Abstract
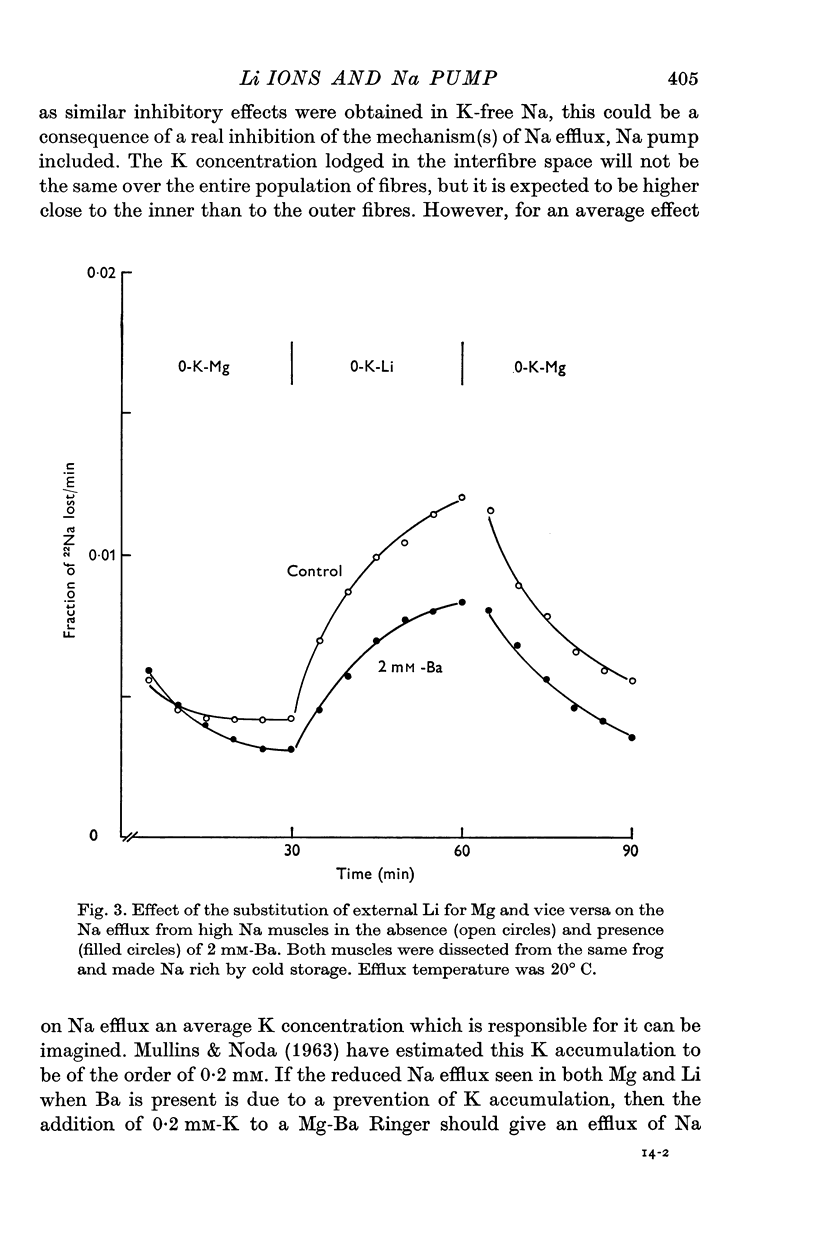
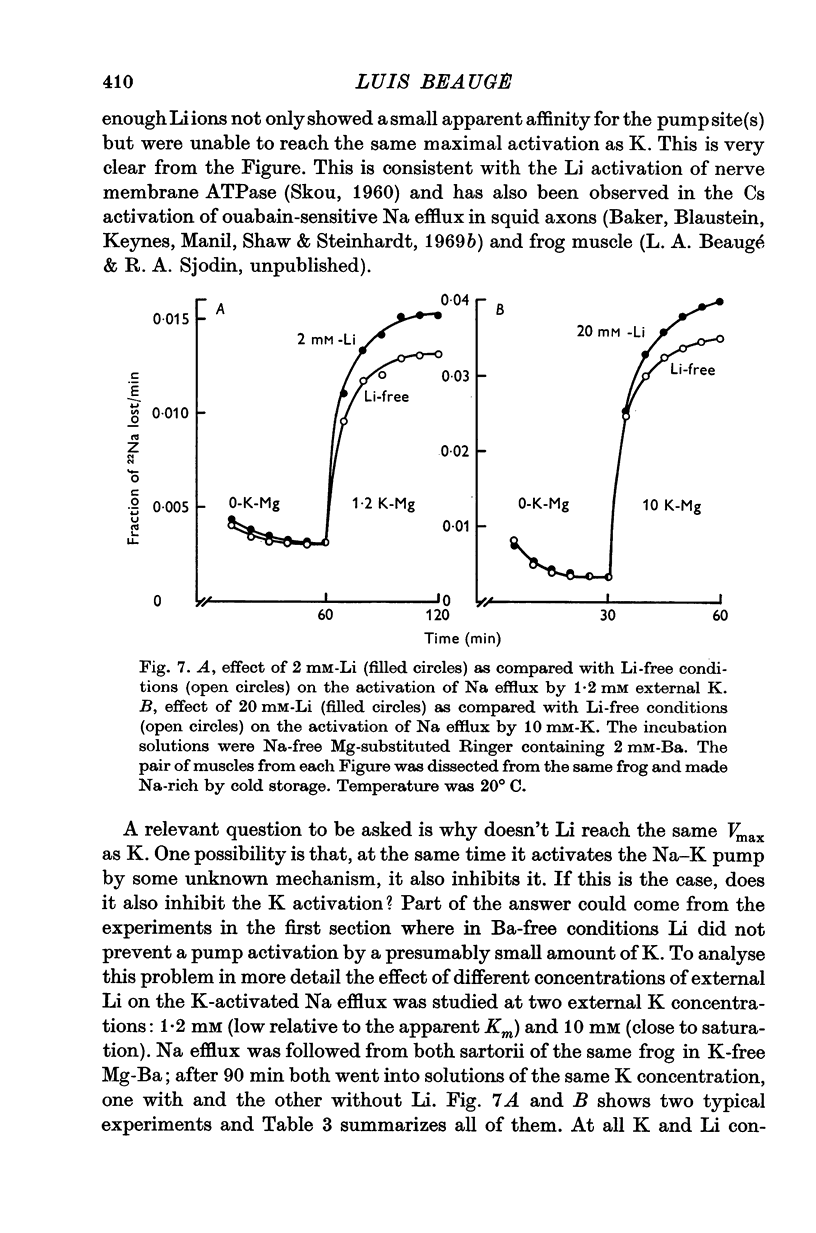
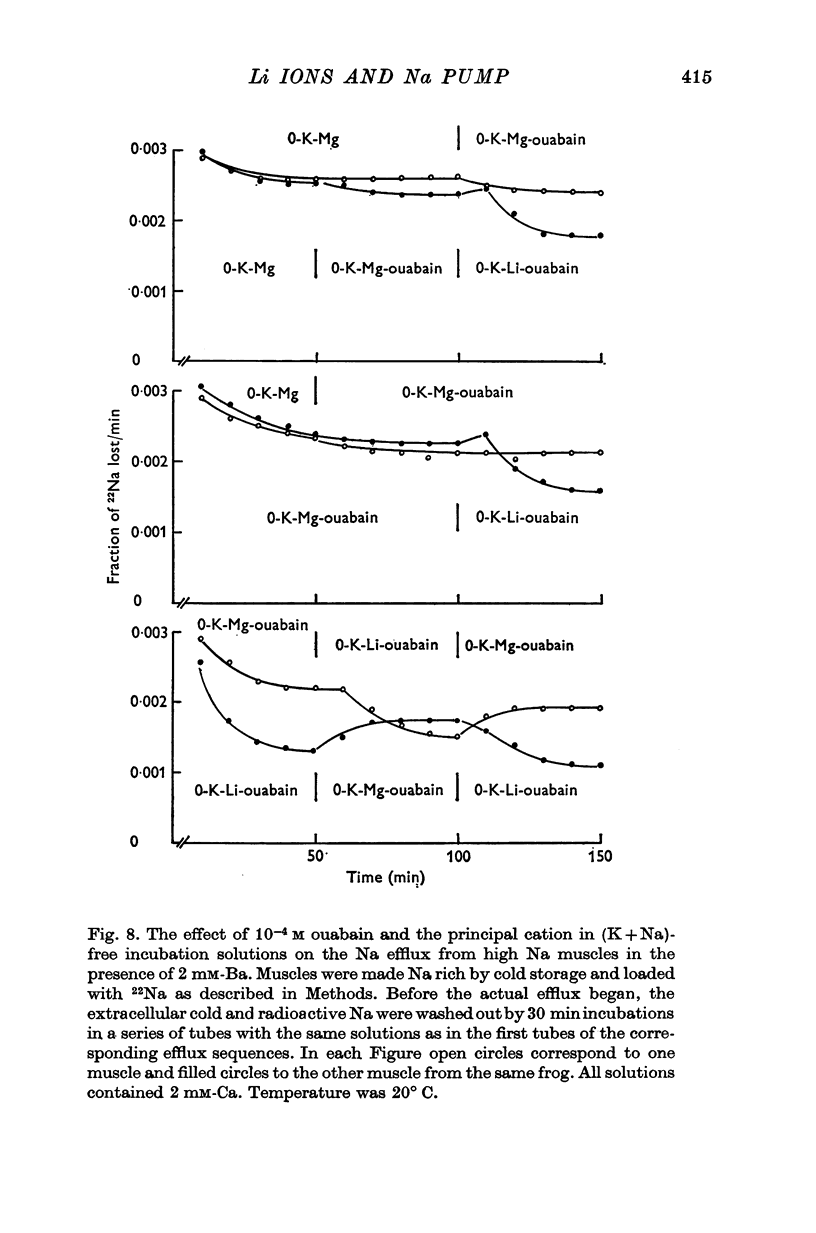
1. The effects of external Li on Na and K efflux as well as those on K influx were studied in high Na muscles from Rana pipiens. 2. In the absence of external Ba, substitution of K-free Li for K-free Mg resulted in an increase of both Na and K efflux. The additional of ouabain produced an inhibition of Na efflux and at the same time a marked increase in the efflux of K. 3. K permeability was greatly reduced by adding 2 mM-Ba to the incubation solutions. Under these conditions, Li gave rise to a ouabain sensitive Na efflux which was 57% of that in the absence of Ba. On the other hand, the efflux of K was only slightly increased and was not affected further by ouabain. 4. The activation curves of Na efflux against the stimulating cation concentration in Na-free Mg-Ba Ringer followed a more or less hyperbolic function for both K and Li. While half-maximal activation was attained at higher concentrations of Li than of K, the maximal efflux in Li was smaller than in K. 5. The extra Na efflux produced by K was increased when Li was added to the media. This increment was not a simple additive effect and was independent of the Li concentration. In addition, at some concentrations Li increased the ouabain-sensitive K influx, whereas at others it reduced it. 6. Reversible changes in membrane permeability to monovalent cations were accomplished by incubating the muscles in the presence of Nystatin, 50 mug/ml. When internal K was reduced to values around 1-2 mumole/g (using Li as a replacement), thus minimizing the possibility of K leaking out of the cells, both Ko and Lio were able to promote a ouabain-sensitive extra efflux of Na. 7. The residual Na efflux in (K+Na)-free solutions was not affected by the removal of Ca from the media in either Mg or Li solutions, both in the absence and the presence of Ba. On the other hand, the values for the residual efflux were higher in Mg (0-00228 min-1) than in Li (0.00135 min-1). 8. These results fully support the notion that Li ions have a K-like activating action on the Na pump in muscles. In addition, they suggest that some other kind of interaction may exist between Li and the Na-K pump.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. A., Ortiz O. Further evidence for a potassium-like action of lithium ions on sodium efflux in frog skeletal muscle. J Physiol. 1972 Nov;226(3):675–697. doi: 10.1113/jphysiol.1972.sp010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. A., Ortiz O. Sodium fluxes in rat red blood cells in potassium-free solutions. Evidences for facilitated diffusion. J Membr Biol. 1973;13(2):165–184. doi: 10.1007/BF01868226. [DOI] [PubMed] [Google Scholar]
- Beaugé L. A., Ortíz O. Sodium and rubidium fluxes in rat red blood cells. J Physiol. 1971 Nov;218(3):533–549. doi: 10.1113/jphysiol.1971.sp009632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. A., Sjodin R. A. The dual effect of lithium ions on sodium efflux in skeletal muscle. J Gen Physiol. 1968 Sep;52(3):408–423. doi: 10.1085/jgp.52.3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass A., Dalmark M. Equilibrium dialysis of ions in nystatin-treated red cells. Nat New Biol. 1973 Jul 11;244(132):47–49. doi: 10.1038/newbio244047a0. [DOI] [PubMed] [Google Scholar]
- Chaplain R. A. The effect of intracellular potassium ions on active sodium efflux in frog sartorius muscle. Experientia. 1973;29(7):794–795. doi: 10.1007/BF01946290. [DOI] [PubMed] [Google Scholar]
- Glen A. I., Bradbury M. W., Wilson J. Stimulation of the sodium pump in the red blood cell by lithium and potassium. Nature. 1972 Oct 13;239(5372):399–401. doi: 10.1038/239399a0. [DOI] [PubMed] [Google Scholar]
- Horowicz P., Taylor J. W., Waggoner D. M. Fractionation of sodium effux in frog sartorius muscles by strophanthidin and removal of external sodium. J Gen Physiol. 1970 Mar;55(3):401–425. doi: 10.1085/jgp.55.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Steinhardt R. A. The components of the sodium efflux in frog muscle. J Physiol. 1968 Oct;198(3):581–599. doi: 10.1113/jphysiol.1968.sp008627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L., Hardy M. A., Jr, Ellory J. C. The uncoupled extrusion of Na+ through the Na+ pump. Biochim Biophys Acta. 1973 Oct 11;323(2):251–266. doi: 10.1016/0005-2736(73)90149-1. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J., NODA K. THE INFLUENCE OF SODIUM-FREE SOLUTIONS ON THE MEMBRANE POTENTIAL OF FROG MUSCLE FIBERS. J Gen Physiol. 1963 Sep;47:117–132. doi: 10.1085/jgp.47.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaghey P. D., Maizels M. Cation exchanges of lactose-treated human red cells. J Physiol. 1962 Aug;162(3):485–509. doi: 10.1113/jphysiol.1962.sp006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Potassium fluxes in dialyzed squid axons. J Gen Physiol. 1969 Jun;53(6):704–740. doi: 10.1085/jgp.53.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post R. L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6530–6540. [PubMed] [Google Scholar]
- Sjodin R. A., Beaugé L. A. An analysis of the leakages of sodium ions into and potassium ions out of striated muscle cells. J Gen Physiol. 1973 Feb;61(2):222–250. doi: 10.1085/jgp.61.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H. Transport of ions across cellular membranes. Physiol Rev. 1949 Apr;29(2):127–155. doi: 10.1152/physrev.1949.29.2.127. [DOI] [PubMed] [Google Scholar]


