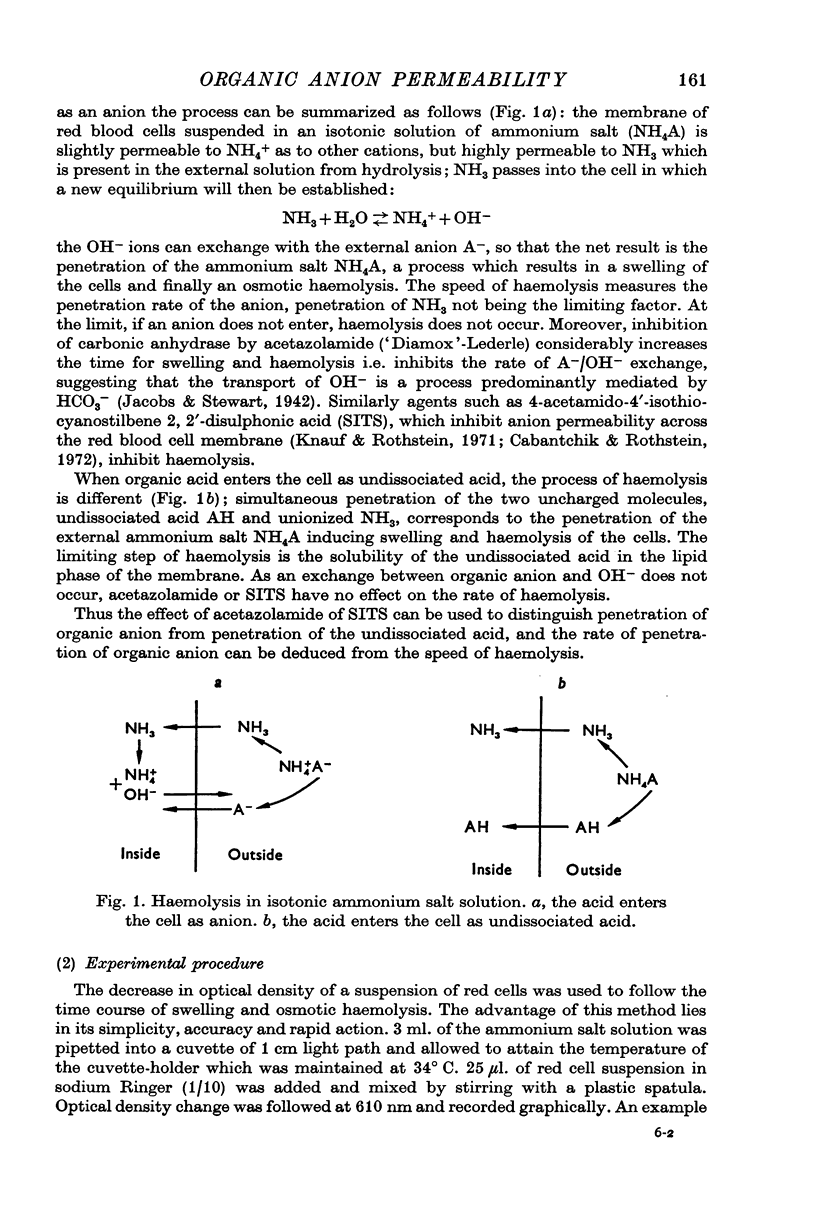
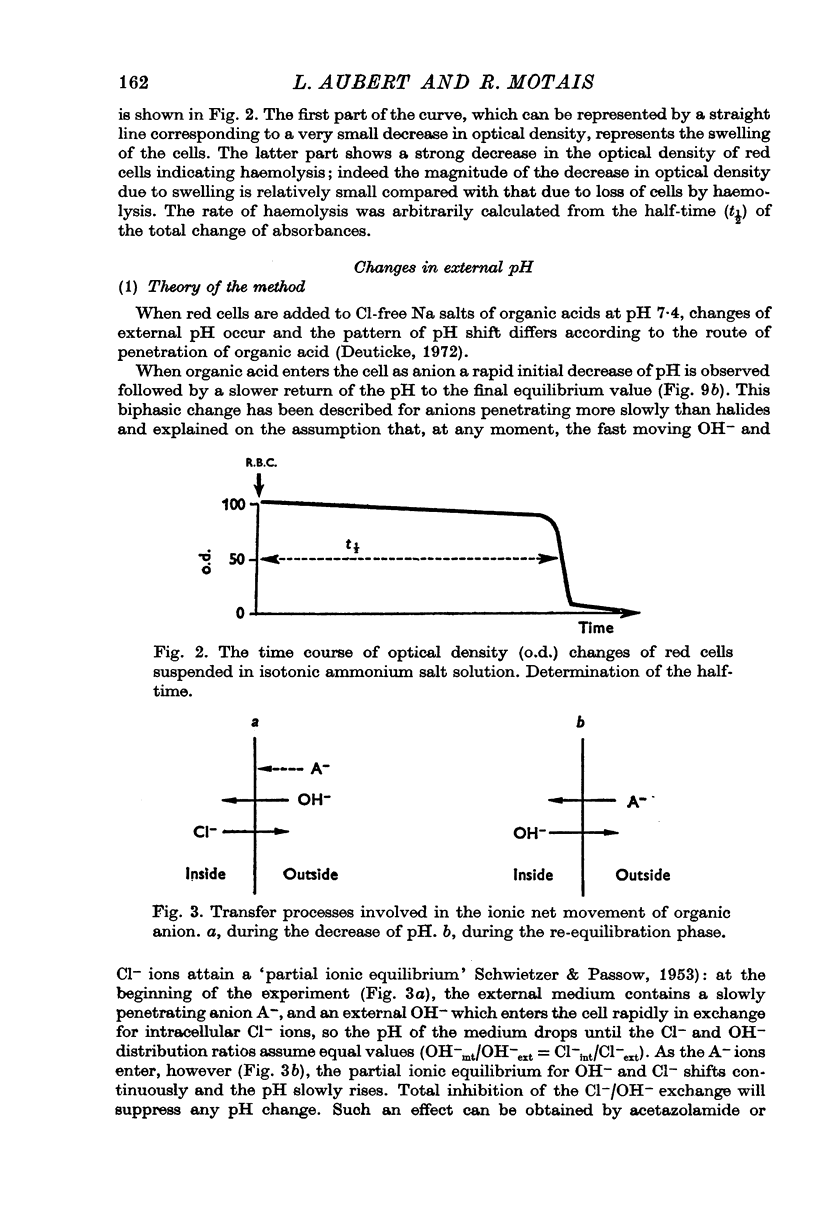
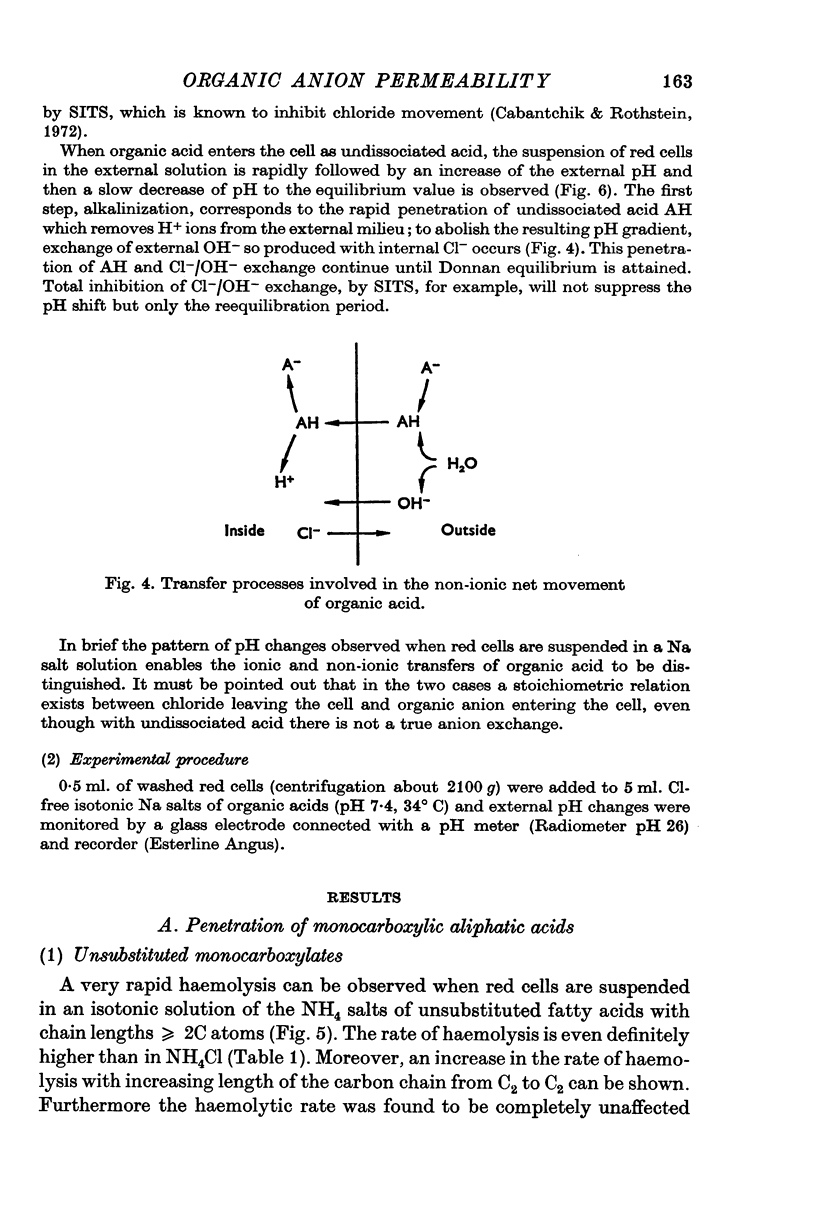
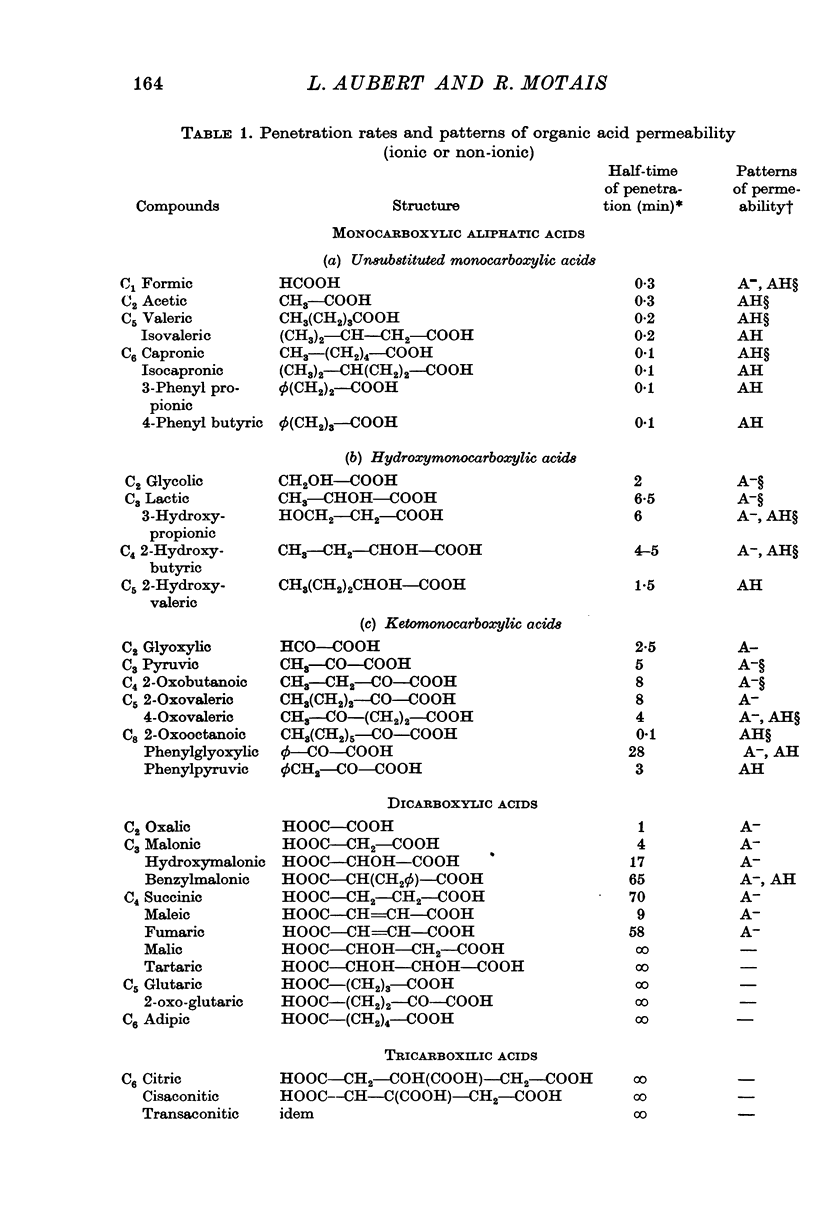
Abstract
1. The penetration of organic anions into bovine red blood cells has been studied under experimental conditions where it could be distinguished from the penetration of undissociated acids which proceeds by diffusion through lipid zones of the membrane. 2. Several lines of evidence suggest that the entry of organic anions cannot be ascribed to simple diffusion across aqueous channels limited by positive charges but needs a specific interaction of the penetrating anion with a component of the membrane. 3. The structural requirements allowing for ionic transfer is a strong polar head for the smallest molecules and in addition an amphiphilic structure for acids with chain length greater than C4. Interaction between substrate and receptor requires at least a three point attachment involving three oxygen atoms in the substrate which react with complementary loci on the receptor to form ionic and hydrogen bonds. Such a three point attachment can be made by a sulphonic group or with carboxylic acid by alpha ketosubstitution, alpha hydroxysubstitution, addition of an amidegroup or addition of a second carboxyl group spatially close to the first. 4. As suggested by the behaviour of the formate anion, in such a transport system any carboxylic acid could interact transiently with the receptor and therefore interfere with the transport of an organic anion even though such ionic interaction with the receptor were insufficient to produce transport of the acid itself.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Anion permeability of the red blood cell. Naturwissenschaften. 1970 Apr;57(4):172–179. doi: 10.1007/BF00592968. [DOI] [PubMed] [Google Scholar]
- GIEBEL O., PASSOW H. [The permeability of erythrocyte membranes for organic anions. On the problem of diffusion through the pores]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:378–388. [PubMed] [Google Scholar]
- Jacobs M. H., Stewart D. R. THE ROLE OF CARBONIC ANHYDRASE IN CERTAIN IONIC EXCHANGES INVOLVING THE ERYTHROCYTE. J Gen Physiol. 1942 Mar 20;25(4):539–552. doi: 10.1085/jgp.25.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P., Sha'afi R. I. Patterns of nonelectrolyte permeability in human red blood cell membrane. J Gen Physiol. 1973 Dec;62(6):714–736. doi: 10.1085/jgp.62.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHANKER L. S., JOHNSON J. M., JEFFREY J. J. RAPID PASSAGE OF ORGANIC ANIONS INTO HUMAN RED CELLS. Am J Physiol. 1964 Aug;207:503–508. doi: 10.1152/ajplegacy.1964.207.2.503. [DOI] [PubMed] [Google Scholar]
- SCHWIETZER C. H., PASSOW H. Kinetik und Gleichgewichte bei der langsamen Anionenpermeabilität roter Blutkörperchen. Pflugers Arch. 1953;256(6):419–445. doi: 10.1007/BF00370058. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]


