Abstract
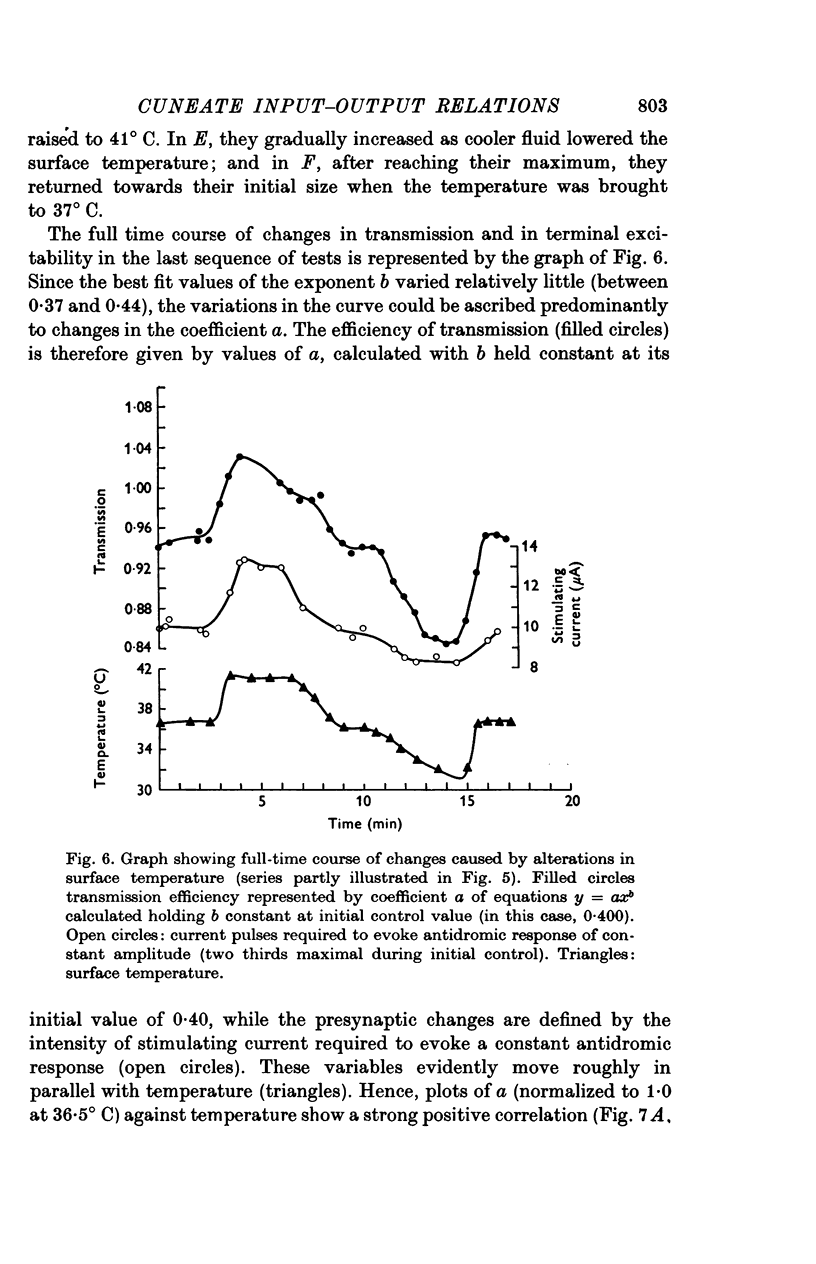
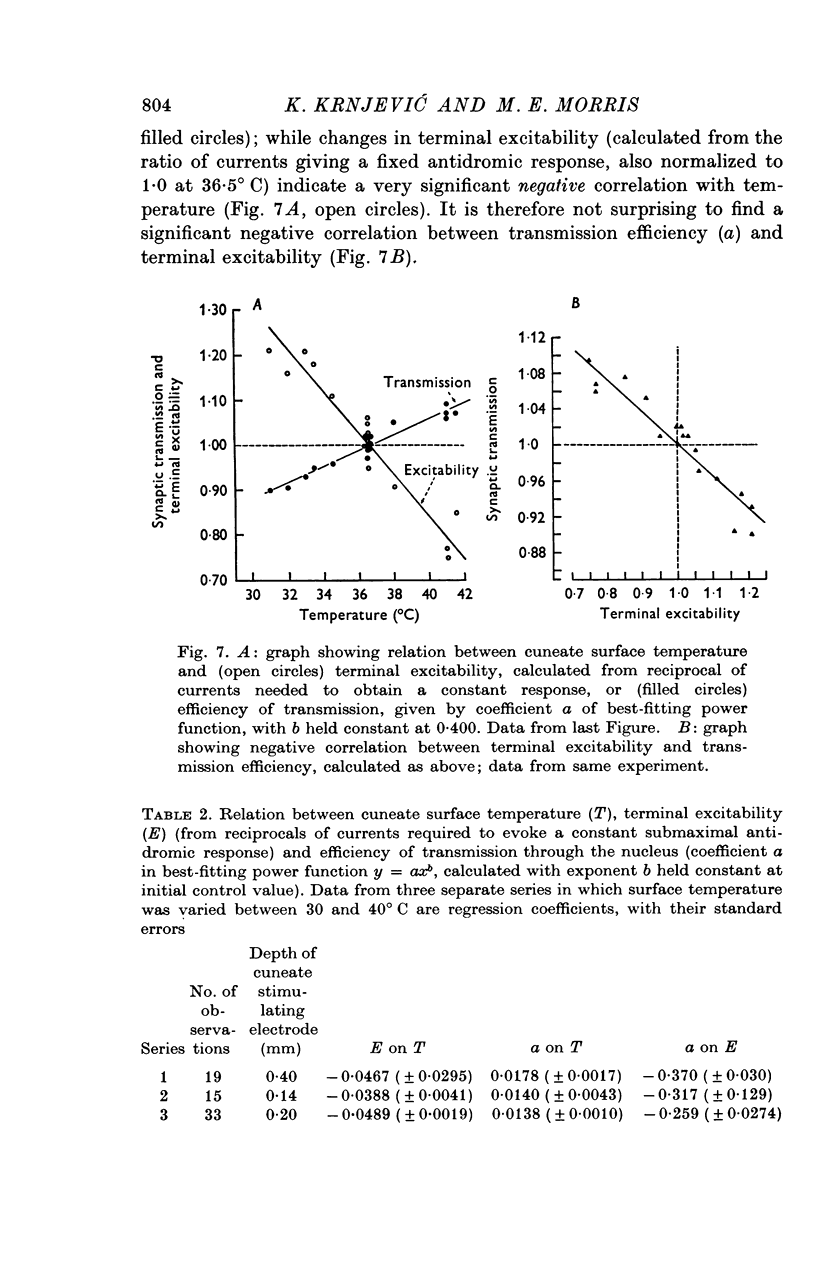
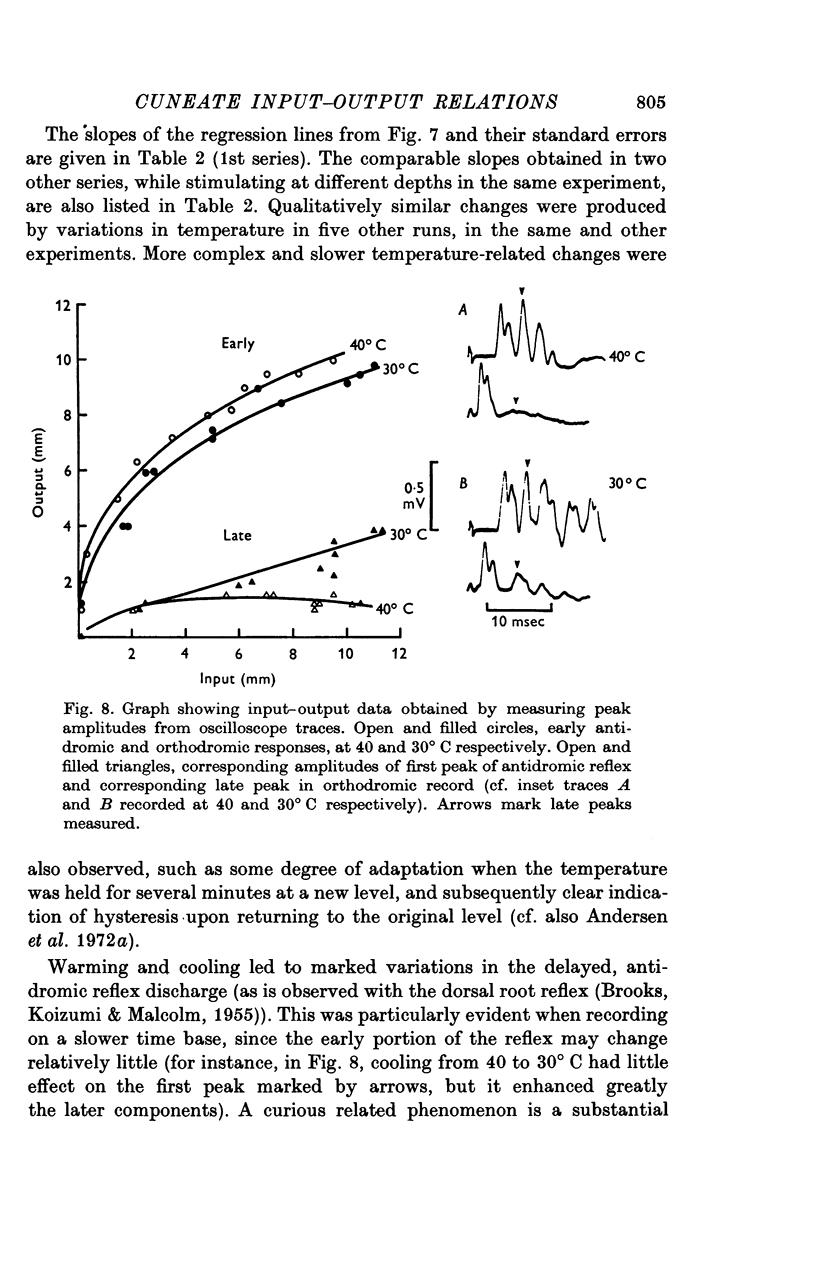
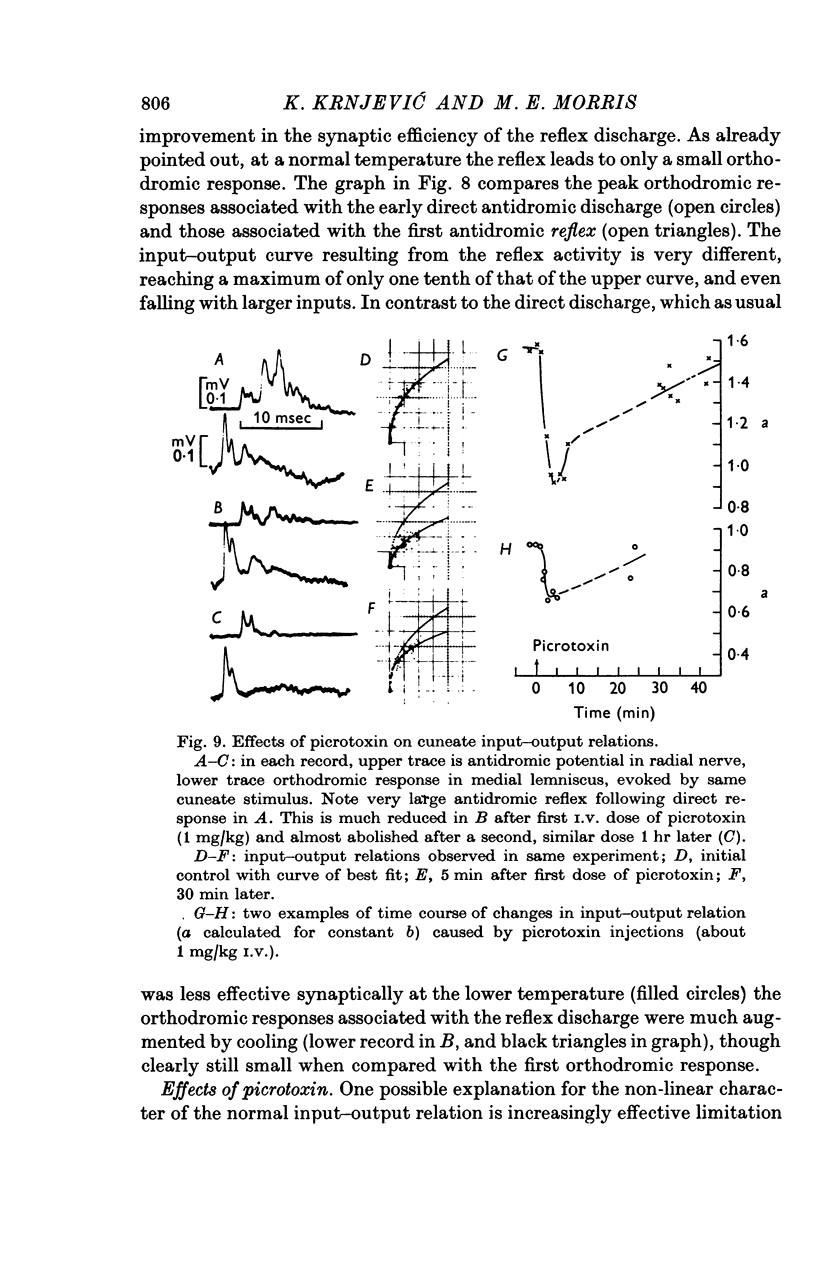
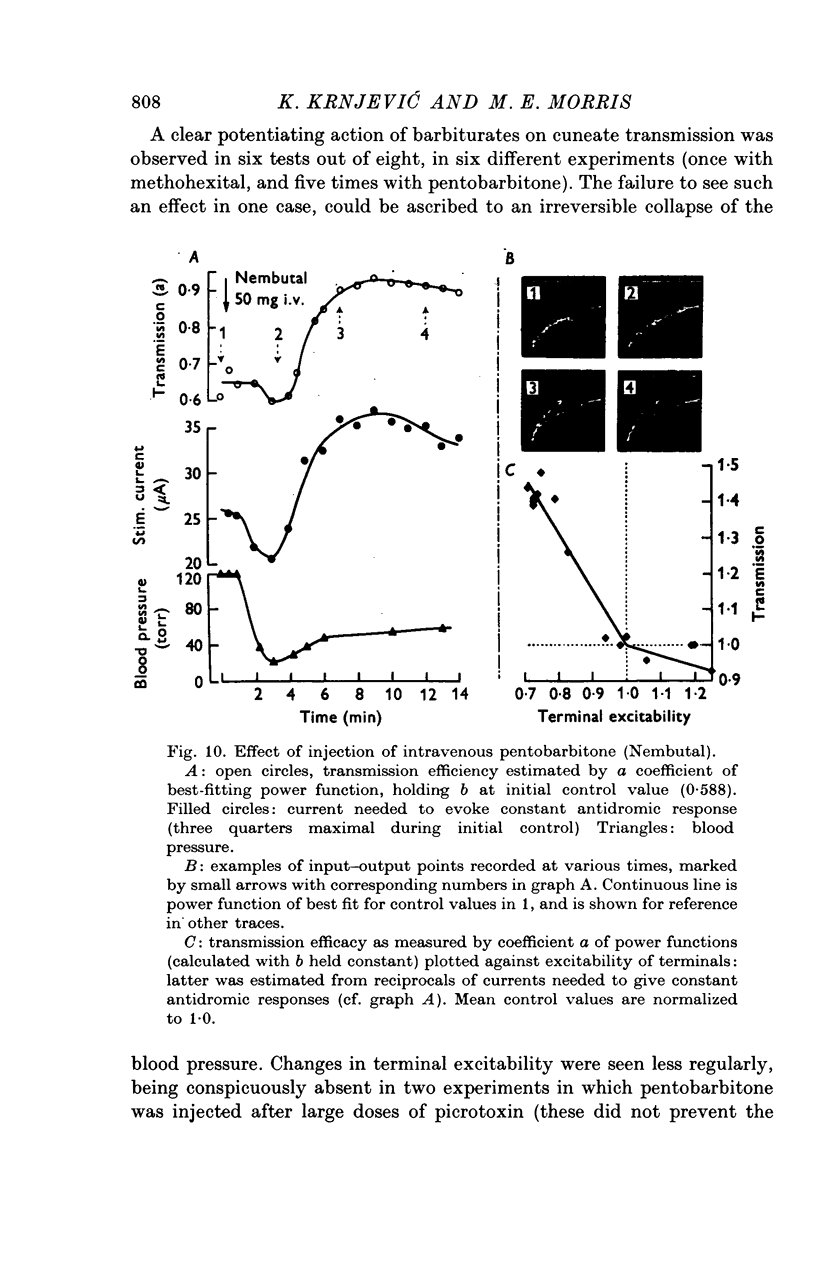
1. In decerebrate cats, micro-electrodes were inserted into the cuneate nucleus to stimulate afferent terminals with single shocks of varying intensities. Estimates of the input and output of the nucleus were obtained by integrating antidromic responses in forelimb cutaneous nerves and orthodromic responses in the medial lemniscus. 2. Input-output curves were normally very non-linear, reflecting the high synaptic potency of small inputs. They were fitted readily by power functions, with exponents averaging 0-50. 3. The normal input-output relation rapidly disappeared after interruption of the blood supply. A loss of synaptic efficiency of small inputs was indicated by curves with exponents of greater than or equal to 1; this was associated with a sharp increase in terminal excitability. 4. Within the range of surface temperature 30-40 degrees C, warming made the input-output curves steeper but reduced terminal excitability, whereas cooling had the opposite effect. The efficiency of transmission was thus inversely correlated with terminal excitability. 5. The non-linear shape of cuneate input-output curves is probably not determined by inhibitory control, since picrotoxin depressed rather than enhanced outputs. 6. On the other hand, pentobarbitone made the input-output curves markedly steeper and tended to lower terminal excitability.
Full text
PDF





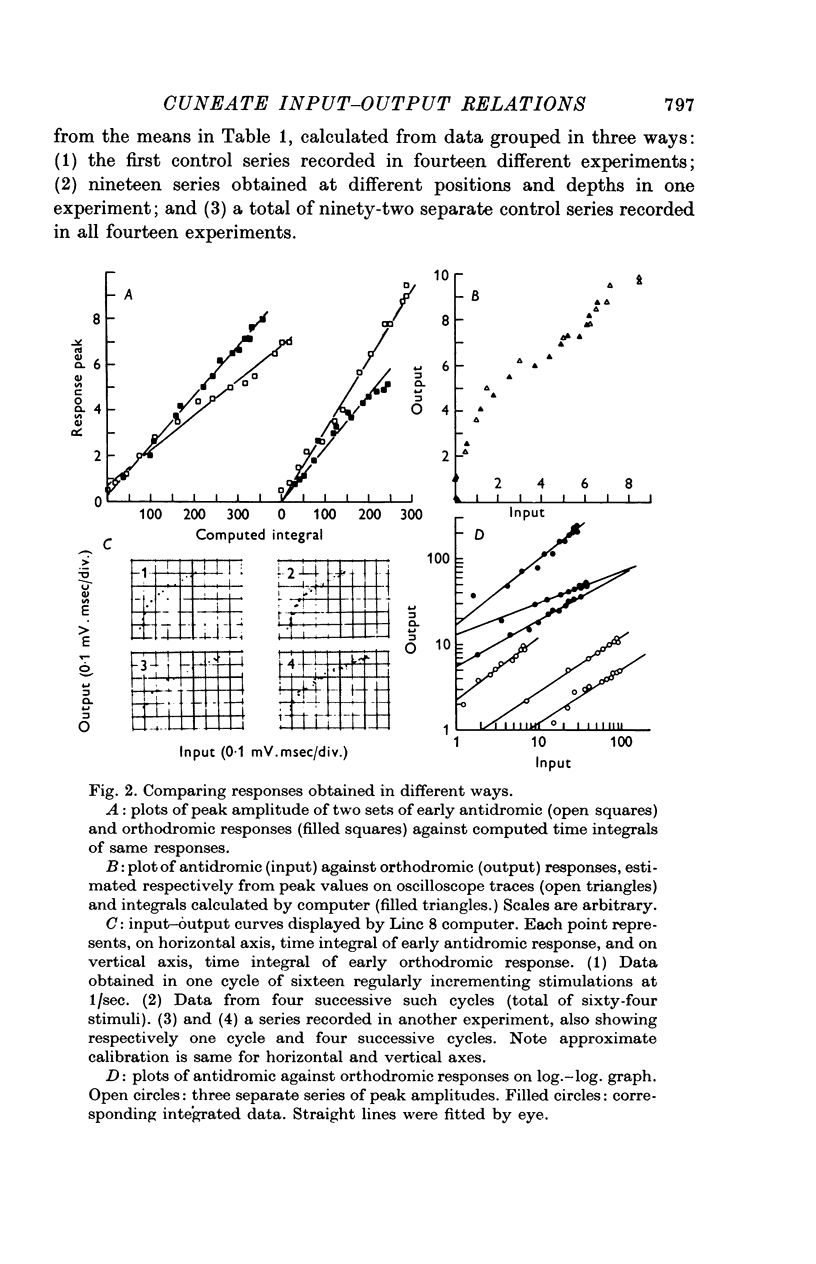

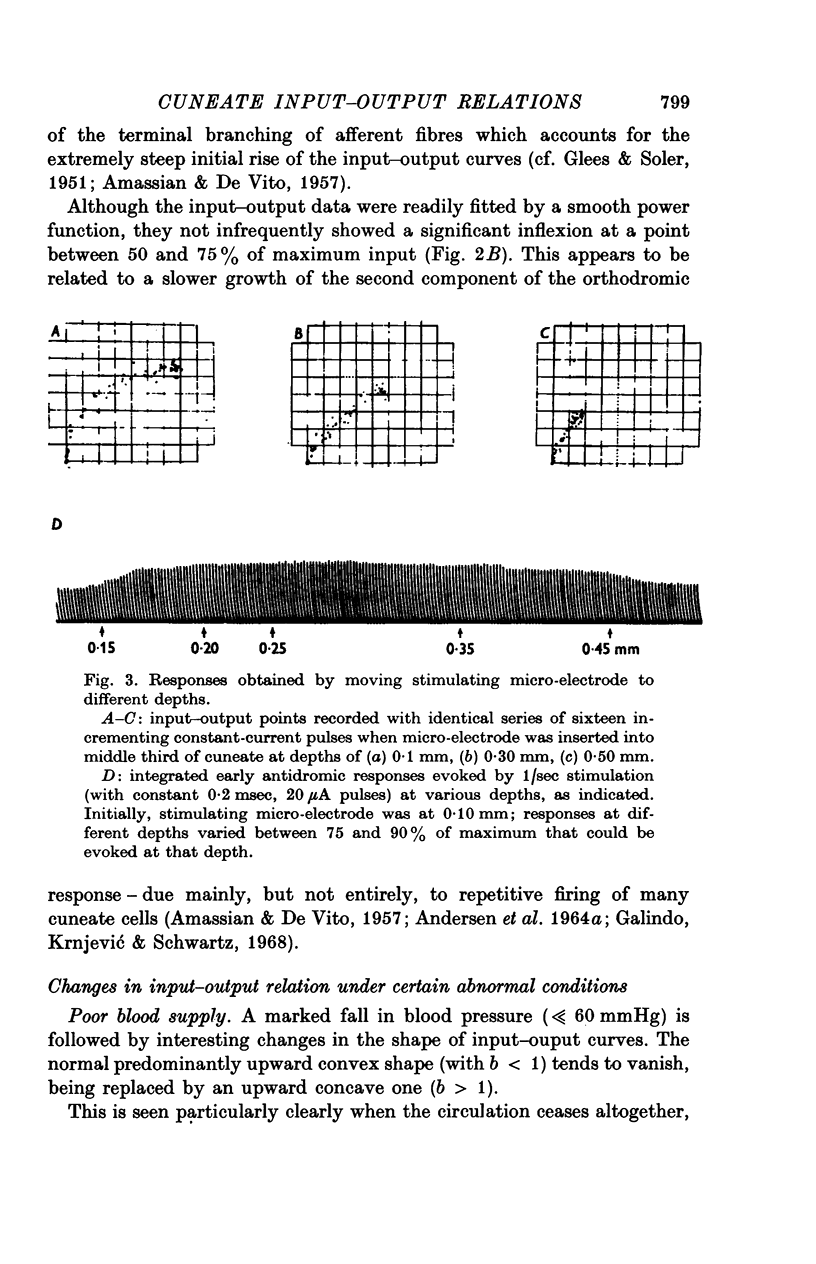
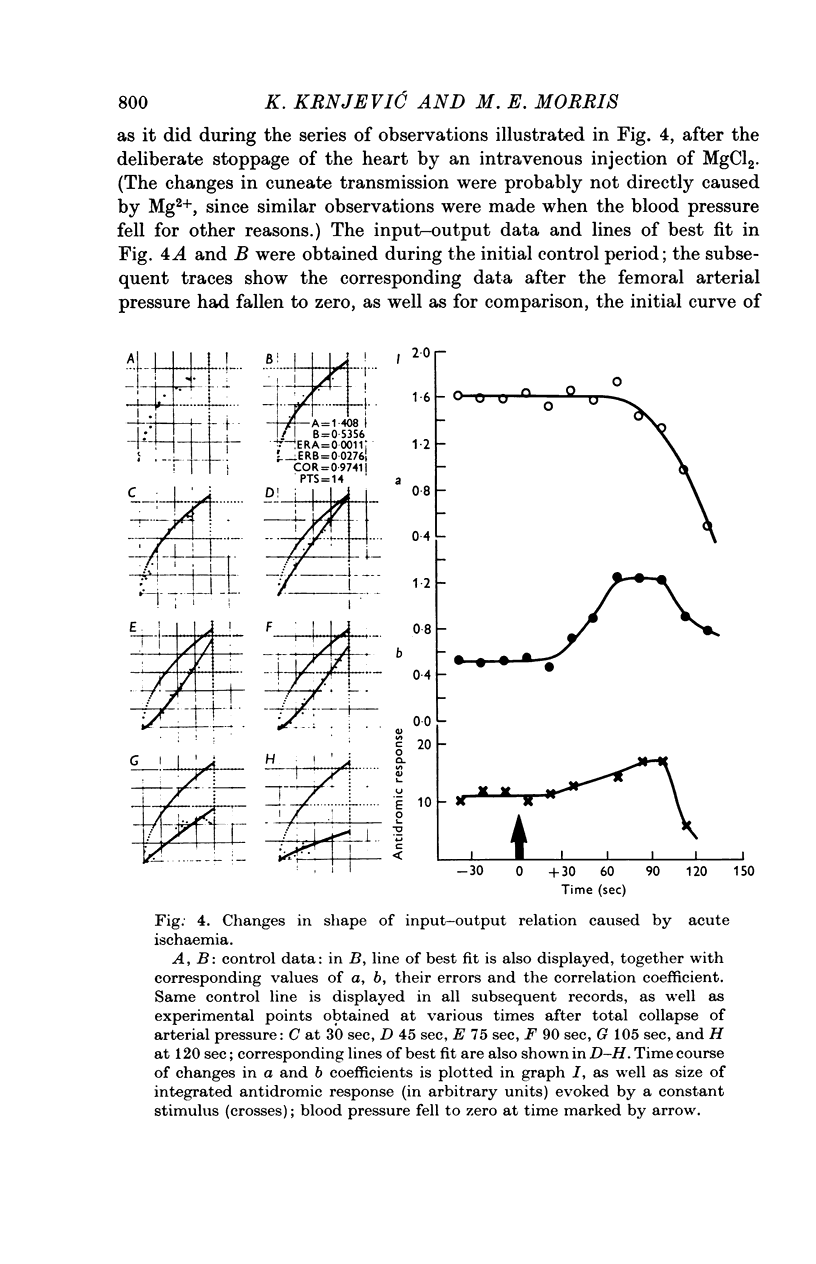

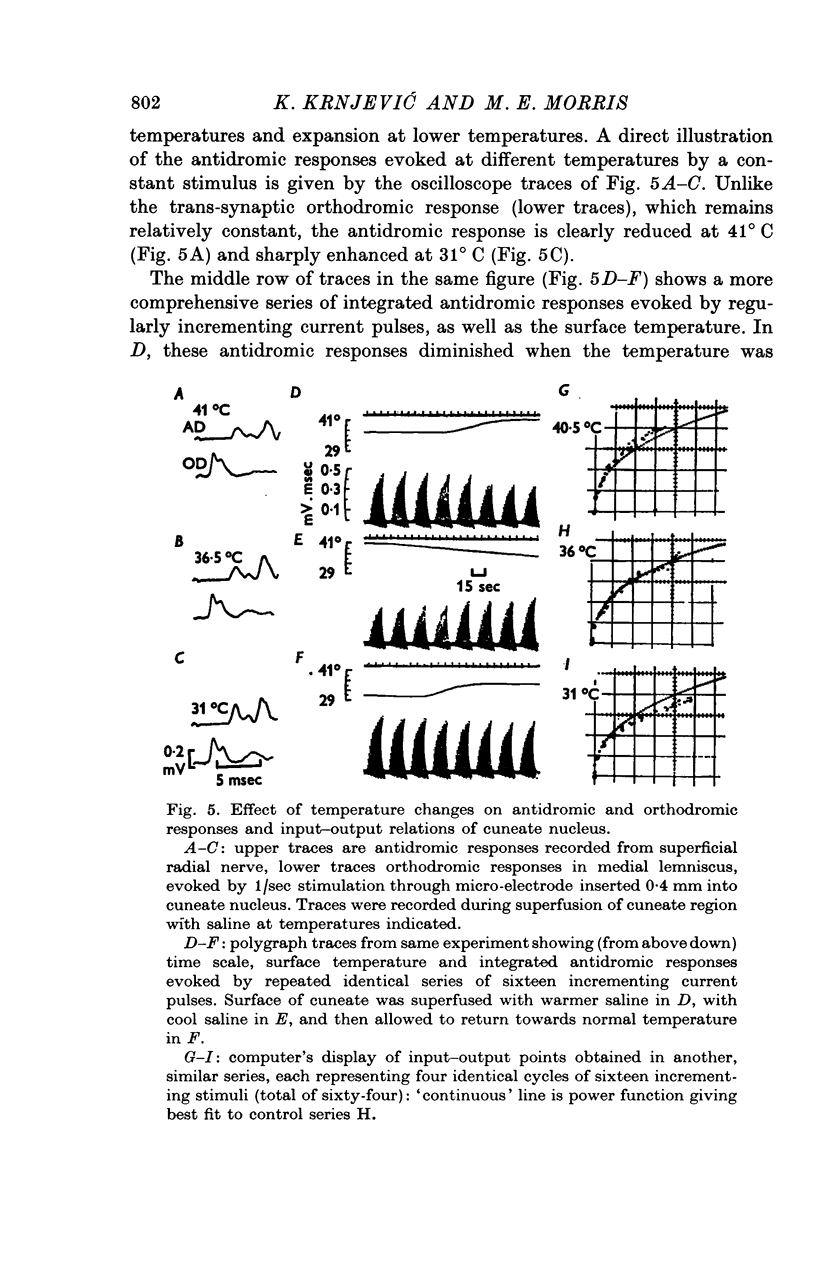








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., OSHIMA T., SCHMIDT R. F. MECHANISMS OF SYNAPTIC TRANSMISSION IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1096–1116. doi: 10.1152/jn.1964.27.6.1096. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. DEPOLARIZATION OF PRESYNAPTIC FIBERS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Jan;27:92–106. doi: 10.1152/jn.1964.27.1.92. [DOI] [PubMed] [Google Scholar]
- Andersen P., Etholm B., Gordon G. Presynaptic and post-synaptic inhibition elicited in the cat's dorsal column nuclei by mechanical stimulation of skin. J Physiol. 1970 Sep;210(2):433–455. doi: 10.1113/jphysiol.1970.sp009219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Gjerstad L., Pasztor E. Effect of cooling on synaptic transmission through the cuneate nucleus. Acta Physiol Scand. 1972 Apr;84(4):433–447. doi: 10.1111/j.1748-1716.1972.tb05194.x. [DOI] [PubMed] [Google Scholar]
- Andersen P., Gjerstad L., Pasztor E. Effects of cooling on inhibitory processes in the cuneate nucleus. Acta Physiol Scand. 1972 Apr;84(4):448–461. doi: 10.1111/j.1748-1716.1972.tb05195.x. [DOI] [PubMed] [Google Scholar]
- Angel A., Unwin J. The effect of urethane on transmission along the dorsal column sensory pathway in the rat. J Physiol. 1970 May;208(1):32P–33P. [PubMed] [Google Scholar]
- BISHOP P. O., McLEOD J. G. Nature of potentials associated with synaptic transmission in lateral geniculate of cat. J Neurophysiol. 1954 Jul;17(4):387–414. doi: 10.1152/jn.1954.17.4.387. [DOI] [PubMed] [Google Scholar]
- BOWSHER D. Projection of the gracile and cuneate nuclei in Macaca mulatta: an experimental degeneration study. J Comp Neurol. 1958 Aug;110(1):135–155. doi: 10.1002/cne.901100106. [DOI] [PubMed] [Google Scholar]
- BROOKS C. M., KOIZUMI K., MALCOLM J. L. Effects of changes in temperature on reactions of spinal cord. J Neurophysiol. 1955 May;18(3):205–216. doi: 10.1152/jn.1955.18.3.205. [DOI] [PubMed] [Google Scholar]
- Banna N. R., Jabbur S. J. Antagonism of presynaptic inhibition in the cuneate nucleus by picrotoxin. Nature. 1968 Jan 6;217(5123):83–84. doi: 10.1038/217083a0. [DOI] [PubMed] [Google Scholar]
- Carli G., Diete-Spiff K., Pompeiano O. Presynaptic and postsynaptic inhibition of transmission of somatic afferent volleys through the cuneate nucleus during sleep. Arch Ital Biol. 1967 Mar;105(1):52–82. [PubMed] [Google Scholar]
- Cesa-Bianchi M. G., Mancia M., Sotgiu M. L. Depolarization of afferent fibers to the Goll and Burdach nuclei induced by stimulation of the brain-stem. Exp Brain Res. 1968;5(1):1–15. doi: 10.1007/BF00239901. [DOI] [PubMed] [Google Scholar]
- Dart A. M., Gordon G. Some properties of spinal connections of the cat's dorsal column nuclei which do not involve the dorsal columns. Brain Res. 1973 Aug 17;58(1):61–68. doi: 10.1016/0006-8993(73)90823-8. [DOI] [PubMed] [Google Scholar]
- Domino E. F. Effects of preanesthetic and anesthetic drugs on visually evoked responses. Anesthesiology. 1967 Jan-Feb;28(1):184–191. doi: 10.1097/00000542-196701000-00019. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Rosén I., Scheid P., Táboríková H. The differential effect of cooling on responses of cerebellar cortex. J Physiol. 1975 Jul;249(1):119–138. doi: 10.1113/jphysiol.1975.sp011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLEES P., LIVINGSTON R. B., SOLER J. Der intraspinale Verlauf und die Endigungen der sensorischen Wurzeln in den Nucleus Gracilis und Cuneatus. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1951;187(3):190–204. doi: 10.1007/BF00341657. [DOI] [PubMed] [Google Scholar]
- GLEES P., SOLER J. Fibre content of the posterior column and synaptic connections of nucleus gracilis. Z Zellforsch Mikrosk Anat. 1951;36(4):381–400. doi: 10.1007/BF00335070. [DOI] [PubMed] [Google Scholar]
- GORDON G., PAINE C. H. Functional organization in nucleus gracilis of the cat. J Physiol. 1960 Sep;153:331–349. doi: 10.1113/jphysiol.1960.sp006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A. Effects of procaine, pentobarbital and halothane on synaptic transmission in the central nervous system. J Pharmacol Exp Ther. 1969 Oct;169(2):185–195. [PubMed] [Google Scholar]
- Galindo A., Krnjević K., Schwartz S. Patterns of firing in cuneate neurones and some effects of Flaxedil. Exp Brain Res. 1968;5(2):87–101. doi: 10.1007/BF00238699. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Temperature dependence of the sodium-potassium permeability ratio of a molluscan neurone. J Physiol. 1970 Nov;210(4):919–931. doi: 10.1113/jphysiol.1970.sp009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., TASAKI I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol. 1958 Aug 29;143(1):114–137. doi: 10.1113/jphysiol.1958.sp006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington T., Merzenich M. M. Neural coding in the sense of touch: human sensations of skin indentation compared with the responses of slowly adapting mechanoreceptive afferents innvervating the hairy skin of monkeys. Exp Brain Res. 1970;10(3):251–264. doi: 10.1007/BF00235049. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S., Renaud L. P. On the pharmacology of ascending, decending and recurrent postsynaptic inhibition of the cuneo-thalamic relay cells in the cat. Br J Pharmacol. 1973 Jul;48(3):396–408. doi: 10.1111/j.1476-5381.1973.tb08348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E. Extracellular accumulation of K+ evoked by activity of primary afferent fibers in the cuneate nucleus and dorsal horn of cats. Can J Physiol Pharmacol. 1974 Aug;52(4):852–871. doi: 10.1139/y74-110. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E. Factors determining the decay of K+ potentials and focal potentials in the central nervous system. Can J Physiol Pharmacol. 1975 Oct;53(5):923–934. doi: 10.1139/y75-126. [DOI] [PubMed] [Google Scholar]
- Krív N., Syková E., Vyklický L. Extracellular potassium changes in the spinal cord of the cat and their relation to slow potentials, active transport and impulse transmission. J Physiol. 1975 Jul;249(1):167–182. doi: 10.1113/jphysiol.1975.sp011009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD D. P. C., McINTYRE A. K. Dorsal column conduction of group I muscle afferent impulses and their relay through Clarke's column. J Neurophysiol. 1950 Jan;13(1):39–54. doi: 10.1152/jn.1950.13.1.39. [DOI] [PubMed] [Google Scholar]
- LOYNING Y., OSHIMA T., YOKOTA T. SITE OF ACTION OF THIAMYLAL SODIUM ON THE MONOSYNAPTIC SPINAL REFLEX PATHWAY IN CATS. J Neurophysiol. 1964 May;27:408–428. doi: 10.1152/jn.1964.27.3.408. [DOI] [PubMed] [Google Scholar]
- MARK R. F., STEINER J. Cortical projection of impulses in myelinated cutaneous afferent nerve fibres of the cat. J Physiol. 1958 Aug 6;142(3):544–562. doi: 10.1113/jphysiol.1958.sp006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCINTYRE A. K., MARK R. F. Synaptic linkage between afferent fibres of the cat's hind limb and ascending fibres in the dorsolateral funiculus. J Physiol. 1960 Sep;153:306–330. doi: 10.1113/jphysiol.1960.sp006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre A. K. Cortical projection of impulses in the interosseous nerve of the cat's hind limb. J Physiol. 1962 Aug;163(1):46–60. doi: 10.1113/jphysiol.1962.sp006958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. E. The action of carbon dioxide on synaptic transmission in the cuneate nucleus. J Physiol. 1971 Nov;218(3):671–689. doi: 10.1113/jphysiol.1971.sp009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. Presynaptic action of barbiturates in the frog spinal cord. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1460–1463. doi: 10.1073/pnas.72.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierau F. K., Klee M. R., Klussmann F. W. Effects of local hypo- and hyperthermia on mammalian spinal motoneurones. Fed Proc. 1969 May-Jun;28(3):1006–1009. [PubMed] [Google Scholar]
- RALL W. Experimental monosynaptic input-output relations in the mammalian spinal cord. J Cell Physiol. 1955 Dec;46(3):413–437. doi: 10.1002/jcp.1030460303. [DOI] [PubMed] [Google Scholar]
- RANDT C. T., COLLINS W. F. Effect of anesthetic agents on spinal cord of cat. Am J Physiol. 1959 Feb;196(2):340–342. doi: 10.1152/ajplegacy.1959.196.2.340. [DOI] [PubMed] [Google Scholar]
- Rustioni A. Non-primary afferents to the cuneate nucleus in the brachial dorsal funiculus of the cat. Brain Res. 1974 Jul 26;75(2):247–259. doi: 10.1016/0006-8993(74)90745-8. [DOI] [PubMed] [Google Scholar]
- Somjen G. Effects of anesthetics on spinal cord of mammals. Anesthesiology. 1967 Jan-Feb;28(1):135–143. doi: 10.1097/00000542-196701000-00015. [DOI] [PubMed] [Google Scholar]
- Thomson T. D., Turkanis S. A. Barbiturate-induced transmitter release at a frog neuromuscular junction. Br J Pharmacol. 1973 May;48(1):48–58. doi: 10.1111/j.1476-5381.1973.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddenberg N. Differential localization in dorsal funiculus of fibres originating from different receptors. Exp Brain Res. 1968;4(4):367–376. doi: 10.1007/BF00235701. [DOI] [PubMed] [Google Scholar]
- WERNER G., MOUNTCASTLE V. B. NEURAL ACTIVITY IN MECHANORECEPTIVE CUTANEOUS AFFERENTS: STIMULUS-RESPONSE RELATIONS, WEBER FUNCTIONS, AND INFORMATION TRANSMISSION. J Neurophysiol. 1965 Mar;28:359–397. doi: 10.1152/jn.1965.28.2.359. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. Effect of barbiturates on 'quantal' synaptic transmission in spinal motoneurones. J Physiol. 1969 Sep;204(1):63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]


