2 |
| 777776404160601 | 1 | 224325153424 (28) | 1 | |||
| 000000000003771 | 1 | Beijing | 322325173523 (67) | 1 | ||
| 774377776413771 | 1 | 1 | 364225223533 (72) | 1 | ||
| 45.6 | 1 | 777777760020611 | 1 | 225225141323 (63) | 1 | |
| 46.2 | 2 | 617776777760401 | 2 | 224325163326 (34) | 2 | |
| 47.5 | 1 | 777737007760771 | 1 | 226325153223 (6) | 1 | |
| 48.6 | 1 | 777777777413731 (c) | 1 | 1 | 154326223513 (76) | 1 |
| 49.1 | 1 | 656573777777600 | 1 | M. bovis | 232224263322 (68) | 1 |
| 50.1 | 2 | 777777777410331 | 1 | 1 | 264225223533 (74) | 1 |
| 777777777000771 | 1 | 1 | 284225223531 (70) | 1 | ||
| 51.5 | 1 | 777777557760771 | 1 | 223326153324 (26) | 1 | |
| 52.5 | 2 | 777777770060771 | 2 | 223325153314 (25) | 2 | |
| 53.6 | 1 | 770000757760771 | 1 | 223125153323 (12) | 1 | |
| 54.1 | 1 | 777777777410000 | 1 | 1 | 264225223533 (74) | 1 |
| 55.2 | 1 | 777777404760771 | 1 | 215125113322 (1) | 1 | |
| 56.3 | 1 | 777777777760771 (h) | 1 | 225125113322 (2) | 1 | |
| 57.2 | 1 | 777777777413731 (c) | 1 | 1 | 264225223533 (74) | 1 |
These isolates have been described as having INS1, INS3 and INS4.\
Lowercase letter in parentheses corresponds to the eight spoligotype clusters which contain isolates with different IS6110 RFLP patterns.\
Spoligotype indicates that the isolates are M. bovis, part of the Beijing family, or a member of genotypic group 1.\
Number in parentheses correlates to the position of the MIRU-VNTR code in Fig. 2.
FIG. 1.
IS6110 RFLP patterns of isolates in this study. The genetic similarity of the isolates depicted in the dendrogram is based on IS6110 RFLP analysis as determined by the BioImage Whole Band Analyzer. RFLP patterns were compared using the Jaccard matching method with an allowed band size deviation of 1%, and the dendrogram showing the relationships between patterns was generated by the unweighted pair group method using arithmetic averages. The IS6110 RFLP patterns were numbered in the order they appear in the dendrogram, with the NTGSN pattern number in parentheses. The numbers 1, 2, and 3 indicate that an isolate with this IS6110 RFLP pattern was PCR positive for the corresponding IS6110 insertion site in strain CDC1551 (INS 1, INS 3, or INS 4); 3? and 4? indicate that the isolate was PCR positive but the predicted RFLP fragment was not apparent in the pattern.
The spoligotype is listed for each isolate in Table 2. Fifty-nine distinct spoligotype patterns were identified. Forty-three spoligotypes represented single isolates; the other 137 isolates were grouped into 16 clusters containing from 2 to 51 isolates having the same spoligotype. Of the 16 spoligotype clusters, 8 contained isolates with multiple IS6110 RFLP patterns (a to h [Table 2]). Based on the observation by Soini et al. (14) that only genotypic group 1 strains contain spacers 33 to 36, 16 of the isolates are group 1. Of these, one isolate had the Beijing spoligotype pattern (20) and one isolate had the Mycobacterium bovis spoligotype (12). None of the 145 isolates with IS6110 at INS 1 (DK1) was in genotypic group 1.
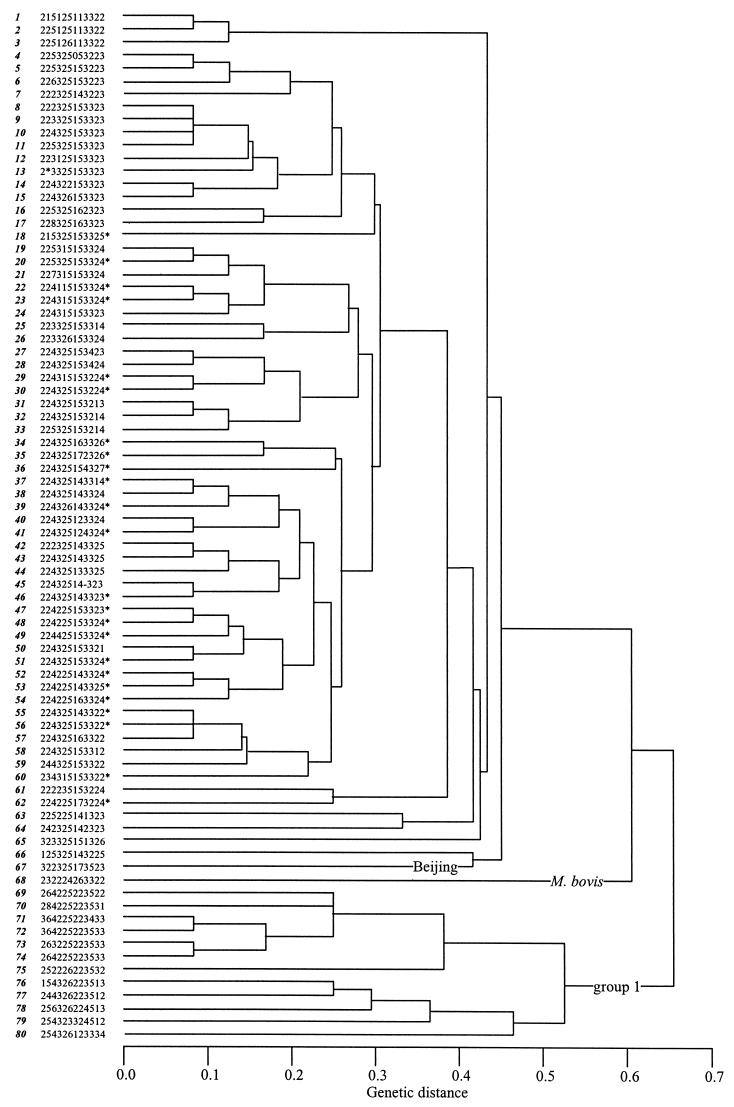
The 12 published MIRU-VNTR loci were used in this study (17). All 12 loci were amplified from the 180 isolates, with the exception of MIRU locus 27 in one isolate (Fig. 2, pattern 45). MIRU locus 4 contains a variable number of 77-bp repeated units (type 1) followed by an invariable 53-bp unit (type 2). This 53-bp unit has been found in all recent clinical isolates of M. tuberculosis and M. bovis previously tested but is lacking in M. tuberculosis strains H37Rv and H37Ra and M. bovis BCG (9, 10, 17). Sequencing of MIRU locus 4 in one of our isolates revealed that it was missing the 3′ 53-bp unit, resulting in an amplicon whose size does not fit the convention described in Table 1 (Fig. 2, pattern 13). MIRU locus 24 contains a variable number of 53-bp repeated units (type 2) followed by an invariable 77-bp unit (type 1). The sequence of MIRU locus 24 in one of our isolates revealed that the 5′ 53-bp unit had recombined with the 3′ 77-bp unit, resulting in a single 77-bp unit. Since only the variable 53-bp MIRU units are counted, this isolate was characterized as having 0 units at MIRU locus 24 (Fig. 2, pattern 4). The diversity of each individual locus is listed in Table 3.
FIG.2.
MIRU-VNTR patterns of isolates in this study. The genetic distance of the isolates depicted in the dendrogram is based on MIRU-VNTR analysis as determined by the SAS/GRAPH module (ET100.exe and cluster.SAS) by the unweighted pair group method using arithmetic averages (7). The patterns were compared by using unweighted loci and treating the allele numbers as nominal values. The MIRU-VNTR patterns were numbered in the order they appear in the dendrogram for cross-reference with Table 2. The MIRU-VNTR patterns of the group 1, Beijing, and M. bovis isolates are labeled, and the patterns of all isolates with a copy of IS6110 at INS 1, INS 3, and INS 4 are indicated with asterisks. The dash in pattern 45 represents the missing MIRU locus 27, which was not amplified from one isolate; the asterisk in pattern 13 indicates the missing 3′ 53-bp unit in MIRU locus 4 in one of our isolates; and the zero in pattern 4 indicates that the isolate is characterized as having zero units at MIRU locus 24 (see the text).
TABLE 3.
Allelic diversity of each MIRU-VNTR locus
| No. of copies | Locus no. at MIRU
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4a | 10 | 16 | 20 | 23 | 24 | 26 | 27b | 31 | 39 | 40 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 1 | 4 | 2 | 0 | 9 | 8 | 0 | 164 | 6 | 2 | 0 | 12 | 2 |
| 2 | 172 | 158 | 14 | 40 | 171 | 1 | 14 | 17 | 3 | 25 | 158 | 21 |
| 3 | 4 | 2 | 9 | 130 | 1 | 1 | 1 | 1 | 170 | 127 | 10 | 95 |
| 4 | 0 | 3 | 131 | 1 | 0 | 1 | 0 | 28 | 4 | 14 | 0 | 47 |
| 5 | 0 | 5 | 22 | 0 | 0 | 168 | 0 | 117 | 0 | 14 | 0 | 9 |
| 6 | 0 | 8 | 2 | 0 | 0 | 9 | 0 | 8 | 0 | 0 | 0 | 5 |
| 7 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 |
| 8 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Allelic diversityc | 0.08 | 0.22 | 0.44 | 0.42 | 0.09 | 0.12 | 0.16 | 0.54 | 0.09 | 0.47 | 0.22 | 0.63 |
The sequence of MIRU 4 in one sample showed that it contained three 77-bp type 1 units.\
Locus MIRU 27 did not amplify in one sample\
Allelic diversity (h) at a locus was calculated as follows: h = 1 − Σ xi2[n/(n − 1)], where xi is the frequency of the ith allele at the locus and n is the number of isolates (11).
Since none of the isolates in this study contained a MIRU locus with more than nine MIRU units, we could represent the MIRU-VNTR pattern as a 12-digit number with the digits corresponding to the copy numbers of the MIRU loci in numerical order (MIRU 2-4-10-16-20-23-24-26-27-31-39-40). Eighty distinct MIRU patterns were observed in this set of isolates. Sixty isolates were unique, and 120 isolates were grouped into 20 clusters (n = 2 to 28 isolates). Analysis of the number of MIRU-VNTR patterns and clusters generated using any combination of 11 of the 12 MIRU loci showed that even in this restricted set of isolates the use of all 12 loci was required for maximum specificity (Table 4). The omission of MIRU 2, 24, 27, or 39 resulted in the identification of at least 77 of the 80 distinct MIRU patterns (95% of the unique isolates). The results obtained following the omission of combinations of these four loci are also shown in Table 4.
TABLE 4.
Specificities of combinations of MIRU loci
| Locus omitted | No. of distinct patterns obtained | No. (%) of unique isolates obtained |
|---|---|---|
| None | 80 | 60 (100) |
| MIRU 2 | 79 | 59 (98.3) |
| MIRU 24 | 79 | 59 (98.3) |
| MIRU 2 and MIRU 24 | 78 | 58 (96.7) |
| MIRU 27 | 78 | 57 (95.0) |
| MIRU 39 | 77 | 57 (95.0) |
| MIRU 24 and MIRU 39 | 77 | 57 (95.0) |
| MIRU 4 | 77 | 56 (93.3) |
| MIRU 2 and MIRU 27 | 77 | 56 (93.3) |
| MIRU 24 and MIRU 27 | 77 | 56 (93.3) |
| MIRU 20 | 76 | 56 (93.3) |
| MIRU 2 and MIRU 39 | 76 | 56 (93.3) |
| MIRU 23 | 76 | 55 (91.7) |
| MIRU 2, MIRU 24, and MIRU 27 | 76 | 55 (91.7) |
| MIRU 2, MIRU 24, and MIRU 39 | 75 | 55 (91.7) |
| MIRU 27 and MIRU 39 | 75 | 54 (90.0) |
| MIRU 31 | 74 | 54 (90.0) |
| MIRU 16 | 73 | 53 (88.3) |
| MIRU 2, MIRU 24, MIRU 27, and MIRU 39 | 73 | 52 (86.7) |
| MIRU 26 | 72 | 52 (86.7) |
| MIRU 10 | 70 | 49 (81.7) |
| MIRU 40 | 67 | 48 (80.0) |
The genetic distances among the isolates based on their MIRU-VNTR patterns can be seen in Fig. 2. The genotypic group 1 isolates were clustered together and were clearly separated from the remaining isolates. The zero-copy isolate (Fig. 2, no. 71) was included in this arm of the dendrogram. The spoligotype for this isolate indicates a large deletion spanning the region of INS 2 and spacers 33 to 36, suggesting that it could actually be a group 1 strain. The 50 isolates with copies of IS6110 at INS 1, INS 3, and INS 4 were genetically closely related by MIRU-VNTR but did not form a separate branch in the dendrogram.
The 20 MIRU clusters and the spoligotype and IS6110 RFLP patterns of isolates in each cluster are listed in Table 5. Clusters A to H contained isolates with identical spoligotypes and IS6110 RFLP patterns. The remaining 12 MIRU clusters could be subdivided by spoligotyping and/or IS6110 RFLP. Cluster I contained isolates with the same spoligotype pattern and similar two-band IS6110 RFLP patterns, and cluster J contained isolates with the same IS6110 RFLP pattern and spoligotype patterns that differed by two deletions (spacers 9 to 24 and spacers 38 and 39). While the isolates in cluster K had multiple IS6110 RFLP patterns, almost all of the isolates had a copy of IS6110 at INS 1, INS 3, and INS 4; the two spoligotypes differed by the deletion of a single spacer. Similarly, the isolates in both clusters L and M contained multiple IS6110 RFLP and spoligotype patterns, but all isolates had a copy of IS6110 at INS 1, and the spoligotypes differed by one deletion in the direct-repeat locus. The isolates in cluster N were divided into two subclusters with identical IS6110 RFLP and spoligotype patterns. The isolates in one of these subclusters had IS6110 insertions at INS 1, INS 3, and INS 4, and the isolates in the other subcluster had the IS6110 insertion at INS 1. The spoligotypes of the two subclusters contain distinct deletions (spacers 4 to 12 or spacers 39 to 42), suggesting they could have been derived from a common progenitor having all sets of these spacers. Clusters O to Q could also be divided by IS6110 RFLP and spoligotyping into subclusters characterized by different insertion sites and divergent spoligotypes, as described for cluster N. Cluster R contains three genotypic group 1 isolates with three different IS6110 RFLP patterns and three different spoligotypes. The relationships among the isolates in clusters S and T are unclear. For cluster S, the spoligotypes can be explained by nondivergent deletions, but this is not the case for cluster T.
TABLE 5.
Isolates clustered by MIRU-VNTR typing
| Cluster | MIRU-VNTR | No. of isolates | Spoligotype | No. of isolates | IS6110 RFLP no. | No. of isolates |
|---|---|---|---|---|---|---|
| A | 222325143223 | 7 | 700036777760731 | 7 | 11 | 7 |
| B | 224325153224 | 3 | 700076700000071 | 3 | 30 | 3 |
| C | 225325153324 | 2 | 700076777760771 | 2 | 32 | 2 |
| D | 224115153324 | 2 | 777740777760771 | 2 | 18 | 2 |
| E | 223325153314 | 2 | 777777770060771 | 2 | 52 | 2 |
| F | 224325143314 | 2 | 777776777760771 | 2 | 18 | 2 |
| G | 224225143325 | 2 | 777776777760771 | 2 | 27 | 2 |
| H | 263225223533 | 2 | 777777774413771 | 2 | 44 | 2 |
| I | 125325143225 | 3 | 777776777760771 | 3 | 02 | 2 |
| 10 | 1 | |||||
| J | 225325153223 | 4 | 777776777760771 | 1 | 02 | 1 |
| 776000017760471 | 3 | 02 | 3 | |||
| K | 224225153324 | 9 | 777736777760771 | 1 | 24 | 1 |
| 777776777760771 | 8 | 18 | 4 | |||
| 15 | 1 | |||||
| 24 | 1 | |||||
| 20 | 1 | |||||
| 26 | 1 | |||||
| L | 224325153323 | 28 | 777746777760601 | 1 | 06 | 1 |
| 777736777760601 | 1 | 04 | 1 | |||
| 037776777760601 | 4 | 02 | 4 | |||
| 777776777760601 | 22 | 02 | 18 | |||
| 16 | 1 | |||||
| 17 | 2 | |||||
| 35 | 1 | |||||
| M | 224325153423 | 12 | 777776777760601 | 1 | 11 | 1 |
| 037776777760601 | 11 | 02 | 11 | |||
| N | 224225153323 | 13 | 777776777760601 | 7 | 02 | 7 |
| 700076777760671 | 6 | 43 | 6 | |||
| O | 224325153324 | 9 | 700076777760771 | 1 | 40 | 1 |
| 700076777760700 | 1 | 41 | 1 | |||
| 700076770000071 | 1 | 30 | 1 | |||
| 700076760000011 | 1 | 30 | 1 | |||
| 777776777760601 | 2 | 02 | 1 | |||
| 35 | 1 | |||||
| 700076777760671 | 3 | 42 | 2 | |||
| 29 | 1 | |||||
| P | 224325153322 | 5 | 700076760000011 | 1 | 31 | 1 |
| 777776777760601 | 4 | 02 | 2 | |||
| 14 | 2 | |||||
| Q | 224325143323 | 5 | 777776777760701 | 1 | 18 | 1 |
| 777776777760601 | 4 | 02 | 3 | |||
| 05 | 1 | |||||
| R | 264225223533 | 3 | 777777777413731 | 1 | 57 | 1 |
| 777777777410331 | 1 | 50 | 1 | |||
| 777777777410000 | 1 | 54 | 1 | |||
| S | 225125113322 | 4 | 777777757760731 | 1 | 01 | 1 |
| 777737777760771 | 1 | 01 | 1 | |||
| 777777777760771 | 2 | 56 | 1 | |||
| 39 | 1 | |||||
| T | 224325163326 | 3 | 777776777720731 | 1 | 33 | 1 |
| 617776777760401 | 2 | 46 | 2 |
The MIRU-VNTR patterns of isolates clustered by IS6110 RFLP and spoligotyping were compared. The 20 clusters can be found in Table 2 by locating the spoligotypes for which there were multiple isolates within each IS6110 RFLP cluster (i.e., the number of isolates in column 4 is >1). Eleven clusters (33 isolates) were not subdivided by MIRU-VNTR typing. The remaining nine clusters (78 isolates) were subdivided by MIRU-VNTR into 21 unique isolates and 11 subclusters (57 isolates). Within each IS6110 RFLP-spoligotype cluster, the MIRU-VNTR patterns varied by as little as an additional MIRU copy at a single locus to the addition of multiple copies at several loci.
The degree of discrimination obtained with each typing method individually and combined is listed in Table 6. A total of 112 distinct types were obtained when the results of all three methods were combined. Ninety isolates were unique, and 90 isolates were grouped into 22 clusters. The combination of IS6110 RFLP and spoligotyping clustered 110 isolates (61%), which is lower than that seen for all low-copy-number isolates in the NTGSN project database (71% clustered isolates). The combination of the two PCR-based methods, spoligotyping and MIRU-VNTR, clustered 103 isolates (57%), whereas the combination of IS6110 RFLP and MIRU-VNTR clustered only slightly fewer isolates (97 isolates; 53.9%).
TABLE 6.
Specificities of genotyping methods
| No. of distinct patterns | No. of unique isolates | No. of clustered isolates | No. of clusters | |
|---|---|---|---|---|
| IS6110 RFLP | 58 | 38 | 142 | 20 |
| Spoligotyping | 59 | 43 | 137 | 16 |
| MIRU-VNTR | 80 | 60 | 120 | 20 |
| IS6110 RFLP and spoligotyping | 90 | 69 | 111 | 20 |
| Spoligotyping and MIRU-VNTR | 99 | 77 | 103 | 22 |
| IS6110 RFLP and MIRU-VNTR | 107 | 83 | 97 | 24 |
| All three methods | 112 | 90 | 90 | 22 |
DISCUSSION
MIRU-VNTR is a PCR-based method for typing M. tuberculosis isolates that requires amplification followed by size analysis of 12 independent loci. The procedure has all of the advantages of PCR-based typing methods—rapid turnaround, no requirement for large quantities of DNA, and the ability to use nonviable samples—and in addition provides a simple digital result. In this study, the amplicons were analyzed on traditional agarose gels, but they can be labeled with fluorescent dyes for high-throughput automated analysis on a DNA analyzer (16). Perfect reproducibility was achieved when 28 samples were analyzed by our laboratory in a blinded fashion and compared with the results obtained by Supply and coworkers for the identical samples (data not shown). Generally, the analysis is straightforward, but some problems remain. Amplification of the repeated units can be difficult, and stutter peaks created by strand slippage during PCR can complicate interpretation of the results. There are also occasional instances when the size convention used to determine the number of MIRU copies does not apply; examples of this were described in Results. The creation of a set of markers for each possible allele would simplify analysis on agarose gels. Overall, we found MIRU-VNTR typing to be much faster than IS6110 RFLP but not as simple as spoligotyping.
VNTR typing of M. tuberculosis complex isolates in most previous studies utilized the five exact tandem repeat (ETR) loci described by Frothingham and Meeker-O'Connell (5). Each locus contains a unique sequence 53 to 79 bp in length that is repeated exactly. Two of the loci used in MIRU-VNTR analysis are also ETR loci: MIRU loci 4 and 31 correspond to ETR loci D and E, respectively (17). The remaining MIRU loci do not contain exact repeated units. MIRU-VNTR typing appears to surpass ETR-VNTR typing in regard to discriminatory power and reproducibility (8, 16). A set of 31 duplicate isolates was used to test the reproducibility of each method. MIRU-VNTR was 100% reproducible, while ETR-VNTR was 97% reproducible. While most of the MIRU-VNTR loci contain repeats of a consensus sequence, the ETR-VNTR loci contain exact repeats, which might increase the probability of strand slippage during PCR, making it slightly harder to obtain reproducible results. Using a second set of 90 M. tuberculosis complex isolates, MIRU-VNTR typing detected 78 patterns and ETR-VNTR typing detected 56 patterns. Since MIRU-VNTR typing utilizes 12 loci while ETR-VNTR typing uses only 5 loci, this increased discriminatory power is not surprising.
It is difficult to directly compare this MIRU-VNTR study with previous studies because of differences in the sample sets. Mazars et al. (11) used 44 epidemiologically unrelated isolates collected in Paris. By design, 82% of the isolates fell into 10 spoligotype-defined clusters and 18% were genotypic group 1 IS6110 low-copy-number isolates. A second study involved 90 M. tuberculosis complex isolates from 38 countries that were selected to represent the diversity of IS6110 RFLP patterns (8, 16). Seventy were M. tuberculosis, and the others were M. bovis, M. bovis BCG, Mycobacterium africanum, Mycobacterium microti, and Mycobacterium canetti. By design, 68 of the 70 M. tuberculosis isolates had unique IS6110 RFLP patterns. Our set of 180 isolates from Michigan contained a single M. bovis isolate, an isolate with the Beijing spoligotype, 15 (8.3%) genotypic group 1 isolates (including the zero-copy isolate), and 163 (90.5%) non-genotypic group 1 IS6110 isolates. In general, the allelic diversity of each MIRU locus was higher in both previous studies, most likely due to the smaller number and greater diversity of isolates in the studies. Not surprisingly, the percentage of unique isolates determined by the three different typing methods was also much lower in our collection. In the Paris collection, 75% were unique by IS6110 RFLP and 77% were unique by MIRU-VNTR. In the international collection, 45% of the M. tuberculosis isolates were unique by spoligotyping and 77% were unique by MIRU-VNTR. In the Michigan collection, only 21% of the isolates were unique by IS6110 RFLP, 24% were unique by spoligotyping, and 33% were unique by MIRU-VNTR. If all three methods are combined, then 100% of the M. tuberculosis isolates in the international collection and 88% of the isolates in the Paris collection have a unique combined genotype, while only 50% of the Michigan isolates were unique.
The previous studies showed that analysis of the genetic relationships among isolates based on the MIRU-VNTR genotypes can be informative. Branches of the dendrogram identified the Beijing, Africa, and Haarlem genotype families (16). Also, there was a clear separation of IS6110 low-copy-number isolates from high-copy-number isolates primarily based on MIRU locus 24: IS6110 high-copy-number M. tuberculosis and M. bovis isolates contained one MIRU 24 copy; IS6110 low-copy-number M. tuberculosis, M. bovis, and M. microti isolates contained two MIRU 24 copies; and M. canetti contained six MIRU 24 copies. Our results further clarify these observations. The M. bovis isolate and the 15 genotypic group 1 low-copy-number isolates had two or three copies of MIRU 24 and were clearly separated from the 163 non-genotypic group 1 isolates and the isolate with the Beijing spoligotype that had one MIRU 24 copy.
It is also important to note that the conclusion that MIRU-VNTR typing performs significantly better than IS6110 RFLP when strains contain a low copy number of IS6110 (17) was based first on a set of eight genotypic group 1 isolates, all with unique spoligotypes (11), and then on a set of 23 isolates, of which 20 were genotypic group 1 M. tuberculosis, M. bovis, and M. bovis BCG or M. canetti (8). In these studies, MIRU-VNTR typing distinguished 29 out of 31 isolates (93%). The two isolates clustered by MIRU-VTNR were M. bovis BCG vaccine strains. In our predominantly non-genotypic group 1 set of isolates, MIRU-VNTR performed better than IS6110 RFLP and spoligotyping, clustering 67% of the isolates compared to 79 and 76%, respectively. Soini et al. (15) proposed that M. tuberculosis isolates of different genetic groups are prevalent in different areas of the world. In their study of IS6110 low-copy-number isolates collected in Houston, Tex., genotypic group 1 isolates were more prevalent among patients born in Asia and genotypic group 2 isolates were more prevalent among patients born in the United States or Mexico. They also noted that the majority of low-copy-number isolates in European studies were from foreign-born patients whereas 36.1% of low-copy-number isolates in Houston were from foreign-born patients and that these isolates were significantly less likely to be clustered than isolates from U.S.-born patients. It should be expected that conclusions regarding genotyping in low-copy-number isolates may be very different from one geographical region to another.
The genetic relatedness of low-copy-number isolates in the United States based on their IS6110 insertion sites was first reported in 1998 (4). Of 126 isolates with two to six copies of IS6110, 118 (93.6%) shared insertion site DK1 (INS 1), and of the 42 isolates with four to five copies of IS6110, 32 (76%) shared a second insertion site, DK3 (INS 4). The decreasing prevalence of these insertion sites in isolates with higher IS6110 copy numbers suggested separate lineages for these two groups of isolates. In our present study, 80% of the isolates had a copy of IS6110 at INS 1 (DK1), and 53% of these isolates also contained IS6110 at INS 3 and INS 4 (DK3), which indicates a distant genetic relatedness among the majority of the isolates in this study. We also found that these insertion sites were detected only in the non-genotypic group 1 isolates, which suggests that there are two distinct groups of IS6110 low-copy-number isolates.
MIRU-VNTR analysis has the potential to become more discriminative by utilizing additional loci. In this study, the addition of loci increased the number of patterns observed (Table 4). While it is possible that new variable MIRU loci will be identified, of the 41 MIRU loci currently identified, only 12 showed any variability across 28 geographically unrelated M. tuberculosis complex genomes (17). However, it would not be difficult to add other types of VNTR loci to the typing method. For example, the addition of ETR-A and ETR-C to the 12 MIRU-VNTR loci increased the number of patterns observed in a set of 90 isolates from 78 to 81 (16).
From this study and others, it is clear that no single method at present will define all unique isolates. In this study, the addition of any second or third typing method defined additional unique isolates. As M. tuberculosis genotyping results accumulate, it is also becoming clear that many variables will factor into deciding the most appropriate primary and secondary typing methods for a given application. All three methods used in this study provide poor discrimination for some isolates, and these weaknesses become apparent in larger-scale studies where common types are found among isolates with no epidemiologic relationships. IS6110 provides the greatest specificity overall but performs poorly with isolates having low copy numbers for IS6110. Spoligotyping has the lowest specificity, and some patterns are quite common, the most striking example being the Beijing pattern. Isolates with this spoligotype pattern show a wide diversity of IS6110 RFLP patterns. Similarly, it is likely that common MIRU-VNTR types will emerge as more isolates are typed. Both spoligotyping and MIRU-VNTR are suited to large-scale ongoing typing, although MIRU-VNTR will be practical only with automated analysis. With either method, identifying isolates with the same pattern provides only a preliminary indication that they may be related, and additional typing will often be required for confirmation.
Acknowledgments
We thank Mitch Yakrus and Andrea Havens for their technical assistance.
REFERENCES
- 1.Bauer, J., A. B. Andersen, K. Kremer, and H. Miorner. 1999. Usefulness of spoligotyping to discriminate IS6110 low-copy-number Mycobacterium tuberculosis complex strains cultured in Denmark. J. Clin. Microbiol. 37:2602-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dale, J. W., D. Brittain, A. A. Cataldi, D. Cousins, J. T. Crawford, J. Driscoll, H. Heersma, T. Lillebaek, T. Quitugua, N. Rastogi, R. A. Skuce, C. Sola, D. van Soolingen, and V. Vincent. 2001. Spacer oligonucleotide typing of bacteria of the Mycobacterium tuberculosis complex: recommendations for standard nomenclature. Int. J. Tuber. Lung Dis. 5:216-219. [PubMed] [Google Scholar]
- 3.de la Salmoniere, Y.-O. G., H. M. Li, G. Torrea, A. Bunschoten, J. van Embden, and B. Gicquel. 1997. Evaluation of spoligotyping in a study of the transmission of Mycobacterium tuberculosis. J. Clin. Microbiol. 35:2210-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fomukong, N., M. Beggs, H. El Hajj, G. Templeton, K. Eisenach, and M. D. Cave. 1998. Differences in the prevalence of IS6110 insertion sites in Mycobacterium tuberculosis strains: low and high copy number of IS6110. Tuber. Lung Dis. 78:109-116. [DOI] [PubMed] [Google Scholar]
- 5.Frothingham, R., and W. A. Meeker-O'Connell. 1998. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem repeats. Microbiology 144:1189-1196. [DOI] [PubMed] [Google Scholar]
- 6.Goyal, M., N. A. Saunders, J. D. A. van Embden, D. B. Young, and R. J. Shaw. 1997. Differentiation of Mycobacterium tuberculosis isolates by spoligotyping and IS6110 restriction fragment length polymorphism. J. Clin. Microbiol. 35:647-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs, D. 1990. SAS/GRAPH software and numerical taxonomy, p. 1413-1418. In Proceedings of the Fifteenth Annual SAS Users Group Conference. SAS Institute, Inc., Cary, N.C.
- 8.Kremer, K., D. van Soolingen, R. Frothingham, W. H. Haas, P. W. M. Hermans, C. Martin, P. Palittapongarnpim, B. B. Plikaytis, L. W. Riley, M. A. Yakrus, J. M. Musser, and J. D. A. van Embden. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 37:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magdalena, J., P. Supply, and C. Locht. 1998. Specific differentiation between Mycobacterium bovis BCG and virulent strains of the Mycobacterium tuberculosis complex. J. Clin. Microbiol. 36:2471-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magdalena, J., A. Vachee, P. Supply, and C. Locht. 1998. Identification of a new DNA region specific for members of Mycobacterium tuberculosis complex. J. Clin. Microbiol. 36:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazars, E., S. Lesjean, A.-L. Banuls, M. Gilbert, V. Vincent, B. Gicquel, M. Tibayrenc, C. Locht, and P. Supply. 2001. High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proc. Natl. Acad. Sci. USA 98:1901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molhuizen, H. O. F., A. E. Bunschoten, L. M. Schouls, and J. D. A. van Embden. 1998. Rapid detection and simultaneous strain differentiation of Mycobacterium tuberculosis complex bacteria by spoligotyping. Methods Mol. Biol. 101:381-394. [DOI] [PubMed] [Google Scholar]
- 13.Plikaytis, B. B., N. Kurepina, C. L. Woodley, W. R. Butler, and T. M. Shinnick. 1999. Multiplex PCR assay to aid in the identification of the highly transmissible Mycobacterium tuberculosis strain CDC1551. Tuber. Lung Dis. 79:273-278. [DOI] [PubMed] [Google Scholar]
- 14.Soini, H., X. Pan, A. Amin, E. A. Graviss, A. Siddiqui, and J. M. Musser. 2000. Characterization of Mycobacterium tuberculosis isolates from patients in Houston, Texas, by spoligotyping. J. Clin. Microbiol. 38:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soini, H., X. Pan, L. Teeter, J. M. Musser, and E. A. Graviss. 2001. Transmission dynamics and molecular characterization of Mycobacterium tuberculosis isolates with low copy numbers of IS6110. J. Clin. Microbiol. 39:217-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for the study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Supply, P., E. Mazars, S. Lesjean, V. Vincent, B. Gicquel, and C. Locht. 2000. Variable human mini-satellite-like regions in the Mycobacterium tuberculosis genome. Mol. Microbiol. 36:762-771. [DOI] [PubMed] [Google Scholar]
- 18.van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Giquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Soolingen, D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J. Intern. Med. 249:1-26. [DOI] [PubMed] [Google Scholar]
- 20.van Soolingen, D., L. Qian, P. E. W. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhasaikan, P. Nymadawa, and J. D. A. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang, Z. H., K. Ijaz, J. H. Bates, K. D. Eisenach, and M. D. Cave. 2000. Spoligotyping and polymorphic GC-rich repetitive sequence fingerprinting of Mycobacterium tuberculosis strains having few copies of IS6110. J. Clin. Microbiol. 38:3572-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]