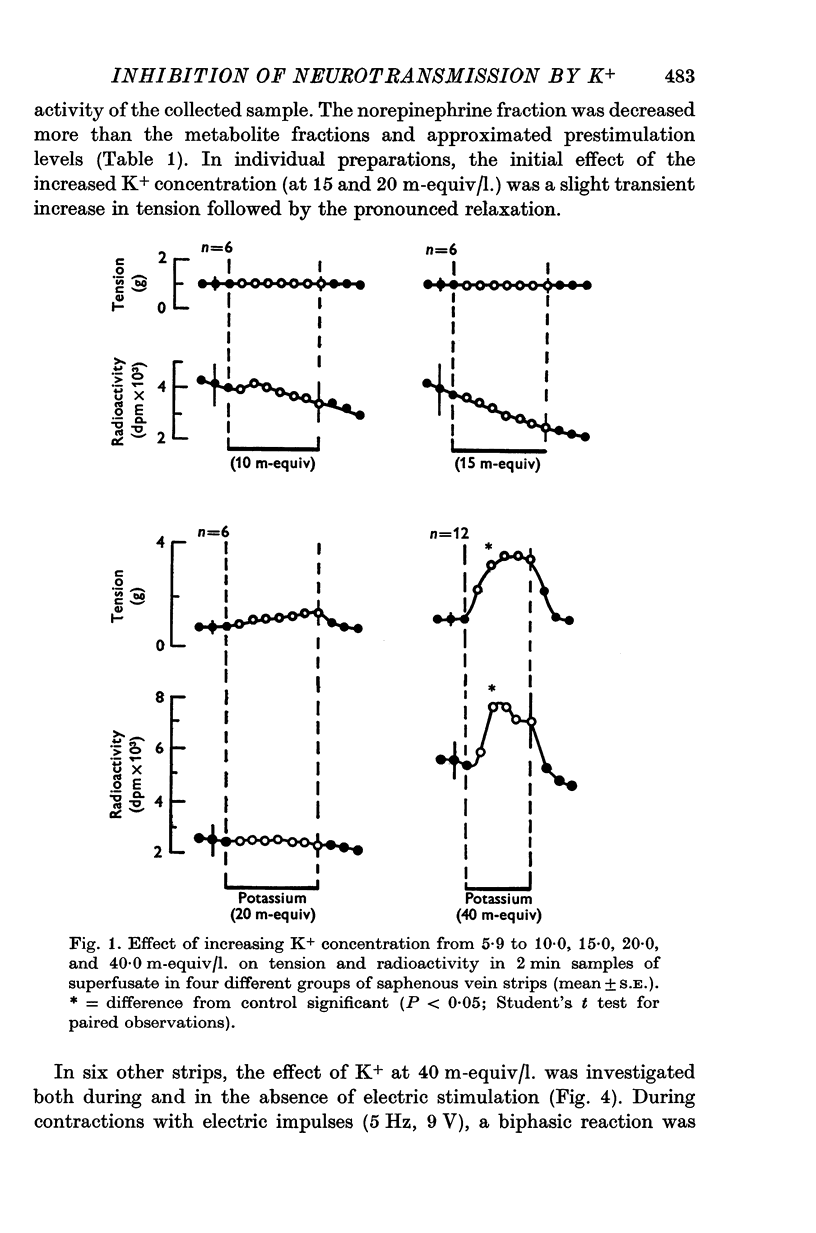
Abstract
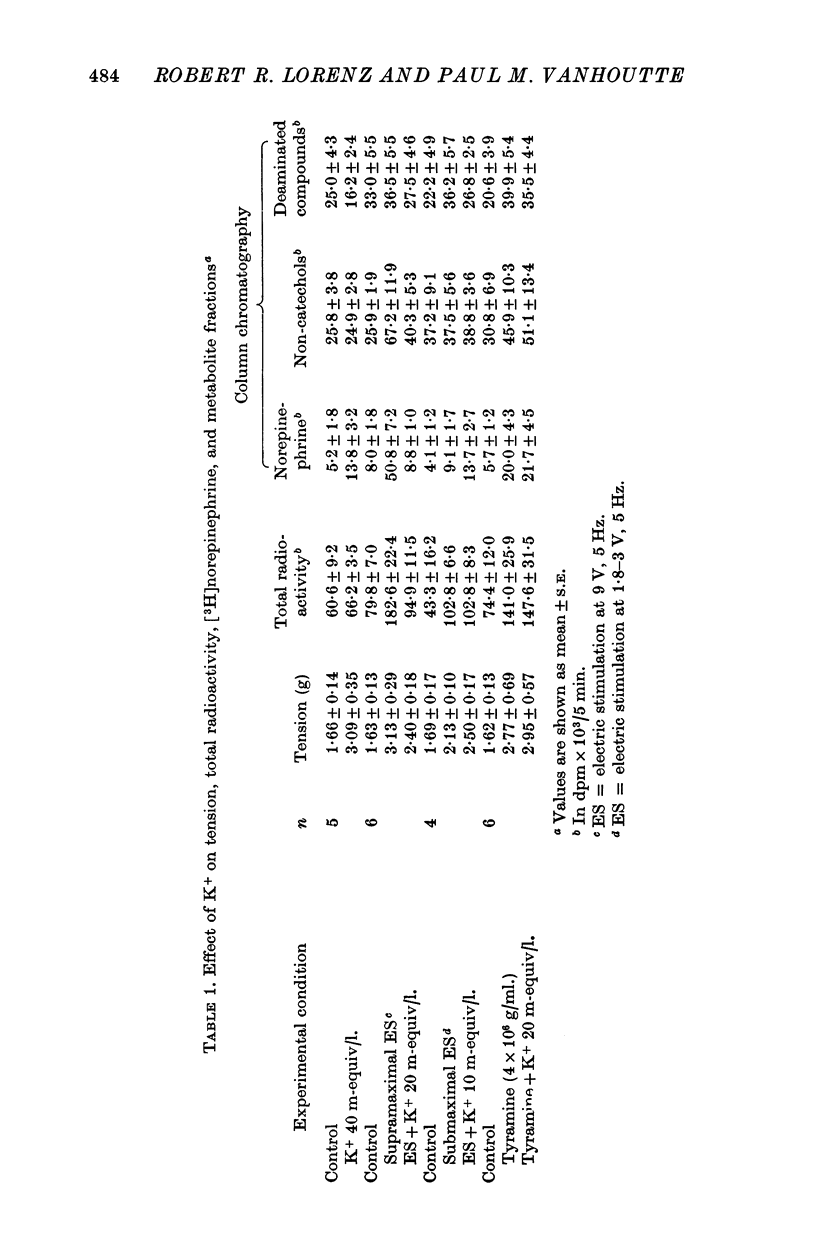
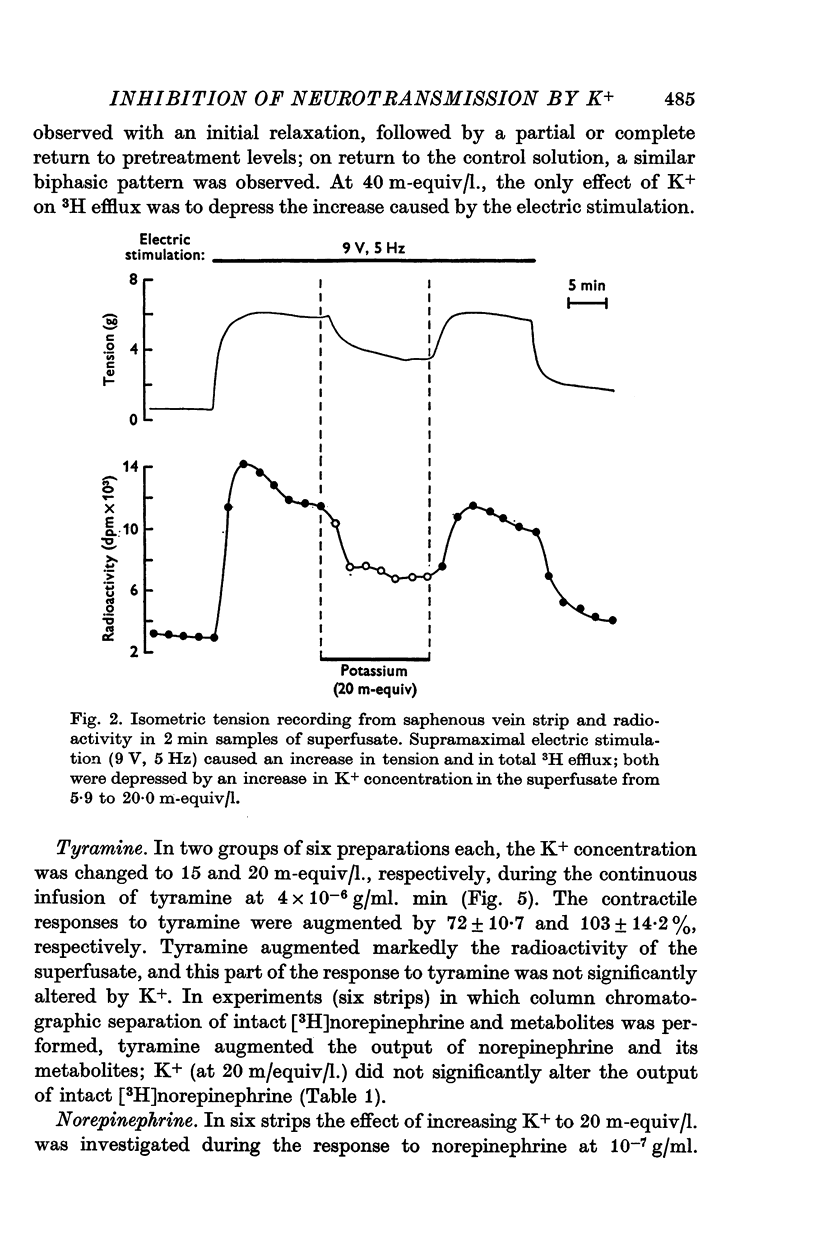
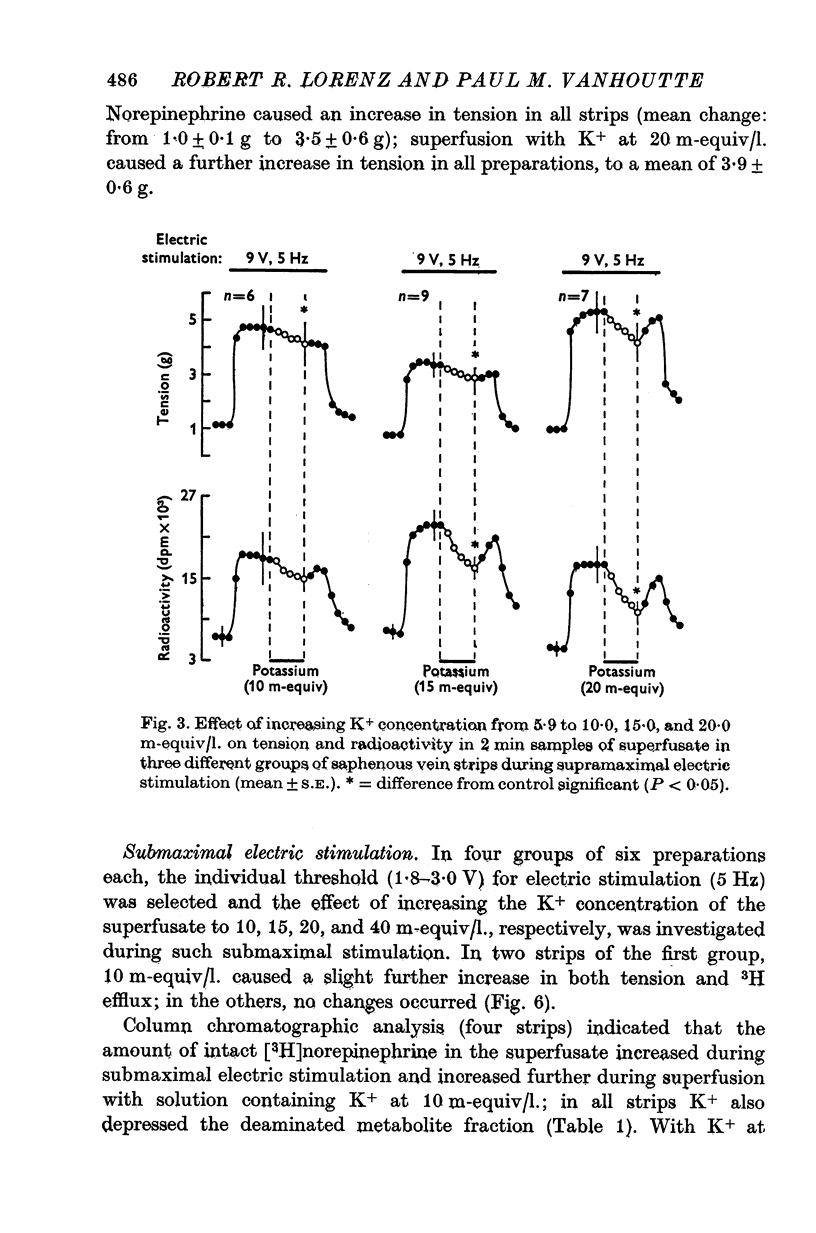
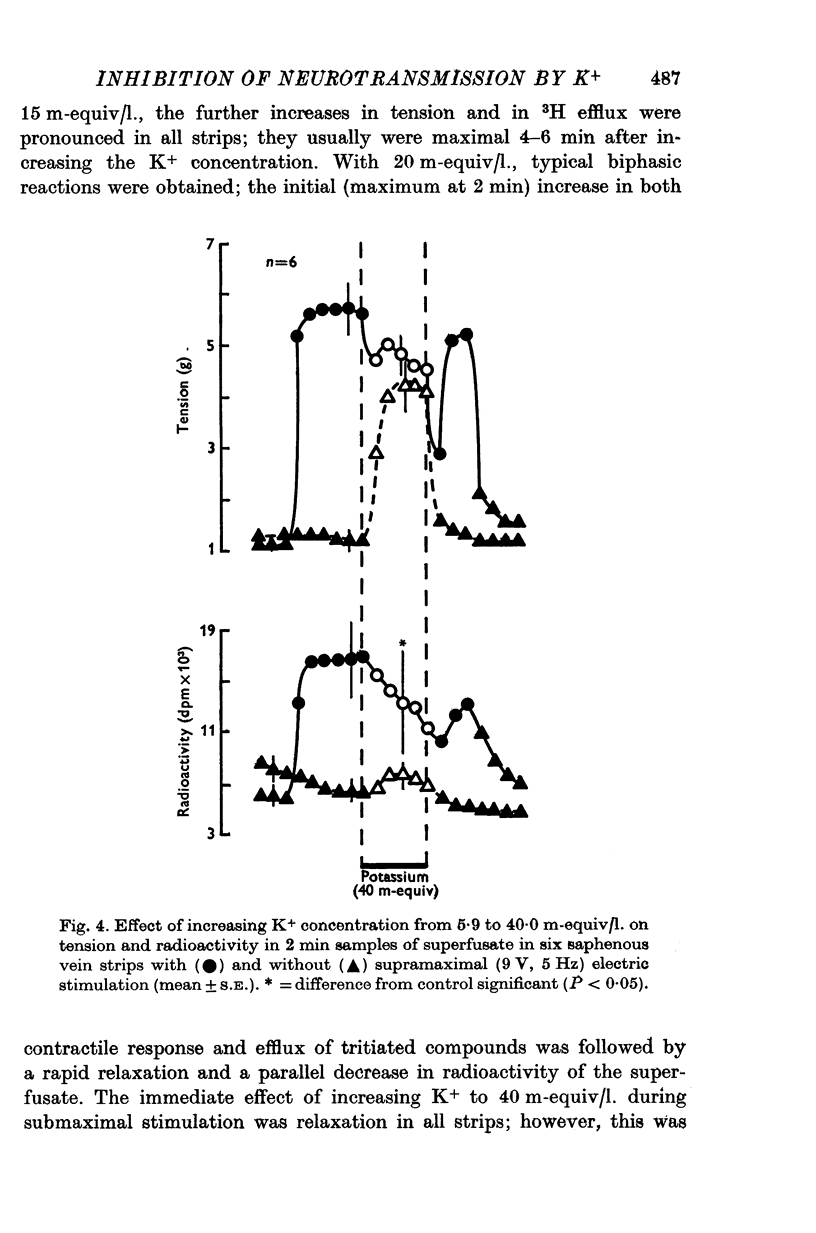
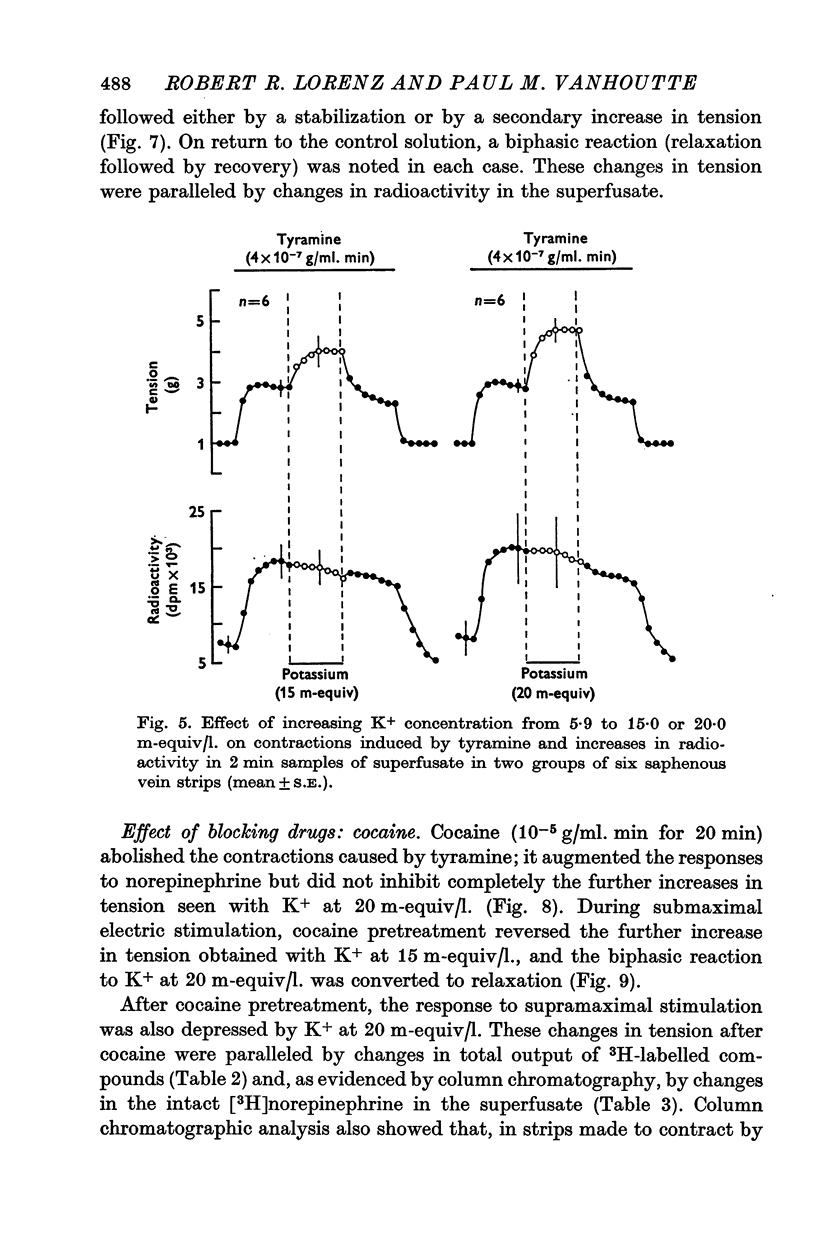
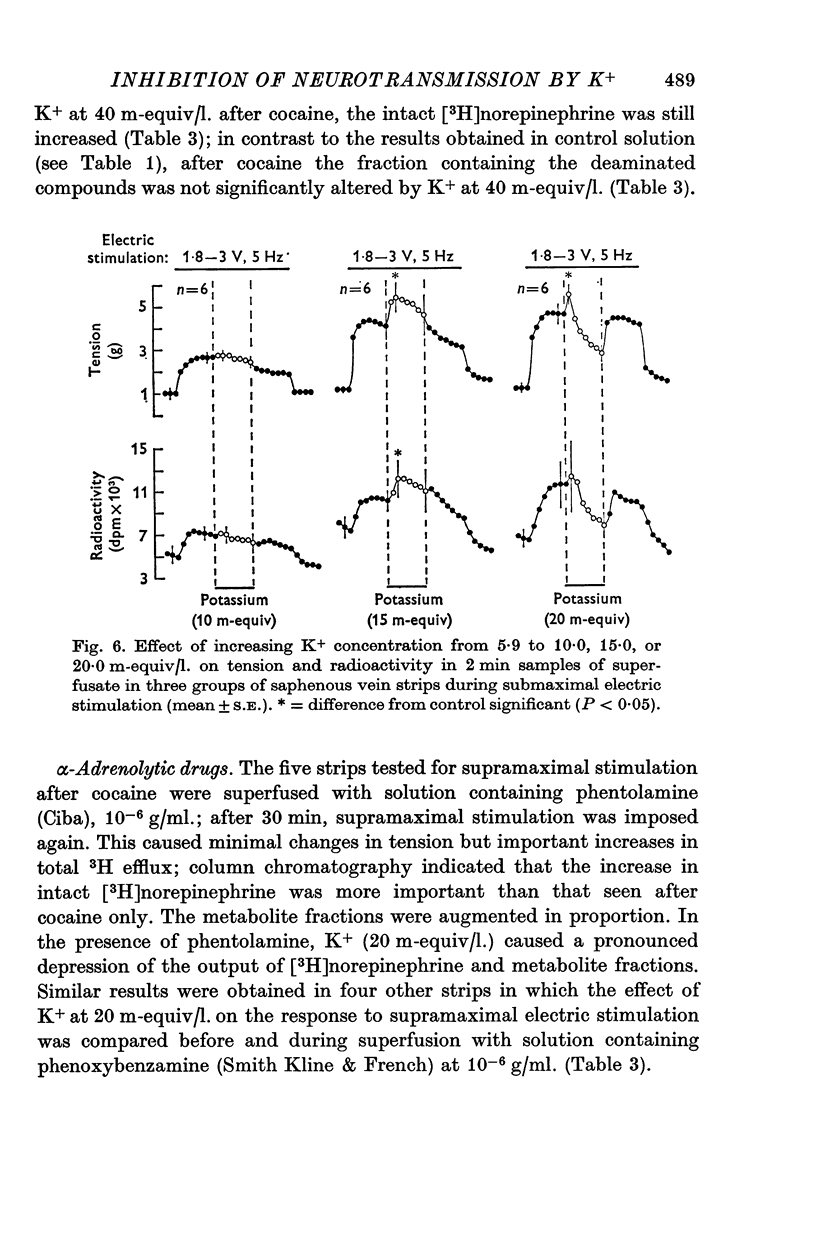
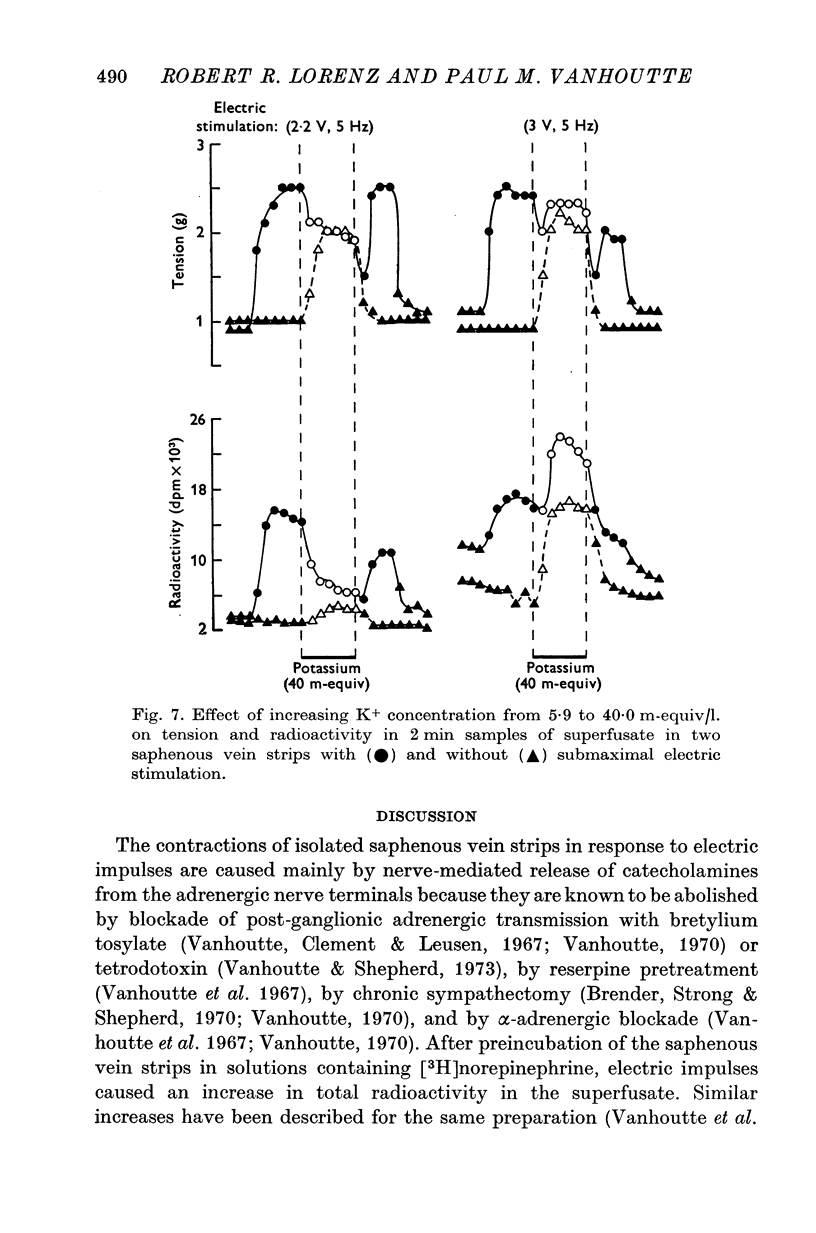
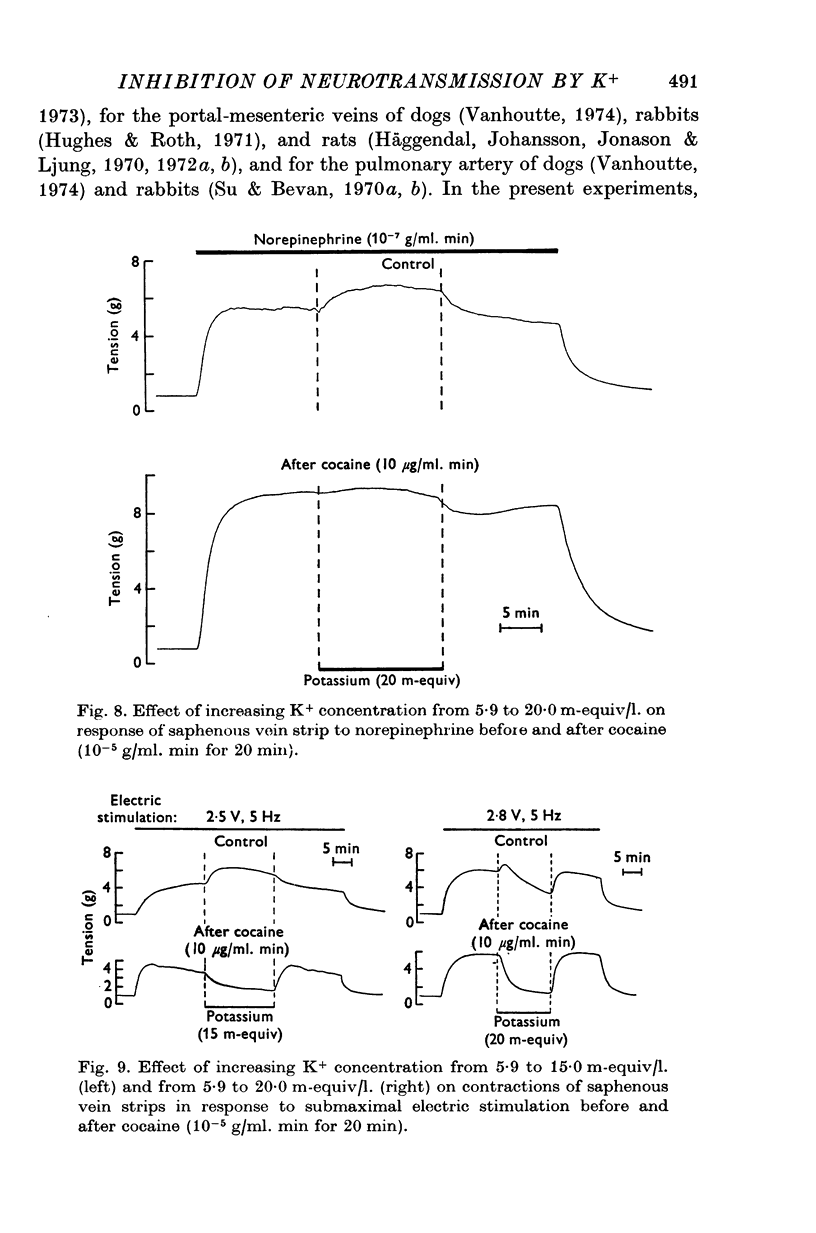
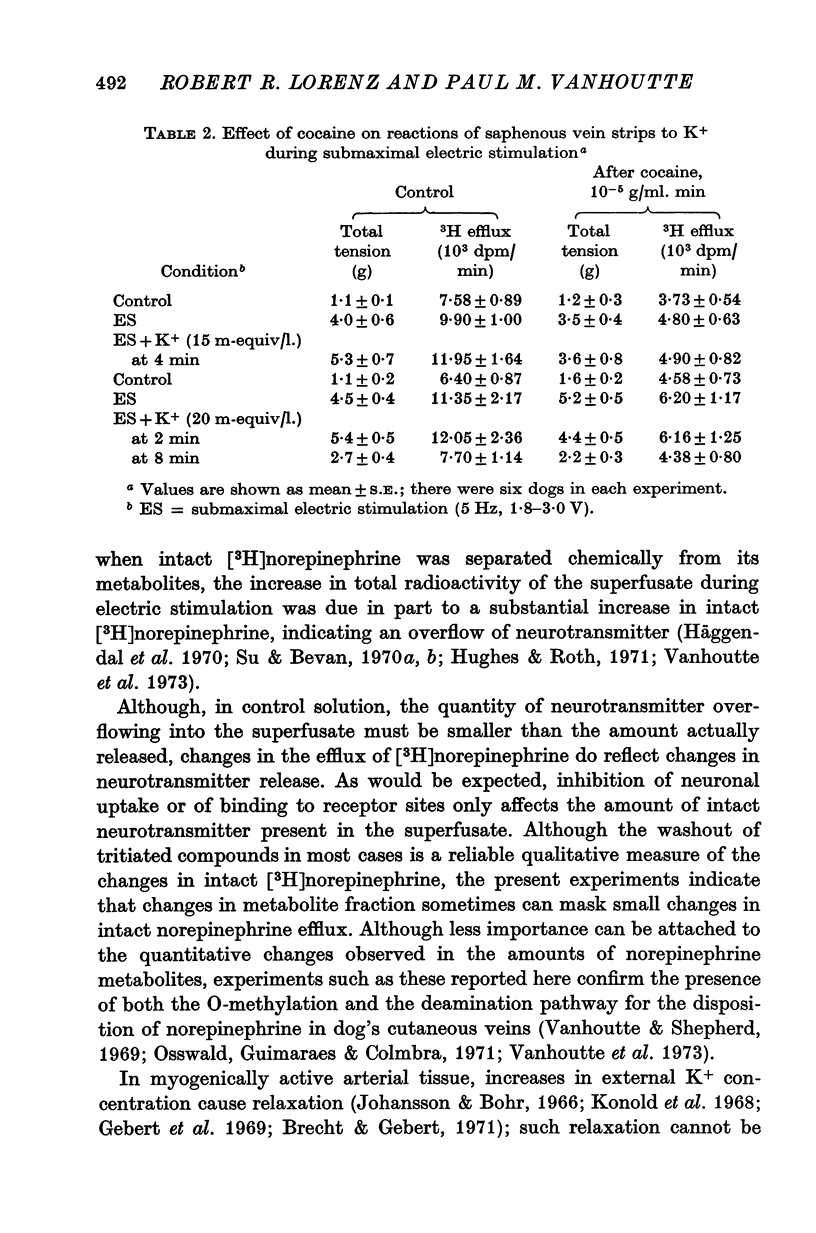
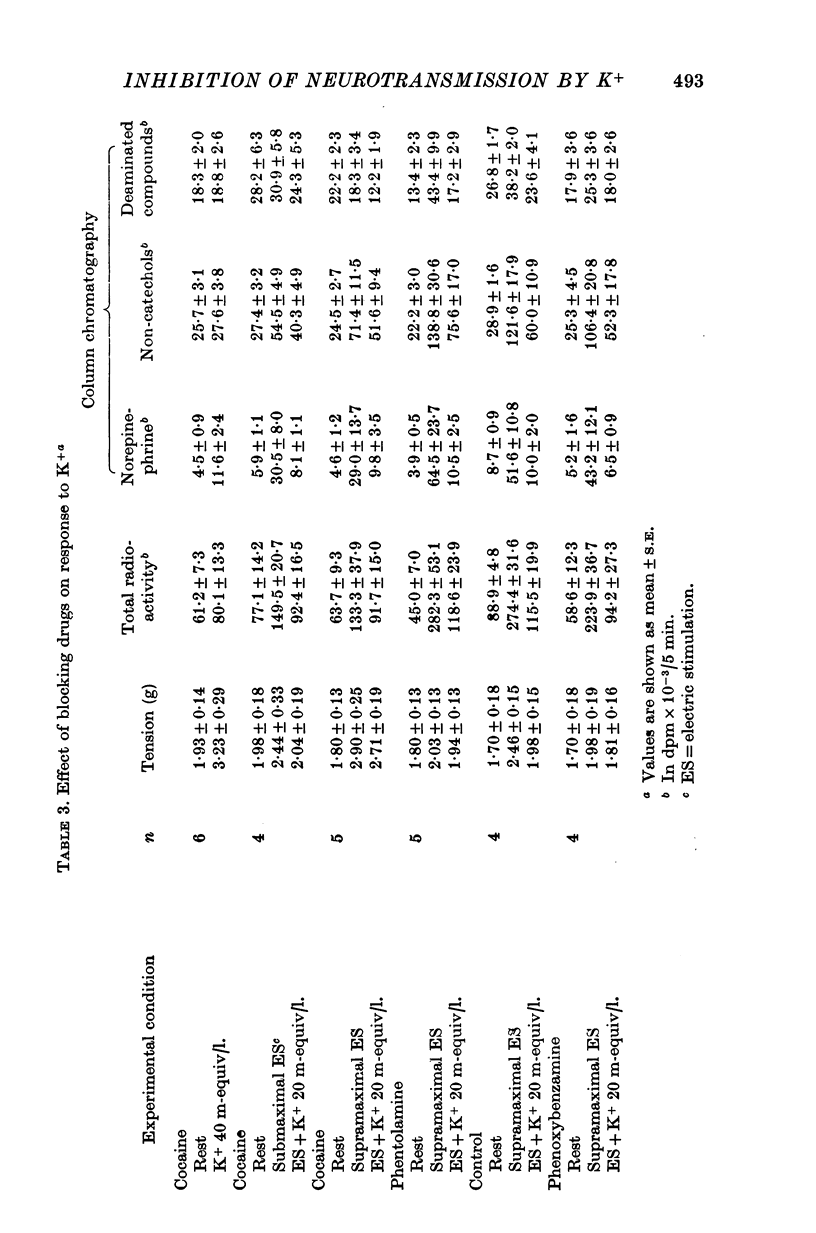
1. In the intact organism, an increase in K+ concentration decreases the reactivity of blood vessels to sympathetic stimulation. The present experiments were designed to determine whether or not K+ interferes with adrenergic neurotransmission. 2. Helical strips cut from dogs' saphenous veins were incubated (4 hr) in Krebs-Ringer solution containing [7-3H]norepinephrine (5 times 10(-8) g/ml). The preparations were mounted for superfusion and isometric tension recording; the superfusate was collected for estimation of total radioactivity and for chromatographic separation of 3H-labelled norepinephrine and metabolites. 3. Supramaximal electric stimulation (5 Hz, 9 V, 2 msec) increased the tension and the [3H]norepinephrine efflux. Increasing the K+ concentration from 5-9 to 1, 15, and 20 m-equiv/l. caused a progressive depression of these contractions and diminished the total 3H efflux in proportion to the relaxation; the decrease in 3H efflux reflected a decrease in intact [3H]norepinephrine. The same increase in K+ concentration did not alter basal tension or basal 3H efflux. 4. Addition of tyramine (4 times 10(-6) g/ml. min) to the superfusate augmented both the tension and the efflux, but these actions were not depresesd by increasing the K+ concentration. 5. Cocaine, phentolamine, and phenoxybenzamine did not prevent the depression by K+ of the response to electric stimulation. 6. These experiments show that K+ causes relaxation of venous smooth muscle constricted by sympathetic stimulation and does so by inhibiting the release of norepinephrine from nerve endings. By contrast, K+ does not inhibit norepinephrine release in response to tyramine. 7. During submaximal electric stimulation (5 Hz, 1-8--3 V, 2 msec), increasing the K+ concentration from 5-9 to 10 and 15 m-equiv/l. potentiated the contractions and increased the [3H]norepinephrine efflux; at 20 m-equil/l, K+ caused transient increases in tension and 3H efflux followed by relaxation and decreased norepinephrine release. After addition of cocaine (10(-5) g/ml. min), K+ only caused relaxation and decrease in 3H efflux, showing that, in addition to inhibition of norepinephrine release, K+ also inhibits the reuptake process. 8. In higher concentrations (40 m-equil/l.), K+ caused both a liberation of norepinephrine and a direct activation of the smooth muscle cells.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Anderson D. K., Roth S. A., Brace R. A., Radawski D., Haddy F. J., Scott J. B. Effect of hypokalemia and hypomagnesemia produced by hemodialysis on vascular resistance in canine skeletal muscle: role of potassium in active hyperemia. Circ Res. 1972 Aug;31(2):165–173. doi: 10.1161/01.res.31.2.165. [DOI] [PubMed] [Google Scholar]
- BEVAN J. A., OSHER J. V. EFFECT OF POTASSIUM ON THE RESTING LENGTH OF VASCULAR SMOOTH MUSCLE OF THE RABBIT AORTA AND ITS RESPONSE TO L-NOREPINEPHRINE. Circ Res. 1963 Oct;13:346–351. doi: 10.1161/01.res.13.4.346. [DOI] [PubMed] [Google Scholar]
- Berti F., Shore P. A. A kinetic analysis of drugs that inhibit the adrenergic neuronal membrane amine pump. Biochem Pharmacol. 1967 Nov;16(11):2091–2094. doi: 10.1016/0006-2952(67)90005-6. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Johnson E. M., Jr, Needleman P. Calcium-dependent norepinephrine release from presynaptic nerve endings in vitro. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2237–2240. doi: 10.1073/pnas.69.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanski D. F., Brodie B. B. The effects of inorganic ions on the storage and uptake of H3-norepinephrine by rat heart slices. J Pharmacol Exp Ther. 1969 Feb;165(2):181–189. [PubMed] [Google Scholar]
- Bose D., Innes I. R. Isoprenaline-induced relaxation of smooth muscle not due to electrogenic sodium pumping. Can J Physiol Pharmacol. 1972 Apr;50(4):378–380. doi: 10.1139/y72-058. [DOI] [PubMed] [Google Scholar]
- Brecht K., Konold P., Gebert G. The effect of potassium, catecholamines and other vasoactive agents on isolated arterial segments of the muscular type. Physiol Bohemoslov. 1969 Jul;18(1):15–22. [PubMed] [Google Scholar]
- Brender D., Strong C. G., Shepherd J. T. Effects of acetylstrophanthidin on isolated veins of the dog. Circ Res. 1970 May;26(5):647–655. doi: 10.1161/01.res.26.5.647. [DOI] [PubMed] [Google Scholar]
- Brown G. L., Feldberg W. The action of potassium on the superior cervical ganglion of the cat. J Physiol. 1936 Mar 9;86(3):290–305. doi: 10.1113/jphysiol.1936.sp003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T., Brace R. A., Scott J. B., Anderson D. K., Haddy F. J. The mechanism of the vasodilator action of potassium. Proc Soc Exp Biol Med. 1972 Jul;140(3):820–824. doi: 10.3181/00379727-140-36560. [DOI] [PubMed] [Google Scholar]
- Clement D., Vanhoutte P., Leusen I. Capacitance reactions of isolated veins to monoamines and acetylcholine. Arch Int Physiol Biochim. 1969 Feb;77(1):73–87. doi: 10.3109/13813456909056649. [DOI] [PubMed] [Google Scholar]
- DODD W. A., DANIEL E. E. Electrolytes and arterial muscle contractility. Circ Res. 1960 Mar;8:451–463. doi: 10.1161/01.res.8.2.451. [DOI] [PubMed] [Google Scholar]
- Dubocovich M. L., Langer S. Z. Negative feed-back regulation of noradrenaline release by nerve stimulation in the perfused cat's spleen: differences in potency of phenoxybenzamine in blocking the pre- and post-synaptic adrenergic receptors. J Physiol. 1974 Mar;237(3):505–519. doi: 10.1113/jphysiol.1974.sp010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMANUEL D. A., SCOTT J. B., HADDY F. J. Effect of potassium on small and large blood vessels of the dog forelimb. Am J Physiol. 1959 Sep;197:637–642. doi: 10.1152/ajplegacy.1959.197.3.637. [DOI] [PubMed] [Google Scholar]
- Enero M. A., Langer S. Z., Rothlin R. P., Stefano F. J. Role of the -adrenoceptor in regulating noradrenaline overflow by nerve stimulation. Br J Pharmacol. 1972 Apr;44(4):672–688. doi: 10.1111/j.1476-5381.1972.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R. F., KIRPEKAR S. M., RIEKER M., SCHWAB A. ACTIONS AND INTERACTIONS OF NOREPINEPHRINE, TYRAMINE AND COCAINE ON AORTIC STRIPS OF RABBIT AND LEFT ATRIA OF GUINEA PIG AND CAT. J Pharmacol Exp Ther. 1963 Oct;142:39–58. [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Drug-induced changes in the release of ( 3 H)-noradrenaline from field stimulated rat iris. Br J Pharmacol. 1971 Sep;43(1):97–106. doi: 10.1111/j.1476-5381.1971.tb07160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Garcia A., Velasco Martin A., Martinez Sierra S., Sanchez Garcia P. Evidence for a different site of action of phenoxybenzamine and desmethylimipramine on the catecholamine uptake system. Experientia. 1972 Jun 15;28(6):671–673. doi: 10.1007/BF01944970. [DOI] [PubMed] [Google Scholar]
- Gebert G., Nguyen-Duong H., Schnizer W., Konold P., Hillenbrand F., Yabu H., Brecht K. The response of isolated arteries and veins to potassium, osmolarity and drugs. Arztl Forsch. 1969 Dec 10;23(12):391–398. [PubMed] [Google Scholar]
- Hughes J., Roth R. H. Evidence that angiotensin enhances transmitter release during sympathetic nerve stimulation. Br J Pharmacol. 1971 Feb;41(2):239–255. doi: 10.1111/j.1476-5381.1971.tb08025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggendal J., Johansson B., Jonason J., Ljung B. Correlation between noradrenaline release and effector response to nerve stimulation in rat portal vein in vitro. Acta Physiol Scand Suppl. 1970;349:17–32. [PubMed] [Google Scholar]
- Häggendal J., Johansson B., Jonason J., Ljung B. Effects of a bicyclic thymoleptic drug (LU 3-010) on neuroeffector function in rat isolated portal veins. J Pharm Pharmacol. 1972 Jul;24(7):557–564. doi: 10.1111/j.2042-7158.1972.tb09057.x. [DOI] [PubMed] [Google Scholar]
- Häggendal J., Johansson B., Jonason J., Ljung B. Effects of phenoxybenzamine on transmitter release and effector response in the isolated portal vein. J Pharm Pharmacol. 1972 Feb;24(2):161–164. doi: 10.1111/j.2042-7158.1972.tb08954.x. [DOI] [PubMed] [Google Scholar]
- Johansson B., Bohr D. F. Rhythmic activity in smooth muscle from small subcutaneous arteries. Am J Physiol. 1966 Apr;210(4):801–806. doi: 10.1152/ajplegacy.1966.210.4.801. [DOI] [PubMed] [Google Scholar]
- KOPIN I. J. STORAGE AND METABOLISM OF CATECHOLAMINES: THE ROLE OF MONOAMINE OXIDASE. Pharmacol Rev. 1964 Jun;16:179–191. [PubMed] [Google Scholar]
- Kirpekar S. M., Furchgott R. F., Wakade A. R., Prat J. C. Inhibition by sympathomimetic amines of the release of norepinephrine evoked by nerve stimulation in the cat spleen. J Pharmacol Exp Ther. 1973 Dec;187(3):529–538. [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Puig M., Wakade A. R. Modification of the evoked release of noradrenaline from the perfused cat spleen by various ions and agents. J Physiol. 1972 Mar;221(3):601–615. doi: 10.1113/jphysiol.1972.sp009770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R. Release of noradrenaline from the cat spleen by potassium. J Physiol. 1968 Feb;194(3):595–608. doi: 10.1113/jphysiol.1968.sp008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konold P., Gebert G., Brecht K. The effect of potassium on the tone of isolated arteries. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(4):285–291. doi: 10.1007/BF00362638. [DOI] [PubMed] [Google Scholar]
- Kuschinsky W., Wahl M., Bosse O., Thurau K. Perivascular potassium and pH as determinants of local pial arterial diameter in cats. A microapplication study. Circ Res. 1972 Aug;31(2):240–247. doi: 10.1161/01.res.31.2.240. [DOI] [PubMed] [Google Scholar]
- Lindmar R., Löffelholz K., Muscholl E. Unterschiede zwischen Tyramin und Dimethylphenylpiperzin in der Ca-Abhangigkeit und im zeitlichen Verlauf der Noradrenalin-Freisetzung am isolierten Kaninchenherzen. Experientia. 1967 Nov 15;23(11):933–934. doi: 10.1007/BF02136230. [DOI] [PubMed] [Google Scholar]
- Norton J. M., Detar R. Potassium and isolated coronary vascular smooth muscle. Am J Physiol. 1972 Feb;222(2):474–479. doi: 10.1152/ajplegacy.1972.222.2.474. [DOI] [PubMed] [Google Scholar]
- Osswald W., Guimarães S., Coimbra A. The termination of action of catecholamines in the isolated venous tissue of the dog. Naunyn Schmiedebergs Arch Pharmakol. 1971;269(1):15–31. doi: 10.1007/BF01422013. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J., Donald D. E. Sympathetic vasoconstrictive responses during exercise- or drug-induced vasodilatation. A time-dependent response. Circ Res. 1968 Jul;23(1):45–60. doi: 10.1161/01.res.23.1.45. [DOI] [PubMed] [Google Scholar]
- Shore P. A. Transport and storage of biogenic amines. Annu Rev Pharmacol. 1972;12:209–226. doi: 10.1146/annurev.pa.12.040172.001233. [DOI] [PubMed] [Google Scholar]
- Skinner N. S., Jr, Costin J. C. Interactions between oxygen, potassium, and osmolality in regulation of skeletal muscle blood flow. Circ Res. 1971 Jan;28(Suppl):73–85. [PubMed] [Google Scholar]
- Skinner N. S., Jr, Costin J. C. Role of O2 and K+ in abolition of sympathetic vasoconstriction in dog skeletal muscle. Am J Physiol. 1969 Aug;217(2):438–444. doi: 10.1152/ajplegacy.1969.217.2.438. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev. 1968 Dec;20(4):197–272. [PubMed] [Google Scholar]
- Starke K. Influence of extracellular noradrenaline on the stimulation-evoked secretion of noradrenaline from sympathetic nerves: evidence for an -receptor-mediated feed-back inhibition of noradrenaline release. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(1):11–23. doi: 10.1007/BF00505064. [DOI] [PubMed] [Google Scholar]
- Starke K., Montel H. Sympathomimetic inhibition of noradrenaline release: mediated by prostaglandins? Naunyn Schmiedebergs Arch Pharmacol. 1973;278(1):111–116. doi: 10.1007/BF00501869. [DOI] [PubMed] [Google Scholar]
- Su C., Bevan J. A. Blockade of the nicotine-induced norepinephrine release by cocaine, phenoxybenzamine and desipramine. J Pharmacol Exp Ther. 1970 Nov;175(2):533–540. [PubMed] [Google Scholar]
- Su C., Bevan J. A. The release of H3-norepinephrine in arterial strips studied by the technique of superfusion and transmural stimulation. J Pharmacol Exp Ther. 1970 Mar;172(1):62–68. [PubMed] [Google Scholar]
- Thoenen H., Huerlimann A., Haefely W. Cation dependence of the noradrenaline-releasing action of tyramine. Eur J Pharmacol. 1969 Apr;6(1):29–37. doi: 10.1016/0014-2999(69)90061-2. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M. Inhibition by acetylcholine of adrenergic neurotransmission in vascular smooth muscle. Circ Res. 1974 Mar;34(3):317–326. doi: 10.1161/01.res.34.3.317. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Lorenz R. R., Tyce G. M. Inhibition of norepinephrine- 3 H release from sympathetic nerve endings in veins by acetylcholine. J Pharmacol Exp Ther. 1973 May;185(2):386–394. [PubMed] [Google Scholar]
- Vanhoutte P. M., Shepherd J. T. Activity and thermosensitivity of canine cutaneous veins after inhibition of monoamine oxidase and catechol-O-methyl transferase. Circ Res. 1969 Nov;25(5):607–616. doi: 10.1161/01.res.25.5.607. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Shepherd J. T. Venous relaxation caused by acetylcholine acting on the sympathetic nerves. Circ Res. 1973 Feb;32(2):259–267. doi: 10.1161/01.res.32.2.259. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P., Clement D., Leusen I. The reactivity of isolated veins to electrical stimulation. Arch Int Physiol Biochim. 1967 Sep;75(4):641–657. doi: 10.3109/13813456709112513. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. Mécanismes adrénergiques et réactivité des veines cutanées du chien aux variations de la température locale. C R Seances Soc Biol Fil. 1970;164(12):2666–2671. [PubMed] [Google Scholar]
- Wende W., Peiper U. Wechselwirkung von Kalium und Noradrenalin auf die Spannungsentwicklung des isolierten Gefässmuskels. Pflugers Arch. 1970;320(2):133–141. doi: 10.1007/BF00588548. [DOI] [PubMed] [Google Scholar]
- White T. D., Paton D. M. Effects of external Na + and K + on the initial rates of noradrenaline uptake by synaptosomes prepared from rat brain. Biochim Biophys Acta. 1972 Apr 14;266(1):116–127. doi: 10.1016/0005-2736(72)90126-5. [DOI] [PubMed] [Google Scholar]


