Abstract
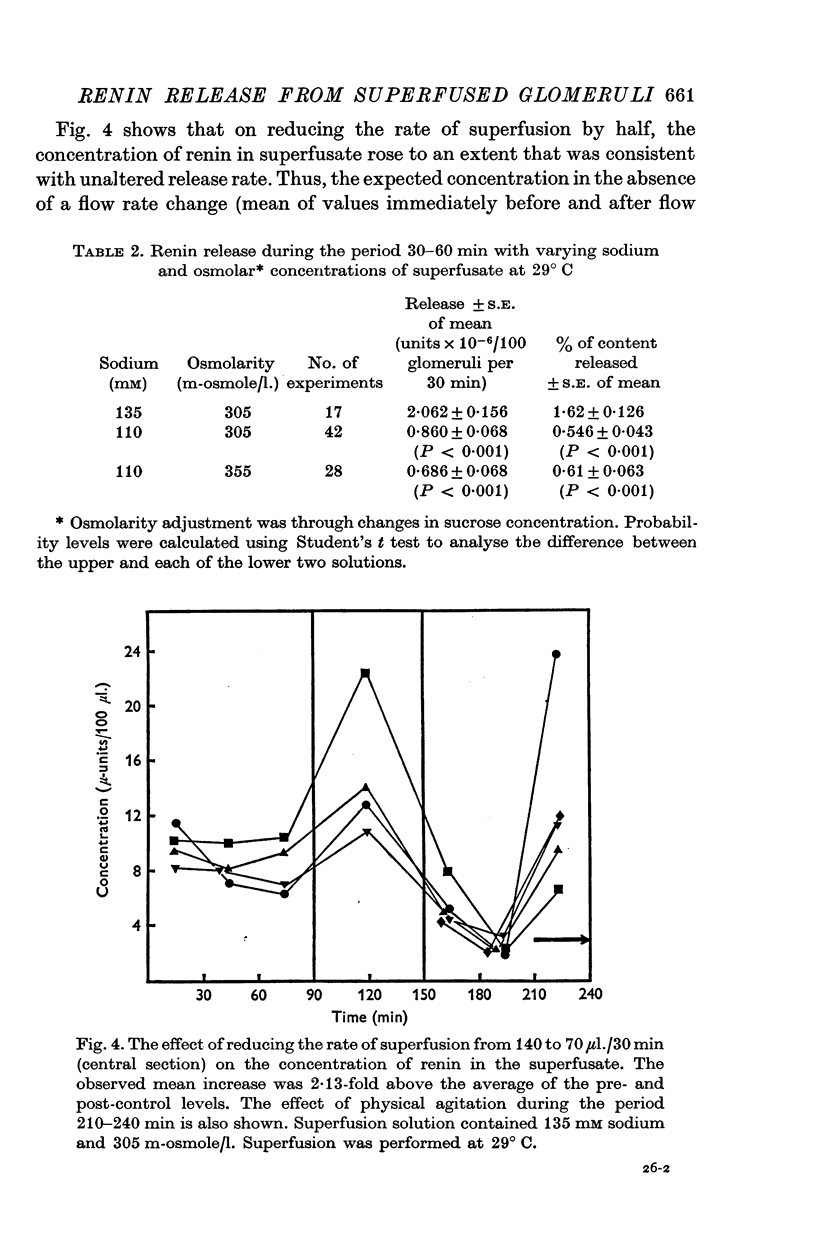
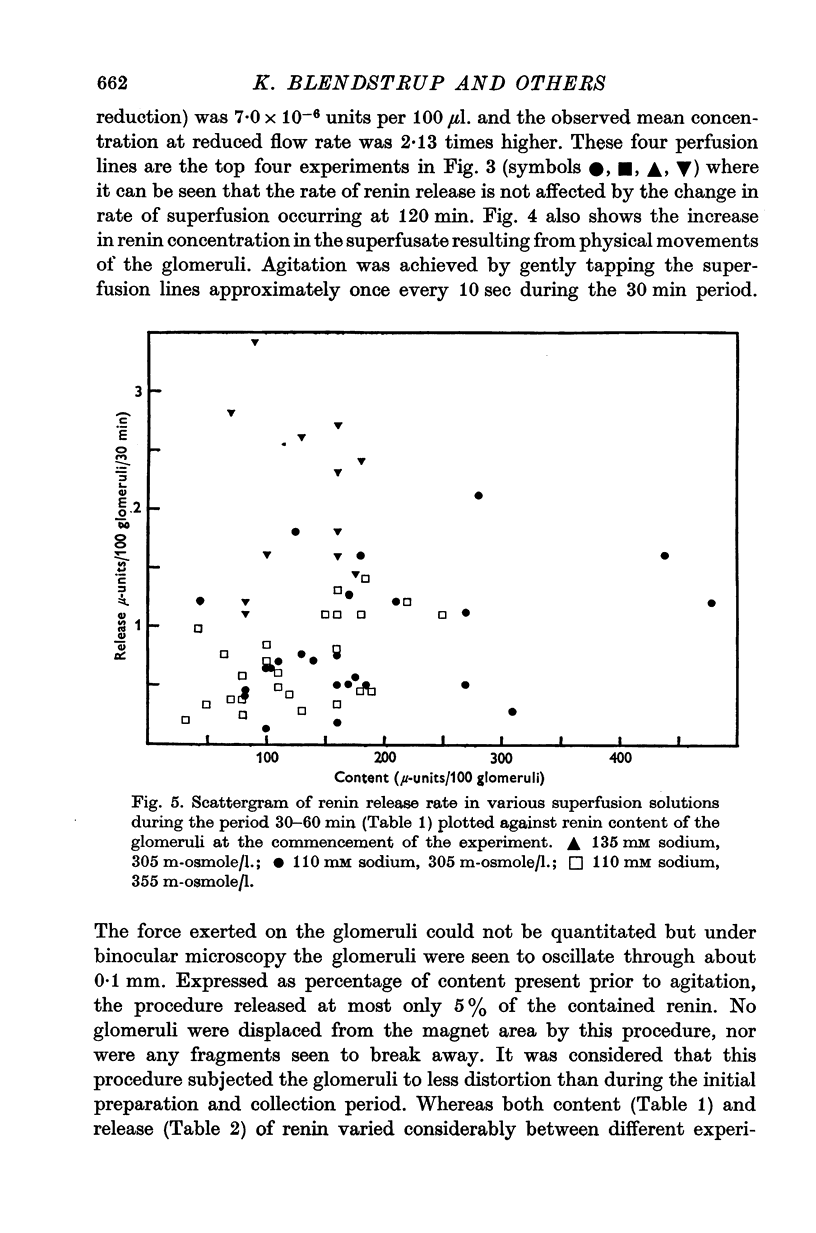
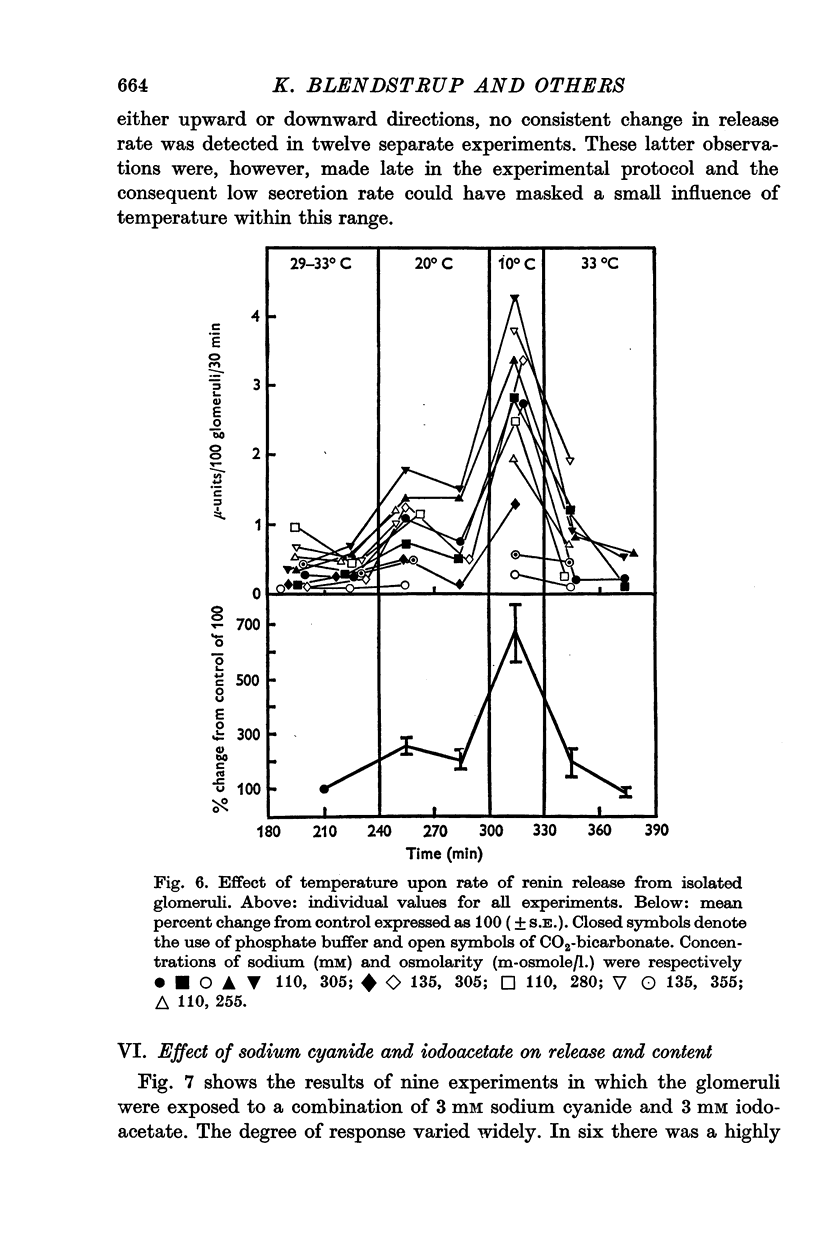
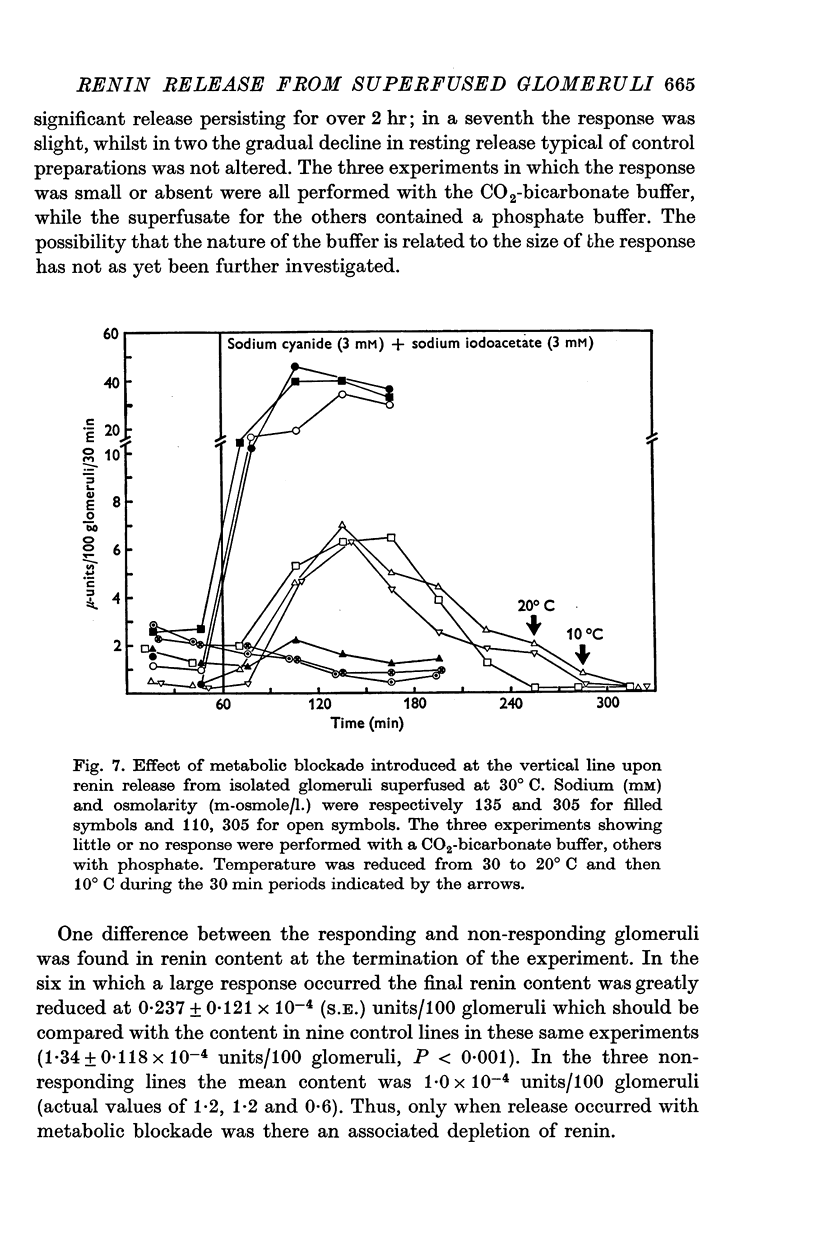
1. A method is described for studying renin release from superfused rat glomeruli following their rapid isolation by a magnetic iron-oxide technique. 2. Microscopically selected glomeruli were free of tubular components. Some possessed vascular pole protrusions of up to 20 mum, unrelated to renin content. 3. Renin content of 102 batches, each of 400 glomeruli, was 1.34 plus or minus 0.08 times 10-4 Goldblatt hog units per 100 glomeruli (plus or minus S.E. of mean). Different osmolarities (305, 355 and 400 m-osmole/1.), sodium concentrations (110 and 135 mM) and buffer compositions of the preparation solution did not alter this value. Renin content per glomerulus in intact kidney was 100-fold higher. 4. At 30 degrees C the contained juxtaglomerular cells released renin at consistent but decreasing rates over 4-6 hr. Initial release rate in 110 mM sodium, 305 m-osmole/1. solutions were 0.86 plus or minus 0.068 times 10-6 units per 100 glomeruli per 30 min (plus or minus S.E. of mean, n = 42) or 0.546 plus or minus 0.046 percent of content per 30 min. In 135 mM sodium, 305 m-osmole/1. solutions, release was 2.4-fold higher (P less than 0.001) and remained elevated for at least 3 hr. When related to renin content per glomerulus resting release rate in vitro was higher by at most one order of magnitude than calculated in vivo values. 5. Release was augmented by gentle physical agitation of the glomeruli. 6. Release rate was inversely ralated to temperature. On reducing temperature from 30 degrees C, release increased 2.6-fold at 20 degrees C and 6.7-fold at 10 degrees C (P less than 0.001, n = 11). The response was reversible. 7. 3 mM sodium cyanide plus 3 mM sodium iodoacetate caused a variable release of renin associated with depletion of content within 4 hr. The response was progressive and reached a peak after 60 min. 8. Sensitivity of renin release to temperature and metabolic blockade indicates that energy is required for retention of renin by the cell. This, together with the release observed with increased sodium concentration at constant osmolarity, suggests a dependence of renin release upon the mechanism controlling the volume of the juxtaglomerular cell or its organelles.
Full text
PDF


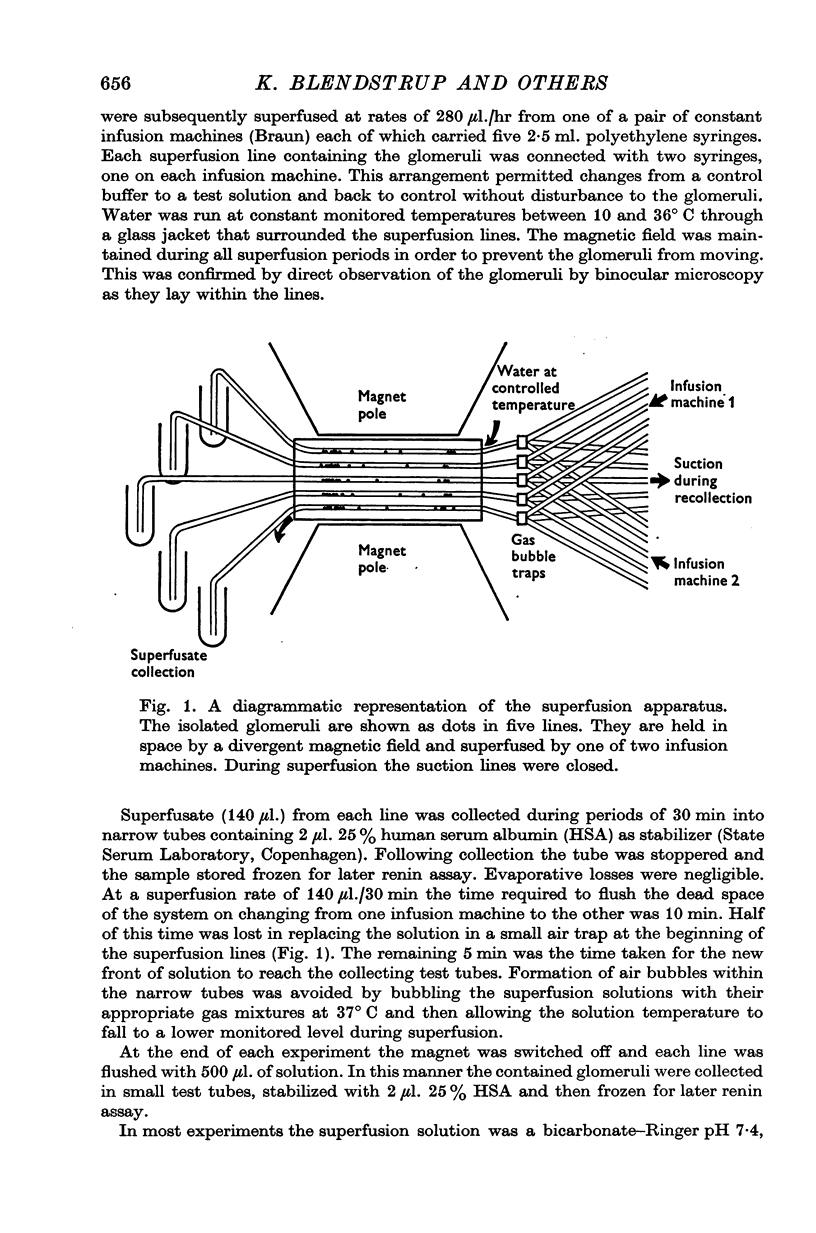

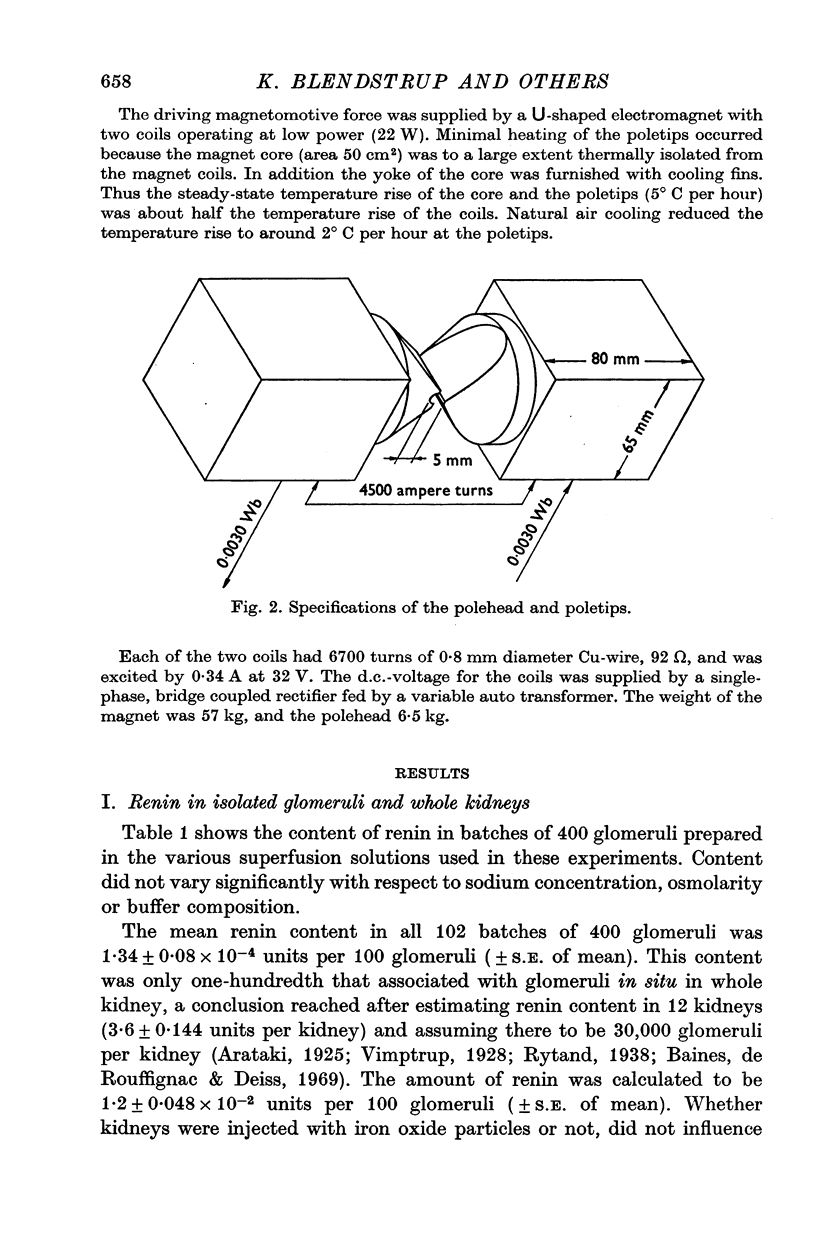
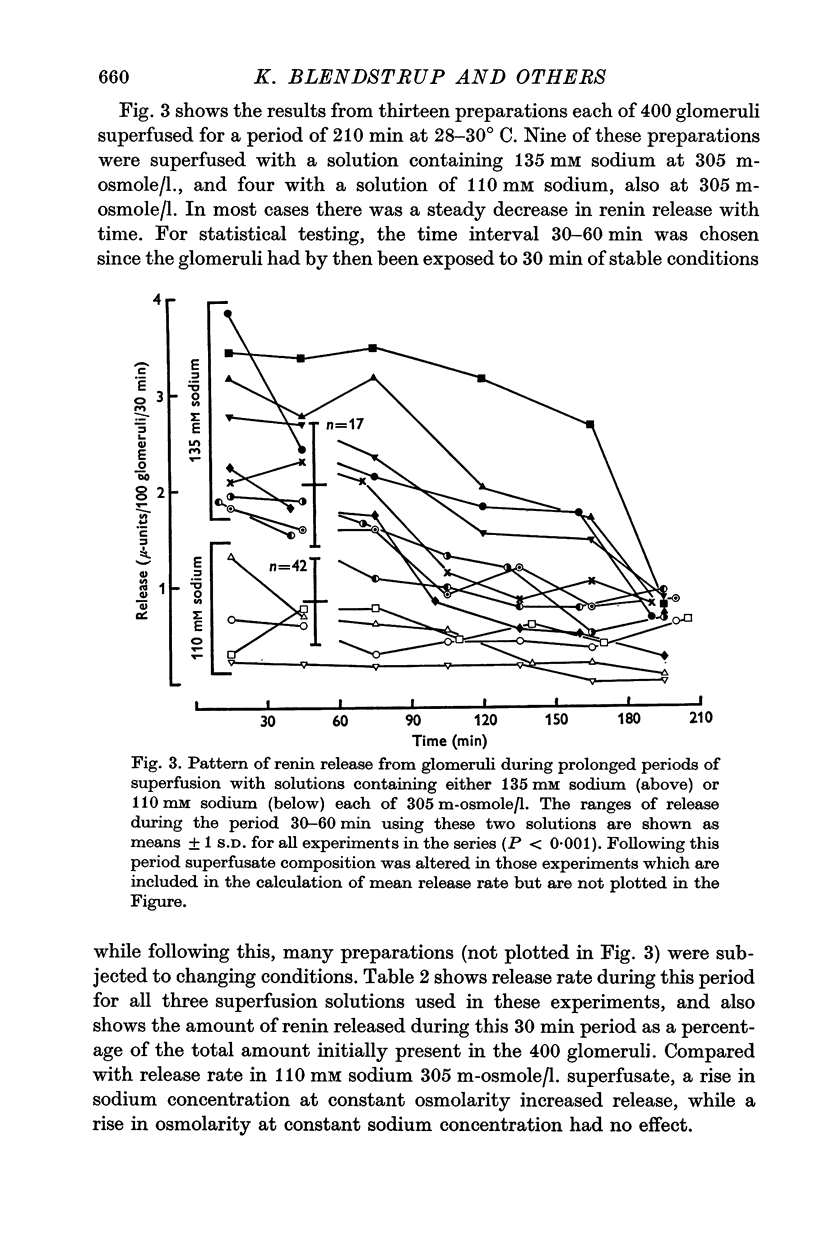








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaine E. H., Davis J. O., Witty R. T. Renin release after hemorrhage and after suprarenal aortic constriction in dogs without sodium delivery to the macula densa. Circ Res. 1970 Dec;27(6):1081–1089. doi: 10.1161/01.res.27.6.1081. [DOI] [PubMed] [Google Scholar]
- Blair-West J. R., Brook A. H., Simpson P. A. Renin responses to water restriction and rehydration. J Physiol. 1972 Oct;226(1):1–13. doi: 10.1113/jphysiol.1972.sp009970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman B., Freeman R. H., Rostorfer H. H. The influence of dietary sodium chloride on in vitro renin release from rat kidney slices. Proc Soc Exp Biol Med. 1971 Oct;138(1):81–88. doi: 10.3181/00379727-138-35836. [DOI] [PubMed] [Google Scholar]
- Brendel K., Meezan E. Properties of a pure metabolically active glomerular preparation from rat kidneys. II. Metabolism. J Pharmacol Exp Ther. 1973 Nov;187(2):342–351. [PubMed] [Google Scholar]
- COOK W. F., PICKERING G. W. The location of renin in the rabbit kidney. J Physiol. 1959 Dec;149:526–536. doi: 10.1113/jphysiol.1959.sp006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. C., Gala R. R. Renin in anesthetized rats. Proc Soc Exp Biol Med. 1973 Sep;143(4):1018–1021. doi: 10.3181/00379727-143-37460. [DOI] [PubMed] [Google Scholar]
- Davis J. O. The control of renin release. Am J Med. 1973 Sep;55(3):333–350. doi: 10.1016/0002-9343(73)90134-4. [DOI] [PubMed] [Google Scholar]
- De Vito E., Gordon S. B., Cabrera R. R., Fasciolo J. C. Release of renin by rat kidney slices. Am J Physiol. 1970 Oct;219(4):1036–1041. doi: 10.1152/ajplegacy.1970.219.4.1036. [DOI] [PubMed] [Google Scholar]
- Faarup P. Renin location in the different parts of the juxtaglomerular apparatus in the cat kidney. 2. Fractions of the afferent arteriole, the cell group of Goormaghtigh, the efferent arteriole and the glomerulus. Acta Pathol Microbiol Scand. 1968;72(1):109–117. doi: 10.1111/j.1699-0463.1968.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Finkielman S., Nahmod V. E. In vitro production of angiotensin I by renal glomeruli. Nature. 1969 Jun 21;222(5199):1186–1188. doi: 10.1038/2221186a0. [DOI] [PubMed] [Google Scholar]
- Gordon R. D., Pawsey C. G. The relative effects of serum sodium concentration and the state of body fluid balance on renin secretion. J Clin Endocrinol Metab. 1971 Jan;32(1):117–119. doi: 10.1210/jcem-32-1-117. [DOI] [PubMed] [Google Scholar]
- LEYSSAC P. P. THE IN VIVO EFFECT OF ANGIOTENSIN AND NORADRENALINE ON THE PROXIMAL TUBULAR REABSORPTION OF SALT IN MAMMALIAN KIDNEYS. Acta Physiol Scand. 1965 May-Jun;64:167–175. doi: 10.1111/j.1748-1716.1965.tb04165.x. [DOI] [PubMed] [Google Scholar]
- Meezan E., Brendel K., Ulreich J., Carlson E. C. Properties of a pure metabolically active glomerular preparation from rat kidneys. I. Isolation. J Pharmacol Exp Ther. 1973 Nov;187(2):332–341. [PubMed] [Google Scholar]
- Michelakis A. M. The effect of sodium and calcium on renin release in vitro. Proc Soc Exp Biol Med. 1971 Jul;137(3):833–836. doi: 10.3181/00379727-137-35677. [DOI] [PubMed] [Google Scholar]
- Newsome H. H., Bartter F. C. Plasma renin activity in relation to serum sodium concentration and body fluid balance. J Clin Endocrinol Metab. 1968 Dec;28(12):1704–1711. doi: 10.1210/jcem-28-12-1704. [DOI] [PubMed] [Google Scholar]
- Ochs H. G., Lamberts B., Saleh M., Heintz R. Reninsekretion in vitro. Vergleich von Nierenschnitten und isolierten Glomeruli. Res Exp Med (Berl) 1973 May 21;160(3):206–212. doi: 10.1007/BF01856784. [DOI] [PubMed] [Google Scholar]
- Peters-Haefeli L. Rate of inactivation of endogenous or exogenous renin in normal and in renin-depleted rats. Am J Physiol. 1971 Nov;221(5):1339–1345. doi: 10.1152/ajplegacy.1971.221.5.1339. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Persson A. E., Agerup B. Tubuloglomerular feedback. Nonlinear relation between glomerular hydrostatic pressure and loop of henle perfusion rate. J Clin Invest. 1973 Apr;52(4):862–869. doi: 10.1172/JCI107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnermann J., Wright F. S., Davis J. M., von Stackelberg W., Grill G. Regulation of superficial nephron filtration rate by tubulo-glomerular feedback. Pflugers Arch. 1970;318(2):147–175. doi: 10.1007/BF00586493. [DOI] [PubMed] [Google Scholar]
- Sokabe H., Nishimura H., Kawabe K., Tenmoku S., Arai T. Plasma renin activity in varying hydrated states in the bullfrog. Am J Physiol. 1972 Jan;222(1):142–146. doi: 10.1152/ajplegacy.1972.222.1.142. [DOI] [PubMed] [Google Scholar]
- Sokabe H., Ogawa M., Oguri M., Nishimura H. Evolution of the juxtaglomerular apparatus in the vertebrate kidneys. Tex Rep Biol Med. 1969 Fall;27(3):867–885. [PubMed] [Google Scholar]
- Vander A. J. Control of renin release. Physiol Rev. 1967 Jul;47(3):359–382. doi: 10.1152/physrev.1967.47.3.359. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Hasegawa T., Tanaka H., Ueda J. Control of renin secretion in the anesthetized dog. 1. Relationship between renin secretion and plasma sodium concentration in the peritoneal dialyzed dog. Jpn Circ J. 1968 Oct;32(10):1373–1377. doi: 10.1253/jcj.32.1373. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Tanaka H., Horiuchi K., Ueda J. Release of renin from dog kidney cortex slices in vitro. Jpn J Pharmacol. 1967 Dec;17(4):685–686. doi: 10.1254/jjp.17.685. [DOI] [PubMed] [Google Scholar]
- Young D. B., Rostorfer H. H. Renin release responses to acute alterations in renal arterial osmolarity. Am J Physiol. 1973 Nov;225(5):1009–1014. doi: 10.1152/ajplegacy.1973.225.5.1009. [DOI] [PubMed] [Google Scholar]
- de Jong W. Release of renin by rat kidney slices; relationship to plasma renin after desoxycorticosterone and renal hypertension. Proc Soc Exp Biol Med. 1969 Jan;130(1):85–88. doi: 10.3181/00379727-130-33493. [DOI] [PubMed] [Google Scholar]


