Abstract
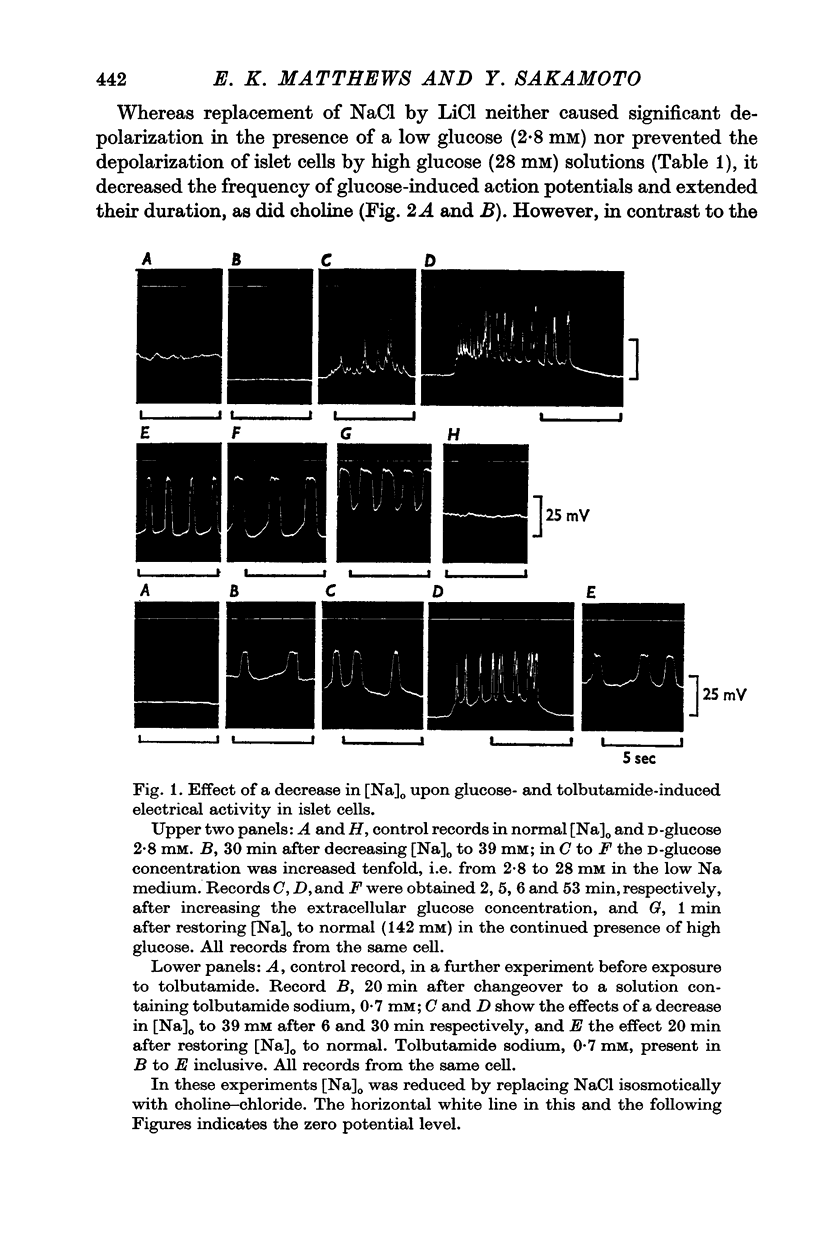
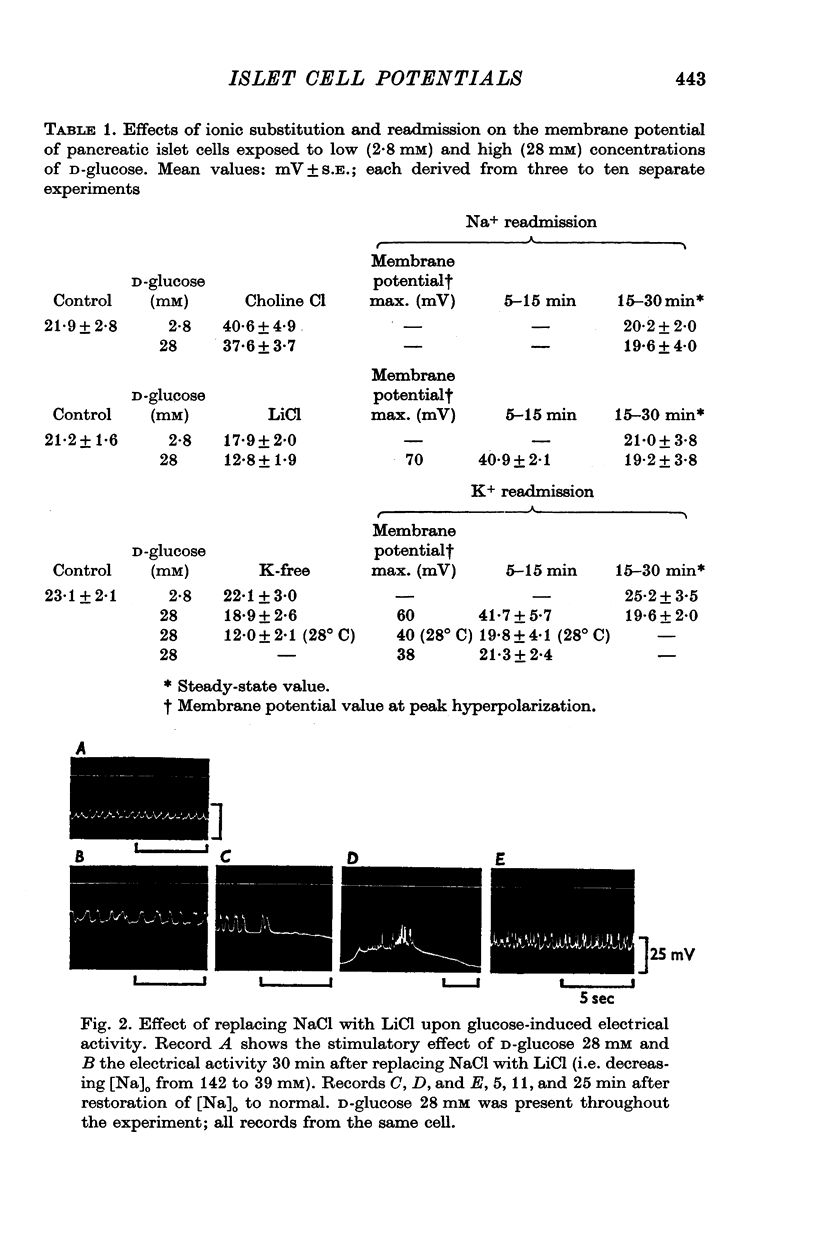
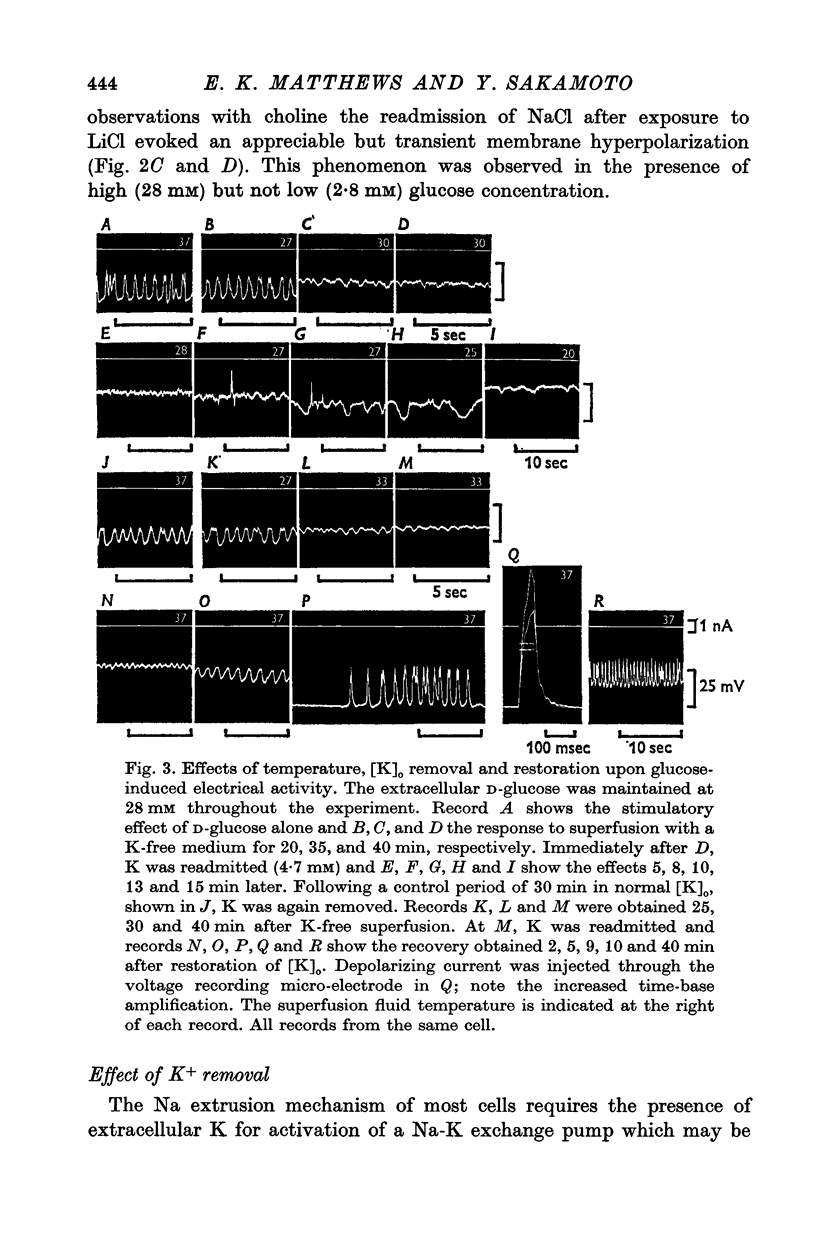
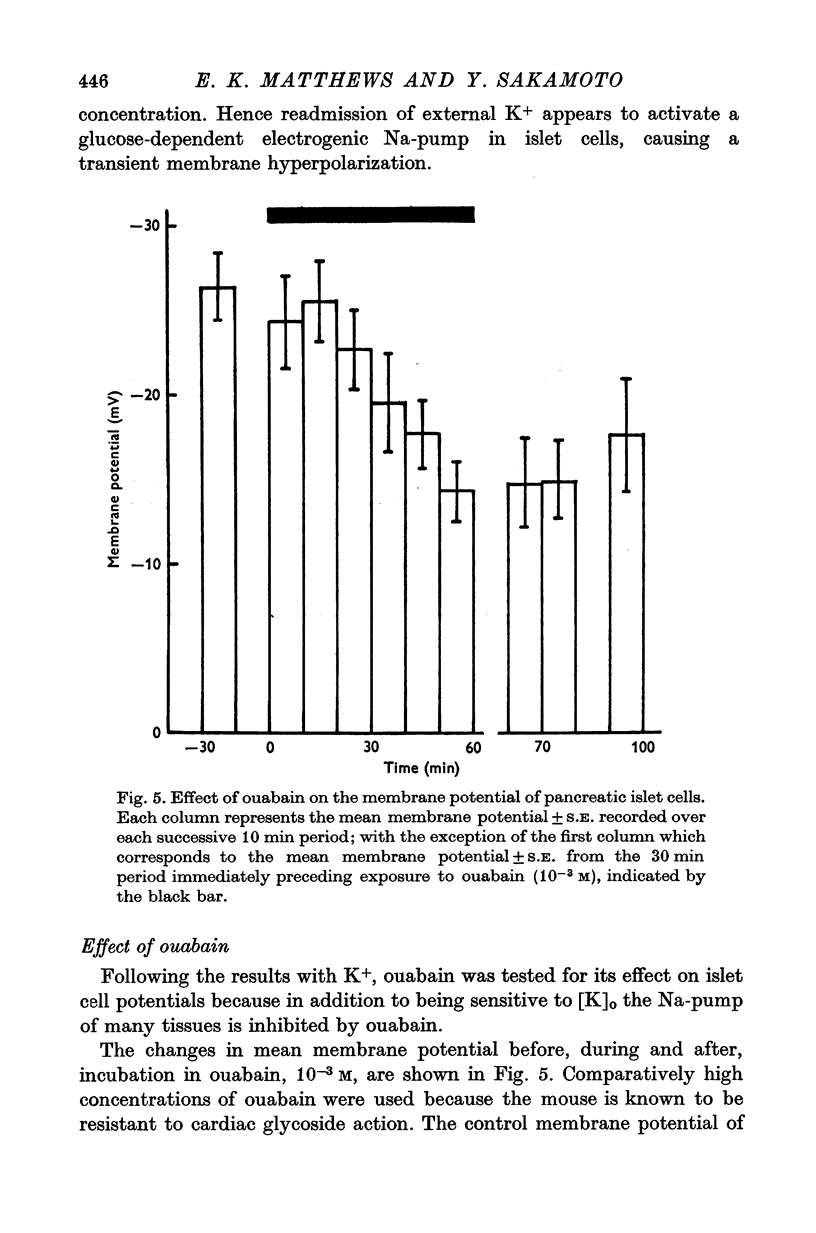
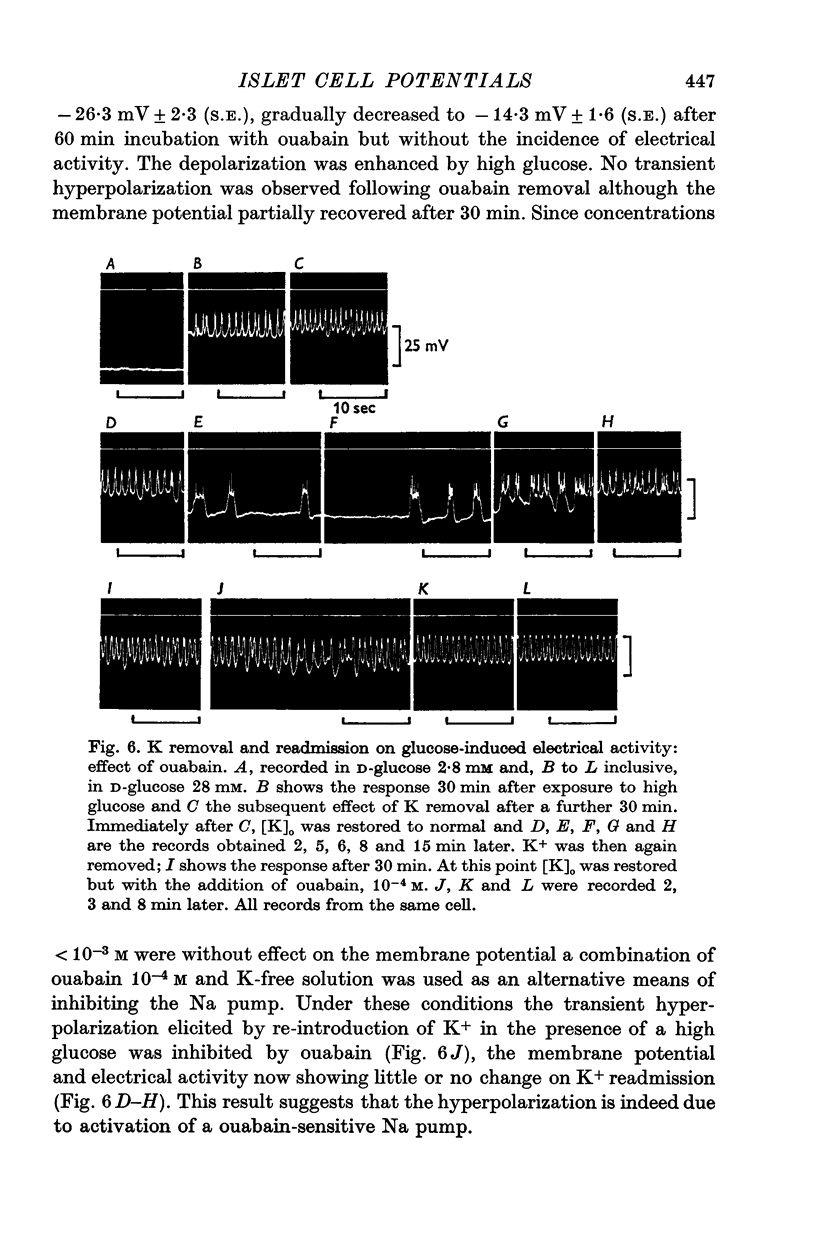
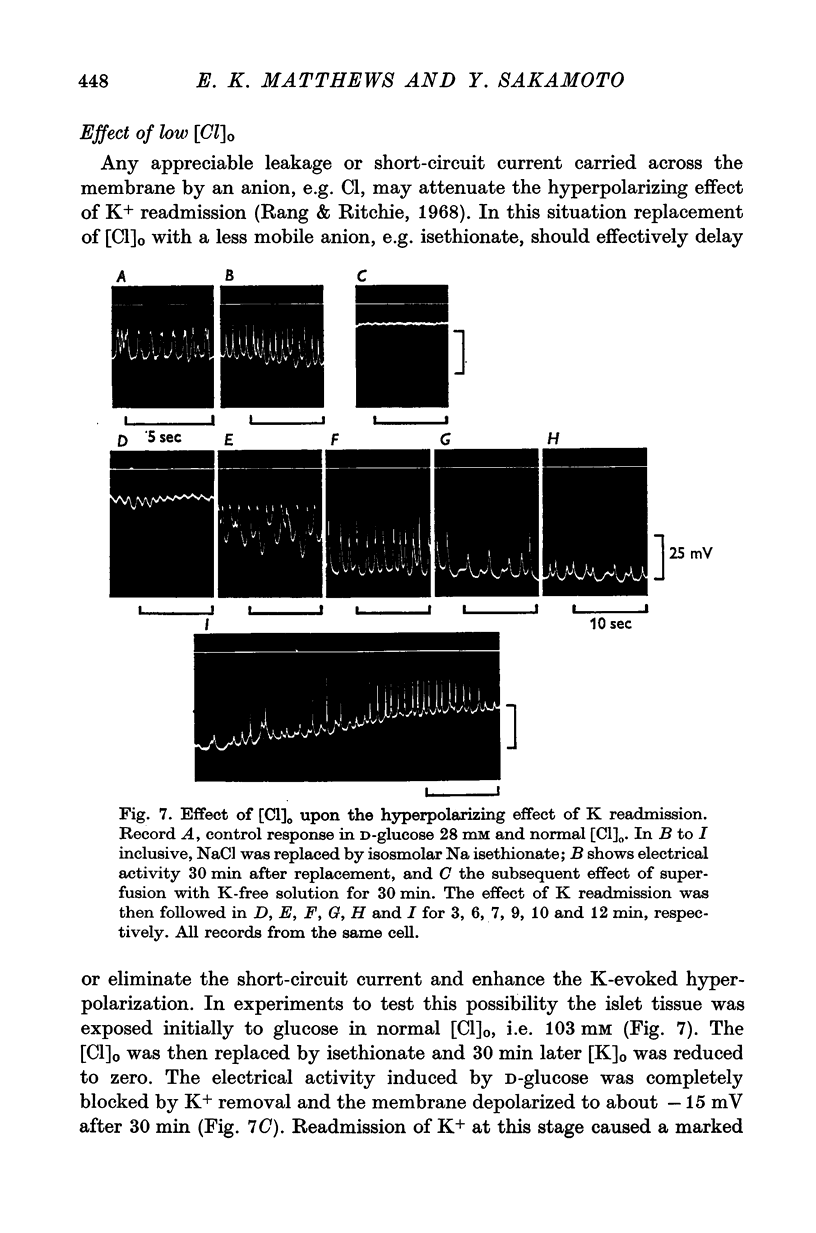
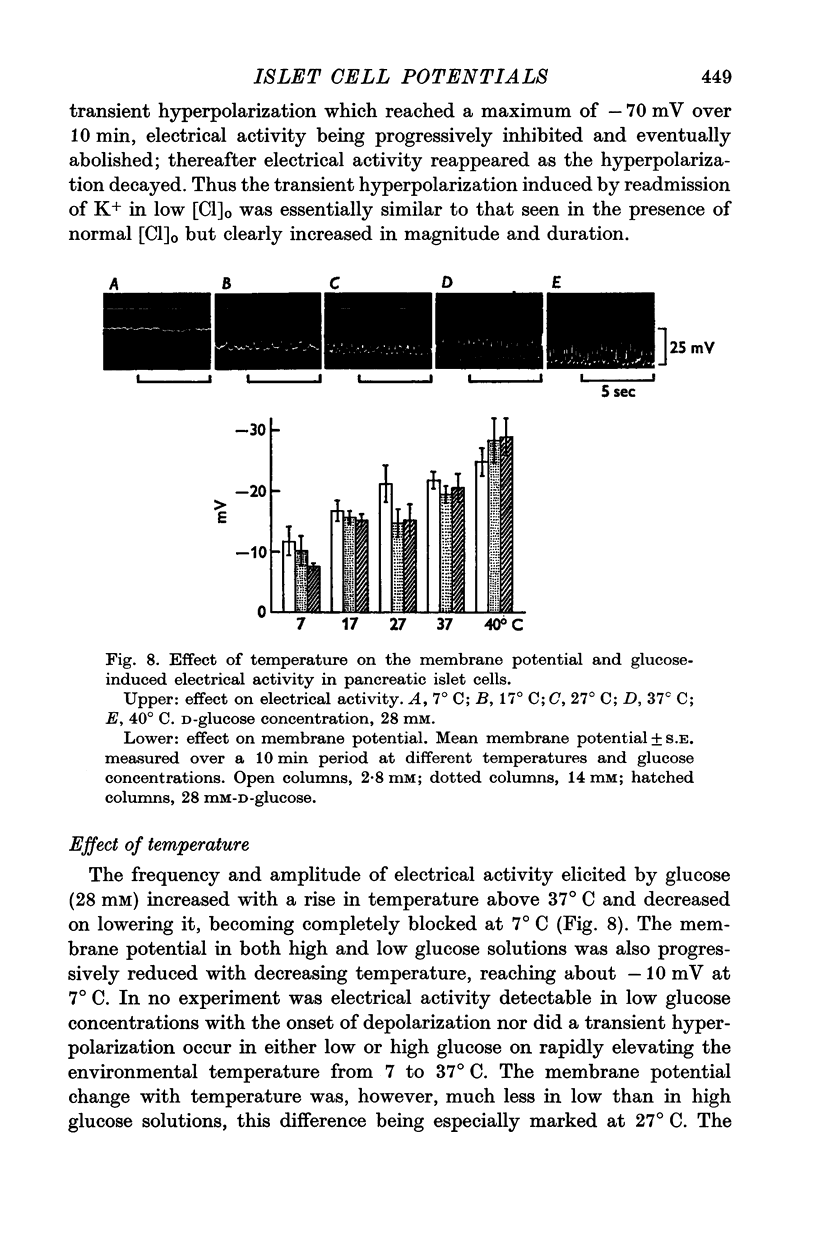
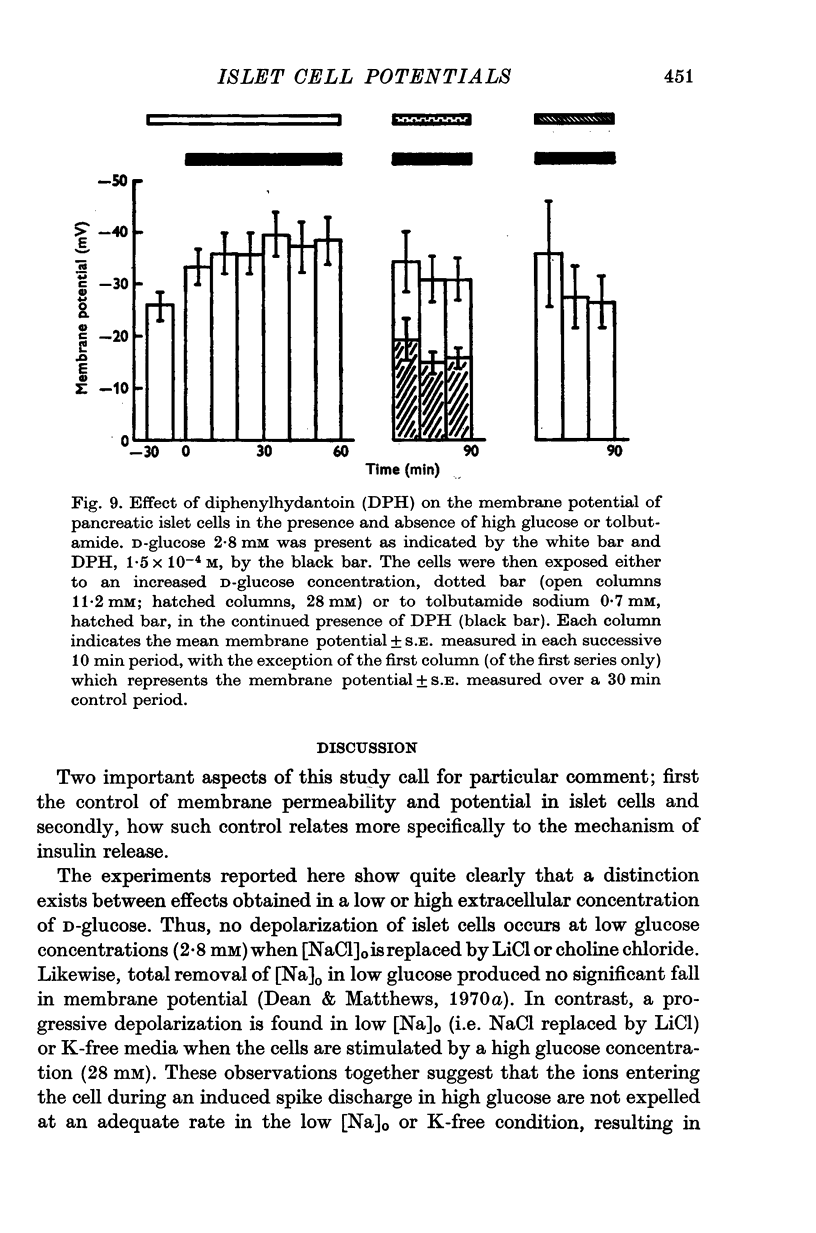
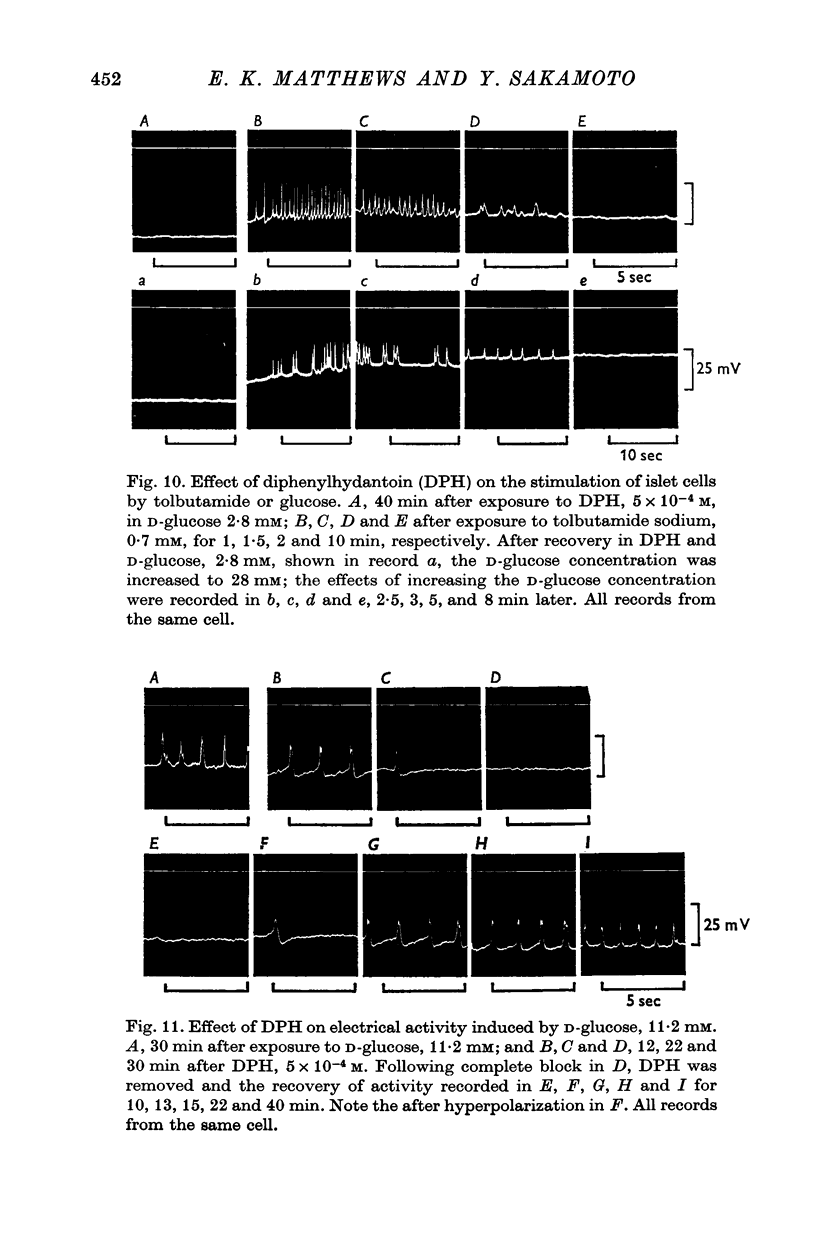
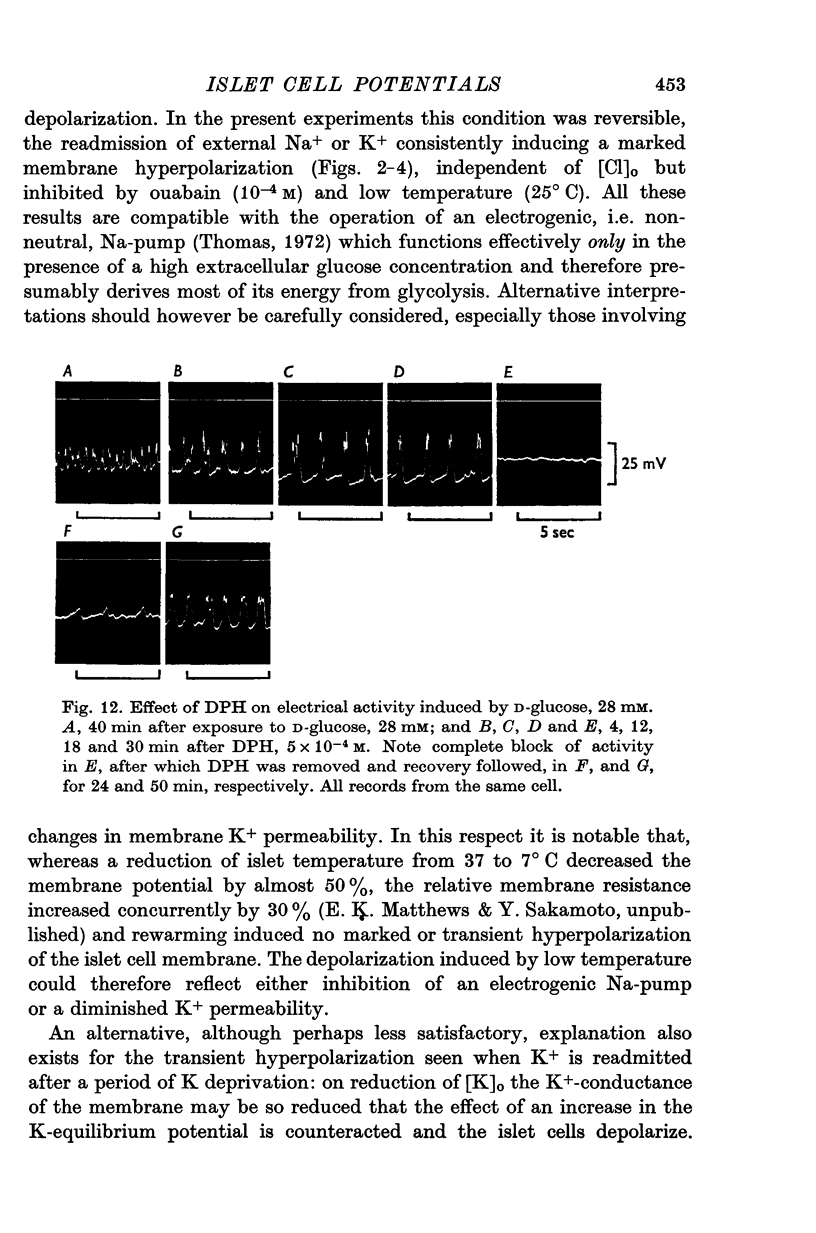
1. Responses of the membrane electrical characteristics of mouse pancreatic islet cells to ionic environmental changes have been used to assess the role of [Na]0 and [K]0 in the control of membrane potential, i.e. by electrodiffusion or via an electrogenic sodium pump. Islet cell electrical properties were measured in vitro with intracellular glass micro-electrodes. 2. Substitution of LiCl for extracellular NaCl did not change the islet cell membrane potential significantly in low (2.8 mM) glucose solutions, but readmission of NaCl caused a transient hyperpolarization (membrane potential maximum: -70 mV) in high glucose; when choline chloride was substituted for NaCl no hyperpolarization was observed on NaCl re-admission. 3. Superfusion with K-free solution gave no marked change in membrane potential during 30 min incubation in either low (2-8 mM) or high (28 mM) glucose concentrations but longer periods of exposure to K-free solutions caused progressive depolarization. 4. Readmission of K+ induced a transient hyperpolarization of up to 30 mV magnitude and 10 min duration in the presence of high (28 mM) but not low glucose (2-8 mM) concentrations. At the level of maximum hyperpolarization the membrane potential reached -60 mV, the electrical activity induced by the high glucose concentration being concurrently completely blocked. Replacement of [Cl]0 by isethionate accentuated these effects. 5. Ouabain, 10(-3) M, or a decrease in temperature from 37 to 7 degrees C depolarized the islet cells and blocked the transient hyperpolarization induced by readmission of K+. 6. Diphenylhydantoin, 1-5 times 10(-4) M, caused a significant hyperpolarization of the islet cells in low glucose (2-8 mM) and inhibited the electrical activity induced by high glucose (28 mM) or tolbutamide (0-7 mM). 7. It is concluded from these results that both an electrogenic and ionic component contribute to the membrane potential of the mouse pancreatic islet cell although electrodiffusional control normally predominates; acceleration of the Na-K exchange pump by diphenylhydantoin inhibits glucose-induced electrical activity. These findings are discussed in relation to the permeability characteristics of the islet cell membrane and the mechanism of insulin release.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broeckaert I. Effect of tri-hydroxymethyl-aminomethane (Tris) on insulin secretion in vitro. Metabolism. 1970 Dec;19(12):1011–1013. doi: 10.1016/0026-0495(70)90023-5. [DOI] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970 Sep;210(2):265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970 Sep;210(2):255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M. Ultrastructural morphometry of the pancreatic -cell. Diabetologia. 1973 Apr;9(2):115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- Fedynskyj N. M., Beck L. V. Tris (hydroxymethyl) aminomethane (THAM) induced stimulation of insulin release by islets of Langerhans previously isolated from rat pancreas. Diabetes. 1970 Aug;19(8):559–562. doi: 10.2337/diab.19.8.559. [DOI] [PubMed] [Google Scholar]
- Fertziger A. P., Liuzzi S. E., Dunham P. B. Diphenylhydantoin (dilantin): stimulation of potassium inflex in lobster axons. Brain Res. 1971 Oct 29;33(2):592–596. doi: 10.1016/0006-8993(71)90146-6. [DOI] [PubMed] [Google Scholar]
- Festoff B. W., Appel S. H. Effect of diphenylhydantoin on synaptosome sodium-potassium-ATPase. J Clin Invest. 1968 Dec;47(12):2752–2758. doi: 10.1172/JCI105956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A. E., HUTTER O. F., NOBLE D. Current-voltage relations of Purkinje fibres in sodium-deficient solutions. J Physiol. 1963 Apr;166:225–240. doi: 10.1113/jphysiol.1963.sp007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. Cations and the secretion of insulin from rabbit pancreas in vitro. J Physiol. 1968 Nov;199(1):177–187. doi: 10.1113/jphysiol.1968.sp008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. The role of sodium and potassium in insulin secretion from rabbit pancreas. J Physiol. 1968 Feb;194(3):725–743. doi: 10.1113/jphysiol.1968.sp008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretagogues. Effects of calcium and sodium on glucose metabolism and insulin release. Biochem J. 1974 Jan;138(1):33–45. doi: 10.1042/bj1380033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizer J. S., Vargas-Gordon M., Brendel K., Bressler R. The in vitro inhibition of insulin secretion by diphenylhydantoin. J Clin Invest. 1970 Oct;49(10):1942–1948. doi: 10.1172/JCI106413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark A. [Isolated mouse islets as a model for studying insulin release]. Acta Diabetol Lat. 1971 Jul-Aug;8(4):649–679. doi: 10.1007/BF01550894. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Sakamoto Y. Electrical characteristics of pancreatic islet cells. J Physiol. 1975 Mar;246(2):421–437. doi: 10.1113/jphysiol.1975.sp010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K. [Electrical activity in islet cells and insulin secretion]. Acta Diabetol Lat. 1970 Sep;7 (Suppl 1):83–101. [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. Cations and the secretion of insulin. Biochim Biophys Acta. 1968 Jan 3;150(1):165–167. doi: 10.1016/0005-2736(68)90022-9. [DOI] [PubMed] [Google Scholar]
- Pincus J. H. Diphenylhydantoin and ion flux in lobster nerve. Arch Neurol. 1972 Jan;26(1):4–10. doi: 10.1001/archneur.1972.00490070022003. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson P. D., Crane P. O. Diphenylhydantoin and movement of radioactive sodium into electrically stimulated cerebral slices. Biochem Pharmacol. 1972 Nov 1;21(21):2899–2905. doi: 10.1016/0006-2952(72)90214-6. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- VASSALLE M. CARDIAC PACEMAKER POTENTIALS AT DIFFERENT EXTRA-AND INTRACELLULAR K CONCENTRATIONS. Am J Physiol. 1965 Apr;208:770–775. doi: 10.1152/ajplegacy.1965.208.4.770. [DOI] [PubMed] [Google Scholar]
- WOODBURY D. M. Effect of diphenylhydantoin on electrolytes and radiosodium turnover in brain and other tissues of normal, hyponatremic and postictal rats. J Pharmacol Exp Ther. 1955 Sep;115(1):74–95. [PubMed] [Google Scholar]
- Williams J. A. Origin of transmembrane potentials in non-excitable cells. J Theor Biol. 1970 Aug;28(2):287–296. doi: 10.1016/0022-5193(70)90056-1. [DOI] [PubMed] [Google Scholar]


