Abstract
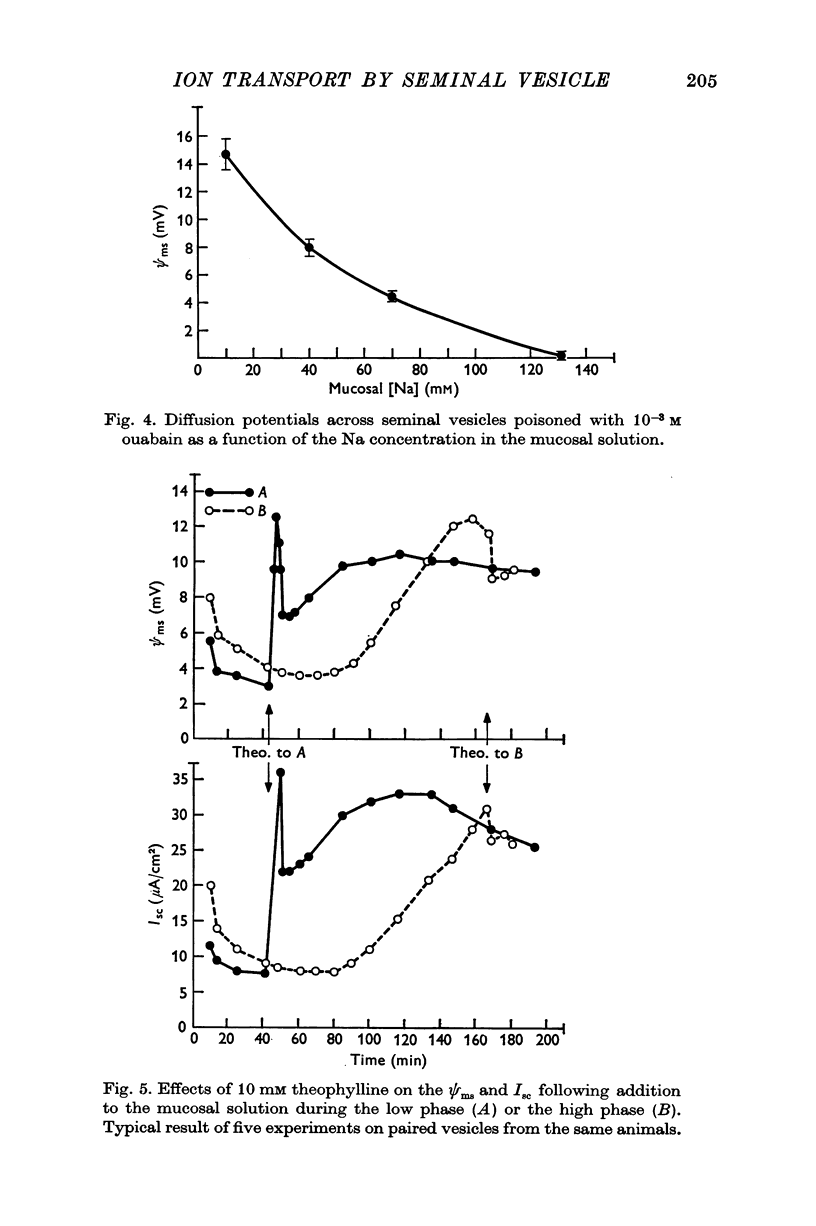
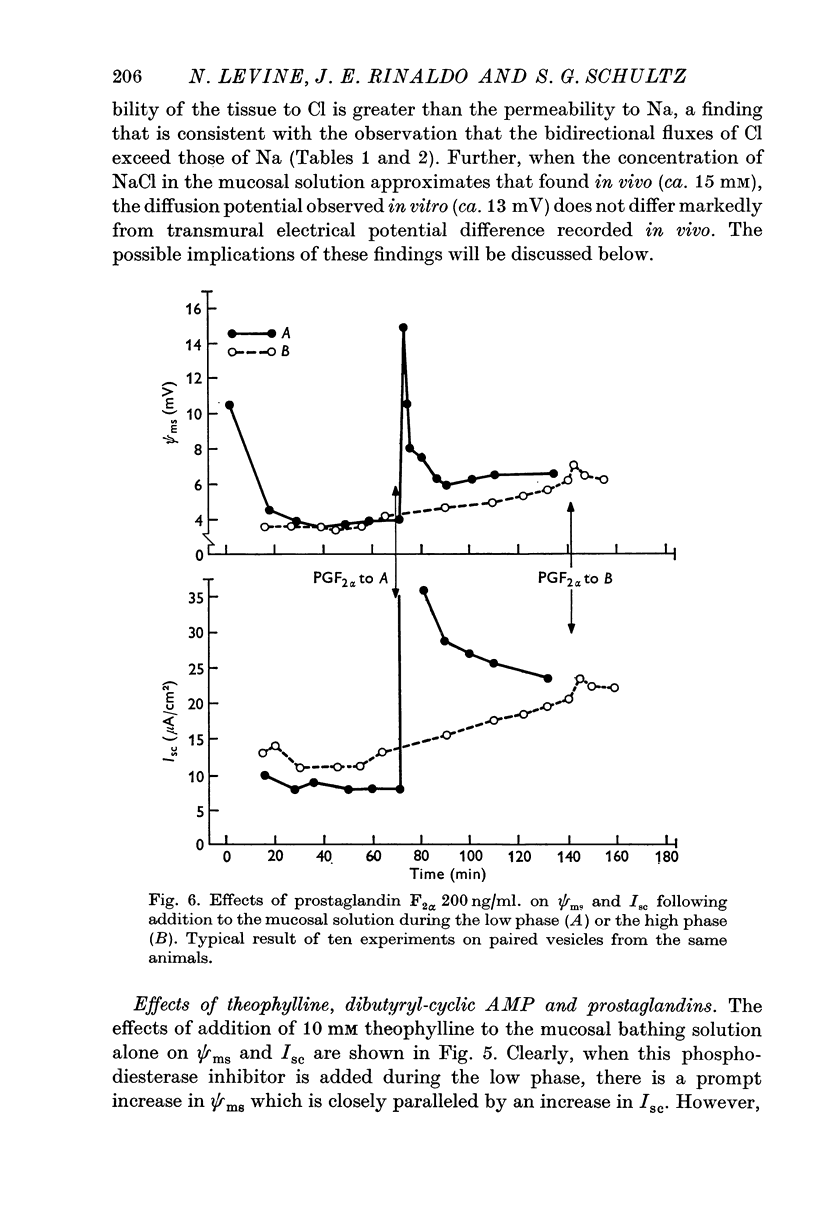
1. The guinea-pig seminal vesicle in vivo is characterized by a transmural electrical potential difference of approximately 11 mV with the lumen electrically negative with respect to the interstitial fluid. The concentrations of Na, Cl and K in the vesicular fluid are 13, 15, and 0-4 mM, respectively. 2. When mounted as a flat sheet in a short-circuit apparatus, guinea-pig seminal vesicles initially undergo a decline in the transmural electrical potential difference and short-circuit current ('low phase') followed by a spontaneous increase in these parameters ('high phase'). 3. During the low phase, net C1 movements across the tissue do not differ significantly from zero, and there is a small 'residual' current that is unaccounted for. 4. During the high phase, there is a significant active C1 secretion into the mucosal solution, not detectable net movement of Na and an unaccounted for or 'residual' current that is equal to that found in the low phase. 5. Theophylline, dibutyryl-3'-5' cyclic adenosinemonophosphate,prostaglandin E1 and prostaglandin F2alpha markedly stimulate the transmural electrical potential difference and short-circuit current during the low phase, but have no effect when added to the bathing solution during the high phase. 6. Diffusion potentials determined across in vitro seminal vesicles suggest that the spontaneous transmural electrical potential difference in vivo may be attributable to the large ionic asymmetries between the vesicular fluid and the plasma. 7. It is postulated that two phases are involved in the elaboration of seminal vesicular fluid. The initial phase, following emptying of the vesicle, is characterized by the secretion of electrolytes, organic molecules and water. Active C1 secretion presumably regulated by intracellular cyclic adenosinemonophosphate and/or prostaglandins may be the driving force for this initial secretion of electrolytes. Following this secretory phase, electrolytes and water are reabsorbed, thereby concentrating the organic components in the vesicular reservoir.
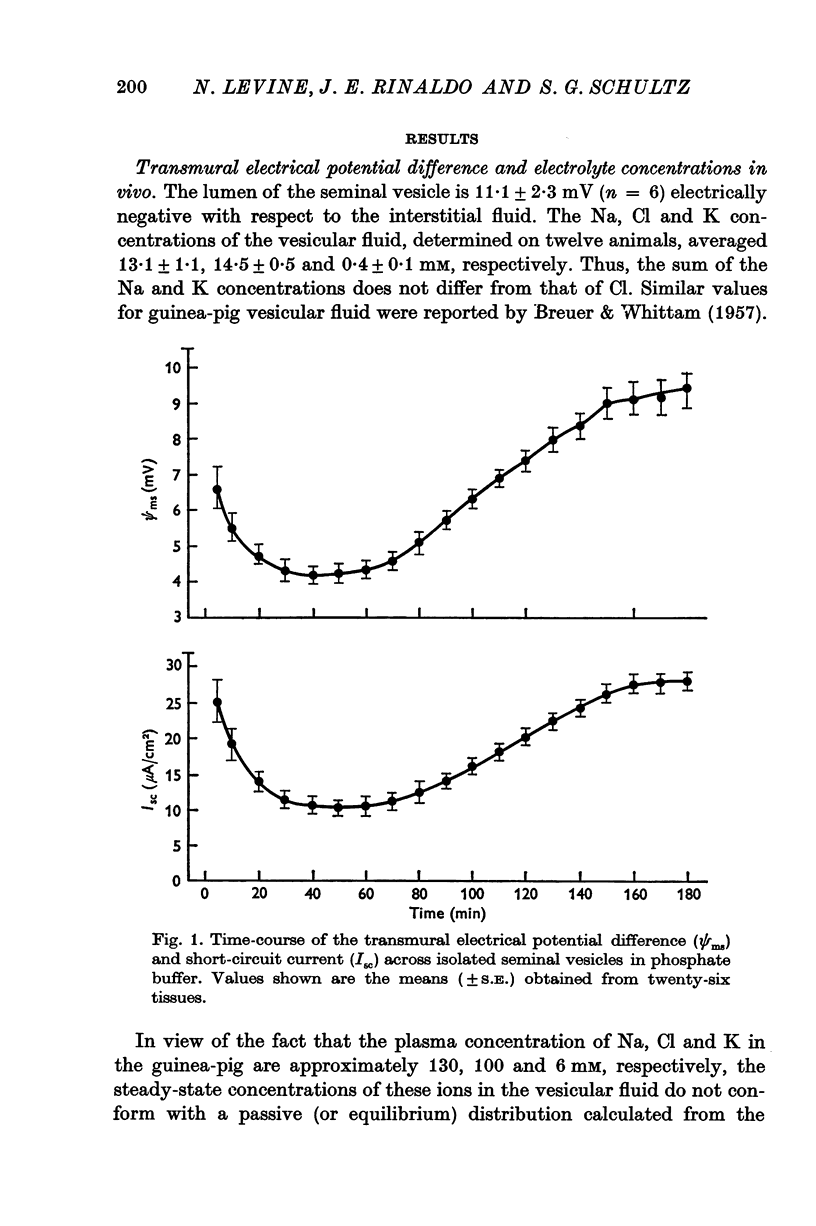
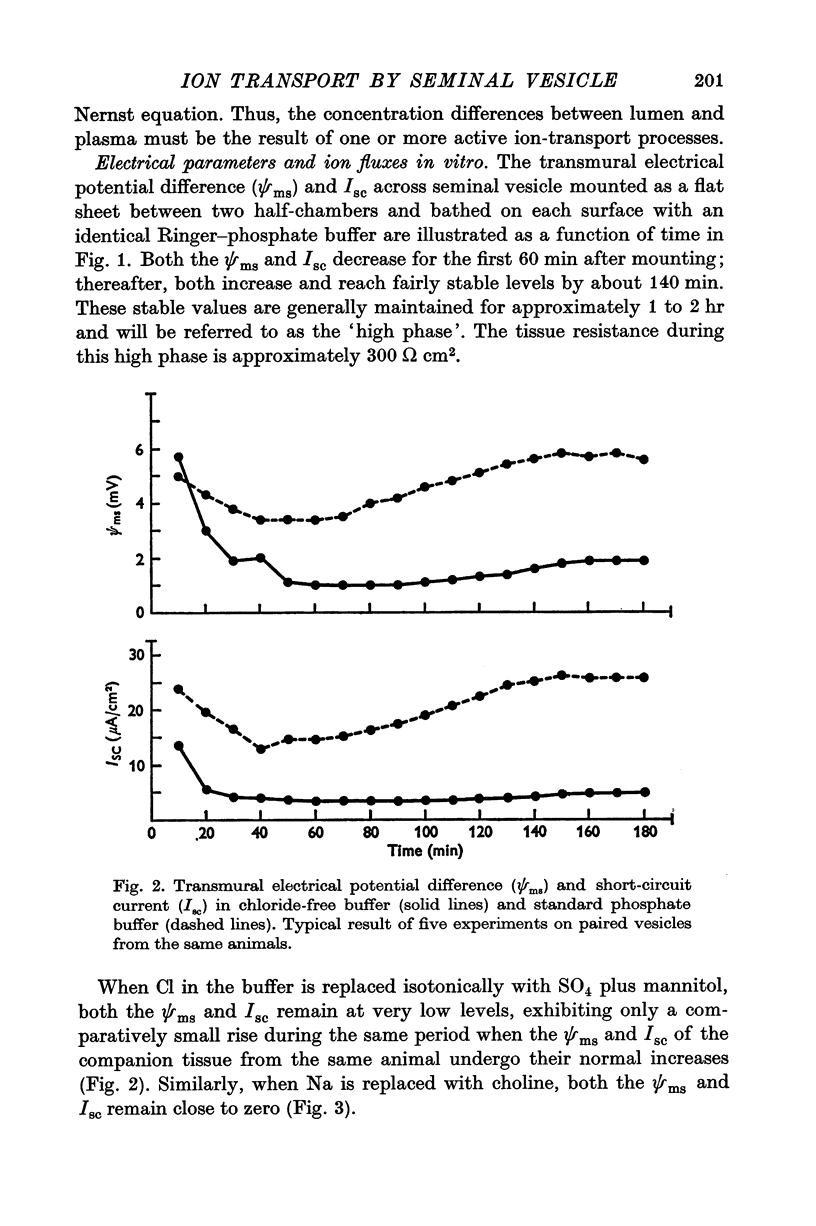
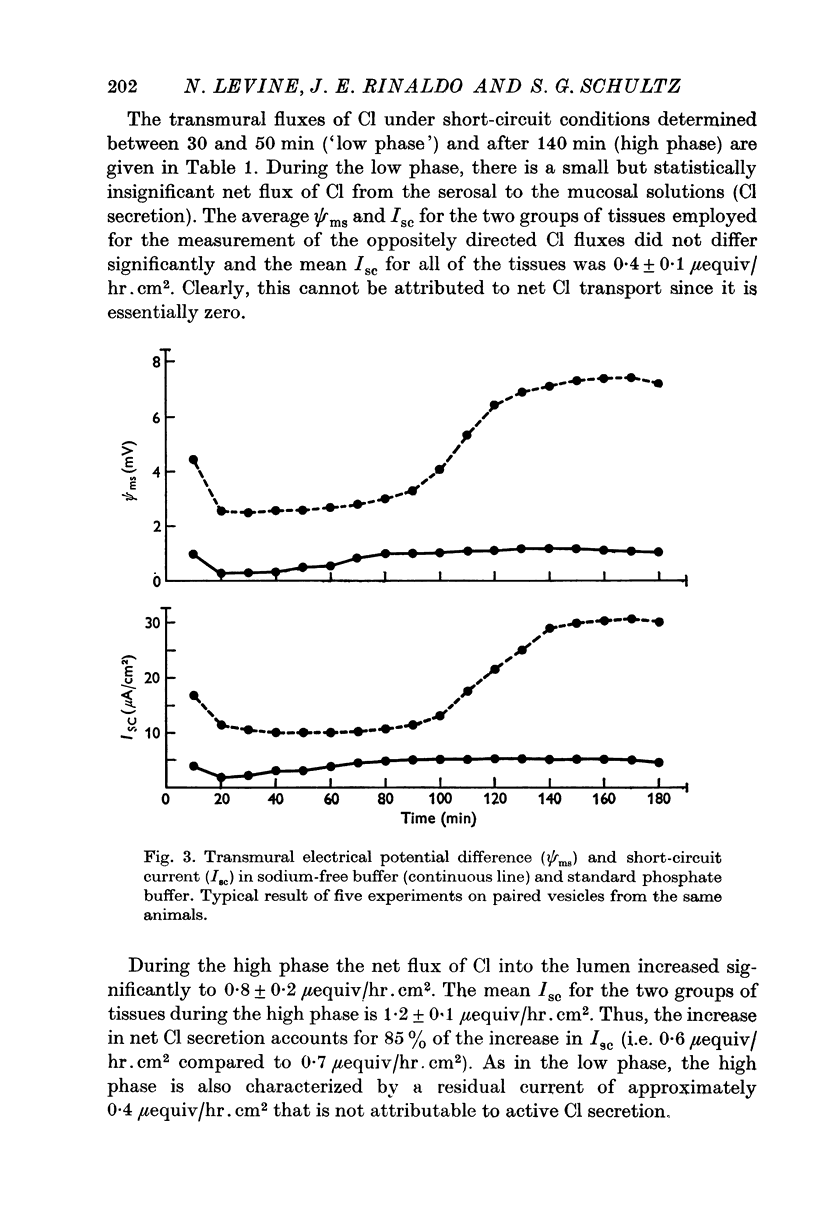
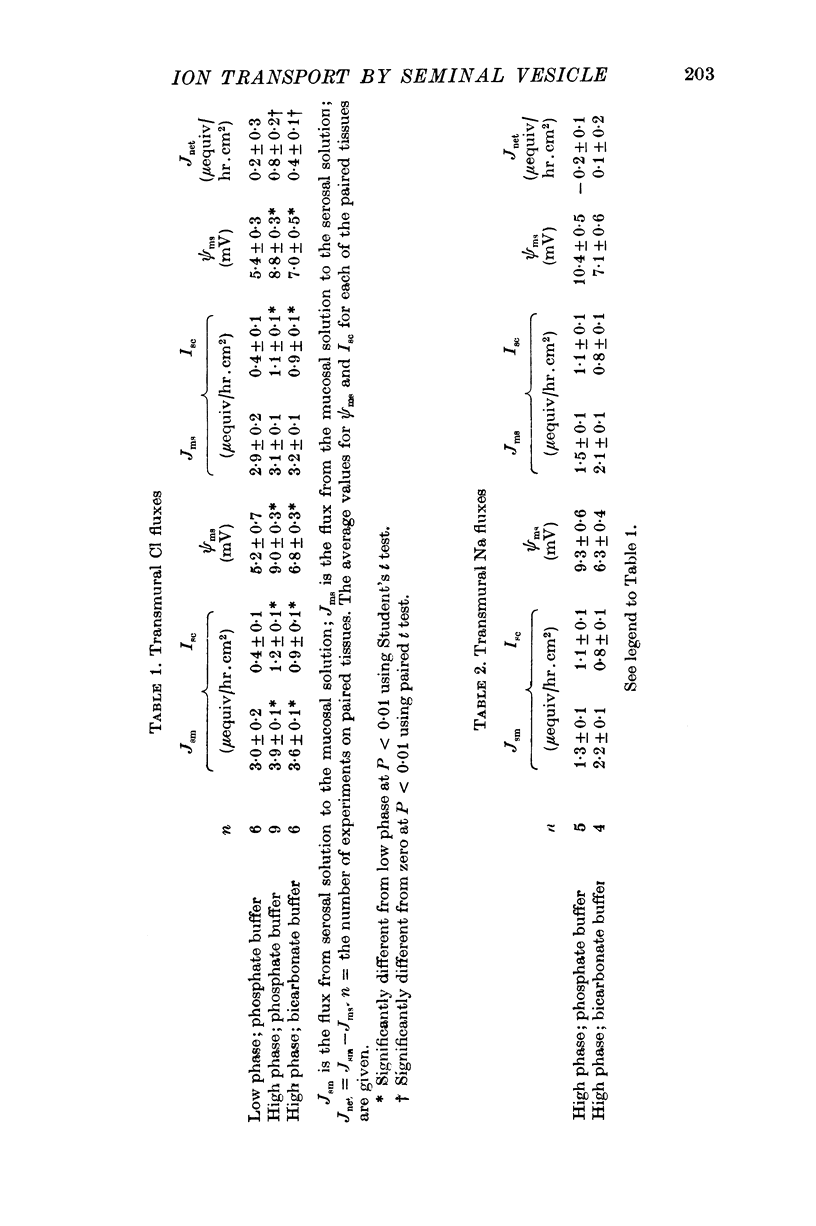
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREUER H. J., WHITTAM R. Ion movements in seminal vesicle mucosa. J Physiol. 1957 Jan 23;135(1):213–225. doi: 10.1113/jphysiol.1957.sp005705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton W. J., Brinster R. L. Active chloride transport in the isolated rabbit oviduct. Am J Physiol. 1971 Aug;221(2):658–661. doi: 10.1152/ajplegacy.1971.221.2.658. [DOI] [PubMed] [Google Scholar]
- Field M. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3', 5'-AMP. Am J Physiol. 1971 Oct;221(4):992–997. doi: 10.1152/ajplegacy.1971.221.4.992. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Nigon K., Alonso D. Adenosine-3',5'-monophosphate: intracellular mediator for methyl xanthine stimulation of gastric secretion. Gastroenterology. 1969 Oct;57(4):377–384. [PubMed] [Google Scholar]
- Levine N., Marsh D. J. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol. 1971 Mar;213(3):557–570. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood D. H., Williams-Ashman H. G. Cholinergic-stimulated alkaline phosphatase secretion and phospholipid synthesis in guinea pig seminal vesicles. J Cell Physiol. 1971 Feb;77(1):7–15. doi: 10.1002/jcp.1040770103. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck R. R., Setchell B. P., Waites G. M., Young J. A. The composition of fluid collected by micropuncture and catheterization from the seminiferous tubules and rete testis of rats. Pflugers Arch. 1970;318(3):225–243. doi: 10.1007/BF00593663. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- WHITTAM R., BREUER H. J. Ion transport and metabolism in slices of guinea-pig seminal-vesicle mucosa. Biochem J. 1959 Aug;72:638–646. doi: 10.1042/bj0720638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadunaisky J. A., Lande M. A., Chalfie M., Neufeld A. H. Ion pumps in the cornea and their stimulation by epinephrine and cyclic-AMP. Exp Eye Res. 1973 May 10;15(5):577–584. doi: 10.1016/0014-4835(73)90069-9. [DOI] [PubMed] [Google Scholar]


