Abstract
Leishmania spp. are intracellular protozoan parasites that cause a wide spectrum of diseases in humans and dogs worldwide. However, monitoring of the Leishmania burden in its different hosts is still based on cumbersome and poorly sensitive methods. Here we have developed a highly accurate real-time PCR assay with which to reproducibly detect and quantify the relative Leishmania major burden in mouse tissue samples. The assay is performed with the LightCycler system using SYBR Green I and primers amplifying a ca. 120-bp fragment from minicircles of the kinetoplast DNA (kDNA). The assay was able to detect as little as 100 fg of L. major DNA per reaction, which is equivalent to 0.1 parasite. The standard curve designed for quantitation of parasites showed linearity over an at least 6-log DNA concentration range, corresponding to 0.1 to 104 parasites per reaction, with a correlation coefficient of 0.979. The assay also proved to have a detection range of the same magnitude as that used for detection of L. donovani and L. amazonensis, but it was 100-fold less sensitive for L. mexicana. When applied to tissues from experimentally infected mice, the real-time PCR assay is not only as sensitive as a conventional PCR assay for detection of Leishmania kDNA but also more rapid. Results indicate that this assay is compatible with the clinical diagnosis of leishmaniasis and will be a great help to scientists who use animals to monitor the efficacy of antileishmanial drugs or vaccines or decipher the unique properties of the life cycle of Leishmania spp .
Leishmania spp. are intracellular protozoa that affect humans and dogs worldwide and are transmitted by the bite of hematophagous sand flies. They cause a large spectrum of diseases, ranging from spontaneously healing skin lesions to fatal visceral symptoms, if left untreated. Two million new human cases arise every year, and at least 350 million people are exposed to the risk of the Leishmania parasite infection (2, 9). Experimental hosts, such as laboratory mice, are largely used to study the immunobiology of these parasites and to screen the efficacy of newly developed drugs and vaccines (4, 11, 20, 27). Most of those studies require detection and quantitation of the Leishmania burdens in different mouse tissues. This is still routinely performed by culture-based techniques (6, 28), which have several limitations, in particular, the amount of time required and the putative presence of viable but noncultivable parasites, such as persistent parasites (1). PCR-based methods for detecting Leishmania species have been developed to amplify rRNA genes, miniexon genes, kinetoplast DNA (kDNA), and repetitive nuclear sequences (15, 18, 24, 26). Recently, we have developed a PCR-based assay with which to quantify the parasite load in mice infected with Leishmania major (23) by using primers from the conserved sequences of kDNA. However, this technique is still cumbersome as it requests agarose gel image analysis.
A more rapid alternative is real-time quantitative PCR, which quantifies DNA (12, 29) and therefore has the potential for accurate microorganism enumeration in medical (14, 16, 17), environmental (25), or food samples (13). Here we describe a highly sensitive and specific method by which to detect and/or quantify L. major in mouse tissues by using the LightCycler (LC) system (30), which was adapted from a previous conventional PCR assay (23). This system combines an air thermocycler and a fluorimeter, enabling rapid-cycle PCR and monitoring of incorporation of the fluorescent dye SYBR Green I in double-stranded DNA. We show also that the assay can be used for detection of L. donovani, L. infantum, L. amazonensis, and L. mexicana.
MATERIALS AND METHODS
Leishmania strains and DNA extraction.
L. major strain NIH173 (MHOM/IR/−/173), L. donovani LV9 (MHOM/ET/1967/Hu3:LV9), L. infantum 2176 (MHOM/FR/1991/LEM 2176), L. amazonensis LV79 (MPRO/BR/1972/M1841), and L. mexicana M379 (MNYC/BZ/1962/M379) were cultured at 26°C in Hosmem-II medium (3) supplemented with 10% heat-inactivated fetal calf serum (Dutscher, Brumath, France), 100 U of penicillin per ml, and 100 μg of streptomycin per ml (Seromed, Berlin, Germany). Stationary-phase promastigotes of the different strains were harvested by centrifugation, washed twice with phosphate-buffered saline, enumerated with a Malassez hemacytometer, pelleted, and stored at −80°C until DNA extraction.
Genomic DNA was extracted from approximately 2 × 107 promastigotes with a DNeasy Tissue Kit (Qiagen, Courtaboeuf, France) in accordance with the manufacturer's protocol. The DNA concentration was estimated by spectrophotometric determination of A260.
Source of mouse tissue DNA.
Tissues were collected from BALB/c mice chronically infected in other studies in our laboratory or from naive mice as controls. Mice were infected intradermally at the ear with 104 metacyclic promastigotes of L. major strain NIH173 and killed at 6 or 12 months postinfection (23). Other mice were infected in the footpad with 2 × 106 amastigotes of L. amazonensis strain LV79 or L. mexicana strain M379 and killed at 12 or 32 weeks postinfection, respectively. Bone marrow and spleens were also collected from mice infected with L. donovani strain LV9 (19). Briefly, mice were inoculated by the intravenous route in the tail vein with 2 × 107 stationary-phase promastigotes and killed 30 days postinoculation.
The following tissues were sampled from infected or uninfected BALB/c mice: ears, retromaxillary or popliteal draining lymph nodes, spleen, liver, femoral bone marrow, blood, footpad, and tail skin (Table 3). Homogenates were prepared as previously described (23). Tissues were removed by using different scissors or scalpels to avoid contamination and were minced with Potter grinders and then carefully homogenized in 1.5-ml microtubes with single-use blue pellet pestles (Polylabo, Paris, France) in phosphate-buffered saline. Aliquots of the homogenates were stored at −20°C until DNA extraction. DNA was extracted from aliquots of homogenates with a DNeasy Tissue Kit.
TABLE 3.
Results of real-time and conventional PCR assays for Leishmania kDNA in mouse tissue samples
| Sample(s) | Species | Source | Result of conventional PCR | Real-time PCR
|
|
|---|---|---|---|---|---|
| CT | Qualita- tive data | ||||
| 1-6 | L. major | Ear | − | >36 | − |
| 7 | L. major | Ear | + | 28.61 | + |
| 8 | L. major | Ear | + | 22.66 | + |
| 9 | L. major | Ear | + | 24.64 | + |
| 10 | L. major | Ear | + | 34.94 | + |
| 11 | L. major | Ear | − | 35.14 | + |
| 12 | L. major | Ear | − | >36 | − |
| 13 | L. major | Lymph node | + | 29.68 | + |
| 14 | L. major | Lymph node | + | 30.68 | + |
| 15 | L. major | Lymph node | + | 23.77 | + |
| 16-18 | L. major | Tail skin | − | >36 | − |
| 19 | L. mexicana | Blood | − | >36 | − |
| 20 | L. mexicana | Blood | + | 31.42 | + |
| 21 | L. mexicana | Blood | − | 33.05 | + |
| 22 | L. mexicana | Bone marrow | + | 28.80 | + |
| 23 | L. mexicana | Bone marrow | + | 21.44 | + |
| 24 | L. mexicana | Lesion | + | 17.94 | + |
| 25 | L. mexicana | Lesion | + | 18.16 | + |
| 26 | L. mexicana | Lesion | + | 16.46 | + |
| 27 | L. mexicana | Liver | + | 23.66 | + |
| 28 | L. mexicana | Liver | + | 24.46 | + |
| 29 | L. mexicana | Lymph node | + | 19.81 | + |
| 30 | L. mexicana | Lymph node | + | 20.70 | + |
| 31 | L. mexicana | Spleen | + | 24.61 | + |
| 32 | L. mexicana | Spleen | + | 20.96 | + |
| 33 | L. mexicana | Tail skin | + | 23.89 | + |
| 34 | L. mexicana | Tail skin | + | 15.36 | + |
| 35 | L. mexicana | Tail skin | + | 18.67 | + |
| 36 | L. amazonensis | Blood | − | >36 | − |
| 37 | L. amazonensis | Lesion | + | 15.81 | + |
| 38 | L. amazonensis | Liver | − | >36 | − |
| 39 | L. amazonensis | Lymph node | + | 25.42 | + |
| 40 | L. amazonensis | Spleen | − | >36 | − |
| 41 | L. amazonensis | Tail skin | − | 29.97 | + |
| 42 | L. donovani | Bone marrow | + | 22.73 | + |
| 43 | L. donovani | Bone marrow | + | 27.87 | + |
| 44 | L. donovani | Bone marrow | + | 28.44 | + |
| 45 | L. donovani | Spleen | + | 19.64 | + |
| 46 | L. donovani | Spleen | + | 19.88 | + |
| 47 | L. donovani | Spleen | + | 23.56 | + |
Primers.
Detection of Leishmania DNA was carried out with previously described primers (23) (forward, 5′-CCTATTTTACACCAACCCCCAGT-3′ [JW11]; reverse, 5′-GGGTAGGGGCGTTC TGCGAAA-3′ [JW12]) that amplify a ca. 120-bp fragment of the minicircle kDNA of L. major, ca. 10,000 copies of which are present in each parasite. These primers match the conserved sequences of the kinetoplast minicircle but do not match human or mouse frequent nucleic acid sequences according to the PCR-Rare software (10). Primers were provided by Genset (Paris, France) as EasyOligos.
Conventional PCR.
A conventional PCR was carried out with an automated thermocycler PCR-Express (Hybaid, Ashford, United Kingdom) as already described (23). Extracted DNA (2 μl) was mixed with a solution containing 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 2 mM MgCl2, 250 μM each dNTP, 10 pmol of each primer, and 0.5 U of Taq polymerase (Promega, Charbonnières, France) in a 40-μl final volume. A hot-start procedure was used to increase specificity. After initial denaturation (4 min at 94°C), 40 cycles of denaturation for 1 min at 64°C, annealing for 30 s at 58°C, and elongation for 30 s at 72°C were carried out and the PCR was terminated by a final extension at 72°C for 10 min. Each sample was tested in duplicate. Negative control tubes that received 2 μl of water instead of DNA extract were included in each PCR run to detect any amplicon contamination. PCR products were visualized after electrophoresis on a 1.5% agarose gel.
Real-time PCR with LC.
A real-time hot-start PCR was performed with the LC FastStart DNA Master SYBR Green I Kit (Roche Diagnostics, Meylan, France) in an LC (Roche Diagnostics). The 12-μl reaction mixture contained 1× LC FastStart DNA Master SYBR Green I, 2 mM MgCl2, 10 μM each primer, and 1.2 μl of template. Times and temperatures are shown in Table 1. For fluorescence signal acquisition, channel F1 was used and the gain was set at 5. For normalization of fluorescent data, the F1/1 ratio was applied.
TABLE 1.
Times and temperatures used for the PCR configuration with SYBR Green I and the LC
| Parameter | Temp (°C) | Time (s) | Slope (°C/s) | Acquisition mode |
|---|---|---|---|---|
| Denaturation | 95 | 8 | 20 | None |
| Amplification (40 cycles) | 95 | 10 | 20 | None |
| 56 | 10 | 20 | None | |
| 72 | 8 | 20 | Single | |
| Melting | 95 | 10 | 20 | None |
| 67 | 30 | 20 | None | |
| 95 | 10 | 0.1 | Continue | |
| Cooling | 40 | 60 | 20 | None |
Data and statistical analysis.
In order to determine the variability of the assays, intraassay and interassay (repeatability) precision was measured. Three replicates of five different concentrations of L. major DNA were tested simultaneously in the same run. The precision among four assays was assessed by using the previous L. major DNA concentrations run on different days. Variability is shown as the mean ± the standard deviation (SD) and reported as the coefficient of variation. Statistical and regression analyses were carried out with Sigma Plot Software (SPSS Inc., Chicago, Ill.).
RESULTS
LC PCR development.
The JW11 and JW12 primers, which amplify a ca 120-bp DNA fragment from L. major kinetoplast minicircles, have already been used (23). A 100-pg sample of DNA extracted from in vitro-grown promastigotes of L. major strain NIH173 was used as the template for establishment of the LC PCR assay, in particular, determination of the optimal annealing temperature and magnesium chloride concentration. Agarose gel electrophoresis of the PCR product confirmed the amplification of a ca 120-bp DNA fragment (data not shown). The reaction volume was minimized to 12 μl including 10% template DNA.
Sensitivity and reproducibility of the assay for L. major.
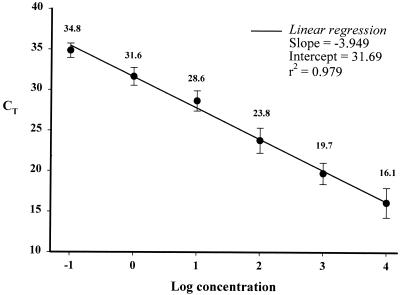
To determine the detection limit of our assay and establish a standard curve that could be used for quantitation, serial dilutions of L. major DNA with final concentrations ranging from 10,000 parasites to 0.01 parasite per reaction were subjected to a real-time PCR analysis. We were able to detect as little as 0.1 parasite, corresponding to 100 fg, per reaction in a 12-μl reaction volume. The mean standard curve, calculated from four independent experiments, was linear over an at least 6-log range of DNA concentrations, with a correlation coefficient of 0.979 (Fig. 1). A negative control consisting of the reaction mixture and water instead of template DNA was added in each run. A melting curve analysis of PCR products showed that the melting temperature of the kDNA amplicon was ca. 84°C while that of nonspecific products was ca. 79.5°C.
FIG. 1.
Sensitivity of the LC PCR assay with 10-fold dilutions of L. major DNA. A plot of mean CT values from four replicates tested on different days against the logarithmic concentration of parasite DNA (ranging from 0.1 to 104 parasite per reaction) is shown. Variability is shown as the mean CT value ± 1 SD.
To analyze the reproducibility and reliability of the real-time PCR assay, we assessed the intraassay and interassay coefficients of variation. Four replicates of five 10-fold DNA concentrations, from 103 parasites per reaction to 0.1 parasite per reaction, were assessed in a single run. The intraassay variations of CT values (the cycle numbers reflecting a positive PCR result differentiated from the background noise) among the replicates were 1.25, 0.22, 0.54, 1.35, and 0.43% for the five different concentrations, respectively. In addition, four replicates of 10-fold L. major DNA dilutions were performed on different days. The interassay variations of CT values for the DNA concentrations ranging from 104 to 0.1 were 11.30, 6.69, 6.51, 4.38, 3.49, and 2.57%, respectively (Fig. 1).
Detection of kDNAs from other Leishmania species.
Primers JW11 and JW12 were also able to amplify a ca 120-bp DNA fragment from promastigotes of L. donovani LV9, L. infantum 2176, L. amazonensis LV79, and L. mexicana M379 by conventional PCR (data shown). Therefore, they were also assessed for amplification of kDNA in our LC assay. The sensitivity of the LC PCR assay for detection of these strains was similar to that of L. major NIH173, except for L. mexicana strain M379, for which the assay was approximately 100 times less sensitive (Table 2). The intraassay variation coefficient was always <1.2%, showing good reproducibility of the assay for those Leishmania species as well.
TABLE 2.
Mean CT values and intraassay SDs of dilution series of promastigote DNAs from different Leishmania species obtained with the LC PCR assay
| Leishmania species | Mean CT value ± SD with following no. of parasites/reaction:
|
||||
|---|---|---|---|---|---|
| 1,000 | 100 | 10 | 1 | 0.1 | |
| L. major NIH173 | 21.06 ± 0.260 | 25.51 ± 0.057 | 29.46 ± 0.160 | 33.05 ± 0.446 | 35.85 ± 0.153 |
| L. donovani LV9 | 17.71 ± 0.017 | 21.75 ± 0.070 | 24.57 ± 0.055 | 28.83 ± 0.228 | 32.29 ± 0.224 |
| L. infantum 2176 | 19.95 ± 0.011 | 24.36 ± 0.272 | 28.90 ± 0.006 | 33.39 ± 0.051 | NDa |
| L. amazonensis LV79 | 19.37 ± 0.121 | 23.42 ± 0.266 | 27.48 ± 0.250 | 32.14 ± 0.075 | 34.18 ± 0.122 |
| L. mexicana M379 | 25.84 ± 0.095 | 30.09 ± 0.121 | 35.01 ± 0.627 | ND | ND |
ND, inconsistent quantification data.
Leishmania detection in mouse tissues: comparison of real-time PCR and conventional PCR.
DNA was extracted from various tissues of BALB/c mice infected with various Leishmania strains (Table 3) and assayed with either a conventional PCR or the LC PCR. In the latter, the standard curve of the respective species was used to generate a relative Leishmania burden based on CT values. In all of the assays, the CT values of negative controls were always >36. Whatever the tissue and the strain were, all of the samples positive by the conventional PCR were also positive by the LC assay and most of the CT values were far below the negative CT value threshold of 36. In addition, a few samples negative by the conventional PCR were positive by the real-time PCR
DISCUSSION
A new molecular real-time PCR assay for detection and quantification of L. major and several other Leishmania species of medical or veterinary importance is described. This assay is based on the LC system with SYBR Green I. This quantitative LC PCR assay allows highly sensitive and reproducible detection and quantitation of the parasite burden over a wide range, at least 6 logs, of parasite concentrations. The very high sensitivity (less than 0.1 parasite per reaction) is partly due to the high copy number of the target minicircle kDNA, which is present at ca. 10,000 copies per parasite. This avoids the use of internal molecular probes and therefore limits the cost of the assay. Including the DNA extraction step, the assay can be performed within 4 to 5 h without risk of contamination, as the reaction capillary remained closed. This is much more rapid than microtitration assays or even a conventional PCR. Using another real-time PCR with the TaqMan system, Bretagne et al. (5) have compared the real-time PCR and culture microtitration for quantification of L. infantum in mouse tissues and shown a good correlation between the two techniques.
Application of the real-time PCR for research and clinical diagnosis in parasitology is just starting and so far concerns mainly Toxoplasma gondii (8, 14, 21) and L. infantum (5). With primers common to several Leishmania species, our assay was also used to determine the relative parasite burdens in mouse tissues infected with L. amazonensis and L. donovani and, to a lesser extent, in mouse tissues infected with L. mexicana based on CT values. Preliminary assays in our laboratory have shown that the PCR yield may be influenced by a tissue DNA concentration above a threshold. Therefore, we are now developing internal standards based on housekeeping genes to determine the parasite burden more accurately.
Identification of a Leishmania infection for laboratory clinical diagnosis by culture or serological techniques requires a long time and has poor specificity. With the development of a real-time PCR assay that can be improved for identification of Leishmania species with internal probes or different primers, as has been done for other pathogenic microorganisms (7, 22, 31), we hope that this assay will replace or supplement the current serology technique. In addition, a large field of application for our assay is monitoring of Leishmania infections in research experiments.
Acknowledgments
We thank the Pasteur Institute for financial support. Part of this study was supported by a grant from the Pasteur-Cerba Laboratory.
REFERENCES
- 1.Aebischer, T. 1994. Recurrent cutaneous leishmaniasis: a role for persistent parasites. Parasitol. Today 10:25-28. [DOI] [PubMed] [Google Scholar]
- 2.Ashford, R. W. 2000. The leishmaniases as emerging and reemerging zoonoses. Int. J. Parasitol. 30:1269-1281. [DOI] [PubMed] [Google Scholar]
- 3.Berens, R. L., and J. J. Marr. 1978. An easily prepared defined medium for cultivation of Leishmania donovani promastigotes. J. Parasitol. 64:160.. [PubMed] [Google Scholar]
- 4.Blackwell, J. M. 1996. Genetic susceptibility to leishmanial infections: studies in mice and man. Parasitology 112Suppl.:S67-S74. [PubMed] [Google Scholar]
- 5.Bretagne, S., R. Durand, M. Olivi, J. F. Garin, A. Sulahian, D. Rivollet, M. Vidaud, and M. Deniau. 2001. Real-time PCR as a new tool for quantifying Leishmania infantum in liver in infected mice. Clin. Diagn. Lab. Immunol. 8:828-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buffet, P. A., A. Sulahian, J. F. Garin, N. Nassar, and F. Derouin. 1995. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 39:2167-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corless, C. E., M. Guiver, R. Borrow, V. Edward-Jones, A. J. Fox, and B. Kaczmarski. 2001. Simultaneous detection of Neisseria meningitides, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J. Clin. Microbiol. 39:1553-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa, J. M., C. Pautas, P. Ernault, F. Foulet, C. Cordonnier, and S. Bretagne. 2000. Real-time PCR for diagnosis and follow-up of Toxoplasma reactivation after allogeneic stem cell transplantation using fluorescence resonance energy transfer hybridization probes. J. Clin. Microbiol. 38:2929-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desjeux, P. 2001. The increase in risk factors for leishmaniasis worldwide. Trans. R. Soc. Trop. Med. Hyg. 95:239-243. [DOI] [PubMed] [Google Scholar]
- 10.Griffais, R., P. M. André, and M. Thibon. 1991. K-tuple frequency in the human genome and polymerase chain reaction. Nucleic Acids Res. 19:3887-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handman, E. 2001. Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 14:229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real-time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 13.Hein, I., A. Lehner, P. Rieck, K. Klein, E. Brandl, and M. Wagner. 2001. Comparison of different approaches to quantify Staphylococcus aureus cells by real-time quantitative PCR and application of this technique for examination of cheese. Appl. Environ. Microbiol. 67:3122-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jauregui, L. H., J. Higgins, D. Zarlenga, J. P. Dubey, and J. K. Lunney. 2001. Development of a real-time PCR assay for detection of Toxoplasma gondii in pig and mouse tissues. J. Clin. Microbiol. 39:2065-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katakura, K., S. I. Kawazu, T. Naya, et al. 1998. Diagnosis of kala-azar by nested PCR based on amplification of the Leishmania mini-exon gene. J. Clin. Microbiol. 36:2173-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearns, A. M., M. Guiver, V. James, and J. King. 2001. Development and evaluation of a real-time quantitative PCR for the detection of human cytomegalovirus. J. Virol. Methods 95:121-131. [DOI] [PubMed] [Google Scholar]
- 17.Komurian-Pradel, F., G. Paranhos-Baccala, M. Sodoyer, P. Chevallier, B. Mandrand, V. Lotteau, and P. André. 2001. Quantitation of HCV RNA using real-time PCR and fluorimetry. J. Virol. Methods 95:111-119. [DOI] [PubMed] [Google Scholar]
- 18.Lachaud, L., J. Dereure, E. Chabbert, et al. 2000. Optimized PCR using patient blood samples for diagnosis and follow-up of visceral leishmaniasis, with special reference to AIDS patients. J. Clin. Microbiol. 38:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang, T., P. Avé, M. Huerre, G. Milon, and J.-C. Antoine. 2000. Macrophage subsets harbouring Leishmania donovani in spleens of infected BALB/c mice: localization and characterization. Cell. Microbiol. 2:415-430. [DOI] [PubMed] [Google Scholar]
- 20.Launois, P., H. Himmelrich, F. Tacchini-Cottier, G. Milon, and J. Louis. 1999. New insight into the mechanisms underlying Th2 cell development and susceptibility to Leishmania major in BALB/c mice. Microbes Infect. 1:59-64. [DOI] [PubMed] [Google Scholar]
- 21.Lin, M.-H., T.-C. Chen, T.-T. Kuo, C.-C. Tseng, and C.-P. Tseng. 2000. Real-time PCR for quantitative detection of Toxoplasma gondii. J. Clin. Microbiol. 38:4121-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan, J. M. J., K. J. Edwards, N. A. Saunders, and J. Stanley. 2001. Rapid identification of Campylobacter spp. by melting peak analysis of biprobes in real-time PCR. J. Clin. Microbiol. 39:2227-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicolas, L., S. Sidjanski, J.-H. Colle, and G. Milon. 2000. Leishmania major reaches distant cutaneous sites where it persists transiently while persisting durably in the primary dermal site and its draining lymph node: a study in laboratory mice. Infect. Immun. 68:6561-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noyes, H. A., H. Reyburn, J. W. Bailey, and D. Smith. 1998. A nested-PCR-based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan. J. Clin. Microbiol. 36:2877-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietilä, J., Q. He, J. Oksi, and M. K. Viljanen. 2000. Rapid differentiation of Borrelia garinii from Borrelia afzelii and Borrelia burgdorferi sensu stricto by LightCycler fluorescence melting curve analysis of a PCR product of the recA gene. J. Clin. Microbiol. 38:2756-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodgers, M. R., S. J. Popper, and D. F. Wirth. 1990. Amplification of kinetoplast DNA as a tool in the detection and diagnosis of Leishmania. Exp. Parasitol. 71:267-275. [DOI] [PubMed] [Google Scholar]
- 27.Sacks, D. L. 2001. Leishmania-sand fly interactions controlling species-specific vector competence. Cell. Microbiol. 3:189-196. [DOI] [PubMed] [Google Scholar]
- 28.Titus, R. G., M. Marchand, T. Boon, and J. A. Louis. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545-555. [DOI] [PubMed] [Google Scholar]
- 29.Wittwer, C. T., M. G. Herrmann, A. A. Moss, and R. P. Rasmussen. 1997. Continuous fluorescence monitoring of rapid cycle DNA amplification. BioTechniques 22:130-138. [DOI] [PubMed] [Google Scholar]
- 30.Wittwer, C. T., K. M. Ririe, R. V. Andrew, D. A. David, R. A. Gundry, and U. J. Balis. 1997. The LightCyclerTM: a microvolume multisample fluorimeter with rapid temperature control. BioTechniques 22:176-181. [DOI] [PubMed] [Google Scholar]
- 31.Woo, T. H. S., B. K. C. Patel, M. Cinco, L. D. Smythe, M. A. Norris, M. L. Symonds, M. F. Dohnt, and J. Piispanen. 1999. Identification of Leptospira biflexa by real-time homogeneous detection of rapid cycle PCR product. J. Microbiol. Methods 35:23-30. [DOI] [PubMed] [Google Scholar]