Abstract
We have analyzed the molecular epidemiology and drug resistance of 121 Mycobacterium tuberculosis isolates from consecutive patients with culture-positive pulmonary tuberculosis attending a university hospital outpatient department in Addis Ababa, Ethiopia. Restriction fragment length polymorphism analysis and spoligotyping were used to analyze the DNA fingerprinting patterns. Fifty-one (41.2%) of the isolates were found in 13 clusters with two or more identical DNA patterns. Two such clusters contained 49.0% of all clustered isolates. In a multivariate logistic regression model, human immunodeficiency virus (HIV)-positive serostatus was significantly associated with clustering of isolates for patients of both sexes (odds ratio [OR], 2.55; 95% confidence interval [CI], 1.17 to 5.80). There was a trend toward increased clustering of isolates from tuberculous women residing in Addis Ababa (OR, 2.10; 95% CI, 0.85 to 5.25). In total, 17 of 121 isolates (14.0%) were resistant to one or more of the antituberculosis drugs isoniazid (8.3%), streptomycin (7.4%), rifampin (2.5%), and ethambutol (1.7%). The high rate of drug-resistant isolates (29.6%) coincided with the peak prevalence of HIV infection (77.8%) in patients 35 to 44 years old. The majority (62.5%) of resistant isolates in this group were found within clusters. The simultaneous accumulation of certain bacterial clones in a patient population likely reflects recent transmission. Hence, we conclude that tuberculosis is commonly caused by recent infection with M. tuberculosis in HIV-positive Ethiopian patients. Furthermore, with the rapidly increasing prevalence of HIV infection in Ethiopia, the burden of tuberculosis, including drug-resistant tuberculosis, is likely to increase. Strengthening of classical tuberculosis control measures by promoting active case finding among HIV-positive adults with tuberculosis is warranted to reduce rates of transmission.
According to recent estimates, 8.4 million new cases of clinical tuberculosis (TB) occurred worldwide in 1999, an increase from the 8 million new cases in 1997 (11, 45). This rise was largely due to a 20% increase in the incidence of TB in African countries that are heavily affected by the human immunodeficiency virus (HIV) infection and AIDS epidemic (45). HIV-infected persons have an increased risk of developing clinical TB (20). This risk was previously attributed mainly to an increased risk of reactivation of a latent infection (15, 34). DNA fingerprinting of the Mycobacterium tuberculosis genome has been used extensively to explore the dynamics of TB transmission (5, 40). In general, the simultaneous accumulation of mycobacterial isolates with identical fingerprints, i.e., clusters, has been attributed to recent infection, whereas isolates with unique fingerprints are most commonly associated with endogenous reactivation (23, 40). Studies from industrialized countries in which DNA fingerprinting of insertion element IS6110 of the M. tuberculosis genome has been used (37) have shown that nearly two-thirds of M. tuberculosis isolates from HIV-infected patients appear in clusters, suggesting recent infection (2, 36). Further support for this suggestion is provided by DNA fingerprinting of nosocomial TB outbreaks, including transmission of multidrug-resistant strains (6, 7, 12). These studies have demonstrated rapid progression to clinical disease in immunosuppressed HIV-infected individuals.
In population-based studies from sub-Saharan Africa, where the rate of M. tuberculosis transmission is very high, the proportion of clustered M. tuberculosis isolates from patients with smear-positive TB has varied from 38 to 47% (18, 28, 29, 44). The proportion of isolates appearing in clusters was even higher (67%) for isolates from male patients in a gold-mining community in South Africa with a particularly high incidence of TB (16). In those studies, however, isolates appearing in clusters were equally distributed among HIV-positive and HIV-negative TB patients.
During the 1990s Ethiopia experienced a severe HIV epidemic, with the prevalence of pregnant women in Addis Ababa infected with HIV rising from 6% in 1989 to 18% in 1997 (13). A TB epidemic has coincided with this development, and in 1999, Ethiopia was ranked number 8 among the 23 countries with the highest total TB burden (45). In 1997, about 30% of all new TB cases were believed to occur in HIV-positive individuals (11). In areas such as Ethiopia where HIV infection is highly endemic, the relative importance of reactivation and recent infection is not known. Information in this field may have important implications for TB control policies (5, 23). Hermans et al. (21) used DNA fingerprinting to describe Ethiopian M. tuberculosis strains. In that study, 48% of strains collected in 1992 and 1993 appeared in clusters, and more than half of the clustered strains had less than four IS6110 bands. Secondary typing of these strains by using the sequences of the PGRS and direct repeat regions as genetic markers decreased the clustering frequency to about 36%. However, no analysis of factors potentially associated with clustering was performed.
As part of ongoing studies on the interaction between pulmonary TB and HIV infection in Addis Ababa, we analyzed the DNA fingerprinting and drug resistance patterns of M. tuberculosis isolates obtained from consecutive patients with suspected pulmonary TB with the objective of determining the factors associated with recent infection and the development of resistance.
MATERIALS AND METHODS
Setting.
Ethiopia is implementing a direct-observation TB control (DOTS) program according to the recommendations of the World Health Organization. In 1998, about 22% of all smear-positive TB patients in Ethiopia were reached by the expanding DOTS program, with a reported cure rate of 74% (45). Patients are identified through passive case finding. Assessment of close contacts of patients with smear-positive pulmonary TB is encouraged, but at present it is not routinely implemented (30; A. Wolde [National Tuberculosis and Leprosy Control Programme], personal communication). The present study was performed at Black Lion University Hospital in Addis Ababa. Patients are referred to Black Lion University Hospital from other health care institutions, but they can also seek care on their own initiative. During the period from March 1996 through February 1997, 1,538 patients were diagnosed with TB; this made up 16.1% of the 9,548 TB patients registered in DOTS program areas during an overlapping period from September 1996 through August 1997.
Patients.
Five hundred twelve consecutive patients aged 15 years or older with suspected pulmonary TB were clinically assessed by medical residents at the medical outpatient department of the Black Lion University Hospital and were included in the study. Inclusion criteria were informed consent and clinical suspicion of pulmonary TB strong enough to warrant a direct sputum smear for acid-fast bacilli (AFB). Exclusion criteria were treatment for TB within the last 3 months. In March 1996 patients were recruited only on Mondays, Wednesdays, and Fridays, owing to laboratory limitations, but from April to November 1996 patients were recruited on all weekdays. According to the registry of the TB laboratory at Black Lion University Hospital, 83% (377 of 453) of the patients examined for AFB during the period with daily recruitment were included in the study population. Of the remaining 17% of the patients, we estimate that 5% had been eligible for the study, whereas 12% did not meet the inclusion criteria. Two research nurses registered the patient's demographic data, residence, and information on possible previous treatment for TB. Posteroanterior chest radiography was performed for all patients.
In the final analysis of the patients, three patients were excluded: two because they were less than 15 years of age and one because of failure to produce sputum for direct microscopy and culture. Thus, 509 patients remained for further analysis.
Case definition.
A patient with a verified case of pulmonary TB was defined as one with relevant clinical symptoms and a sputum culture positive for M. tuberculosis.
Laboratory procedures.
Direct microscopic examination of sputum for AFB after Ziehl-Neelsen staining was done with three consecutive sputum samples (one obtained during a spot check and two obtained in the morning) at the Black Lion University Hospital TB laboratory. The sputum samples were kept at 4°C and, after microscopy for the detection of AFB, were sent to the TB laboratory at the Armauer Hansen Research Institute (AHRI) in Addis Ababa for further examination. At AHRI the three samples from each patient were pooled, digested with sodium hypochlorite, concentrated, and then reexamined by Ziehl-Neelsen staining (3). Mycobacterial culture on conventional Löwenstein-Jensen (LJ) egg medium and LJ medium containing 0.6% sodium pyruvate was performed with all sputum samples. Before culture the samples were digested and decontaminated of nonmycobacterial microorganisms by the sodium lauryl sulfate method (17). The tubes were incubated at 37°C in 5% CO2 for 1 week and thereafter at 37°C in air for another 7 weeks and were checked once a week for mycobacterial growth. Growth of mycobacteria was confirmed by detection of a typical colonial morphology and by microscopy for AFB.
Cultures confirmed to be positive by Ziehl-Neelsen staining at AHRI were transported at room temperature on LJ egg medium to the Swedish Institute for Infectious Disease Control (SMI), Stockholm, Sweden, for further characterization. Subculturing was performed on LJegg medium. Cultures with atypical macroscopic or microscopic characteristics were tested with nucleic acid probes specific for the M. tuberculosis complex and the Mycobacterium avium complex by using the Accuprobe system (Gen-Probe, San Diego, Calif.).
Drug susceptibility testing of all isolates was performed by the radiometric respirometry method, according to the BACTEC system (Becton-Dickinson, Sparks, Md.) (35). The drugs tested included streptomycin (4 mg/liter), isoniazid (0.2 mg/liter), ethambutol (5 mg/liter), and rifampin (2 mg/liter).
A standardized method was used for the analysis of IS6110 restriction fragment length polymorphisms (RFLPs) (37). M. tuberculosis DNA was extracted and digested with the restriction endonuclease PvuII, and the resulting fragments were separated by electrophoresis before they were submitted to Southern blotting. Hybridization was performed with a 245-bp PCR fragment of the IS6110 sequence as a probe; the fragment was nonradioactively labeled with peroxidase and was subsequently visualized by use of an enhanced chemiluminescence kit (Amersham International plc, Little Chalfont, United Kingdom).
The IS6110 DNA patterns were analyzed with Gelcompar software (version 3.1b; Applied Maths BVBA, Kortrijk, Belgium) following scanning of the radiographs at 74.8 dots/cm (190 dots/in.; HP Scanjet IIcx/T; Hewlett-Packard, Camas, Wash.). On the basis of the molecular sizes of the hybridization fragments and the number of IS6110 copies for each isolate, the fingerprinting patterns were compared by the unweighted pair group method of arithmetic averaging by using the Dice coefficient. Dendrograms were constructed to show the relatedness among the strains by using a previously described algorithm (37), and similarity matrices were generated to visualize the relatedness between the banding patterns of all isolates.
Isolates with less than five bands by RFLP analysis were further characterized by spoligotyping, which is based on the amplification of the polymorphic direct repeat locus (25). These isolates were also tested biochemically for niacin production, as described by Wayne (43). The hybridization patterns of the amplified DNA were obtained by using multiple synthetic spacer oligonucleotides which are covalently bound to a membrane (Isogen Bioscience BV, Maarssen, The Netherlands). PCR and hybridization were performed as described previously (25).
Serum samples were screened for the presence of HIV antibodies by using an enzyme-linked immunosorbent assay (ELISA) for both HIV type 1 (HIV-1) and HIV-2 (Enzygnost anti-HIV-1/HIV-2; Behringwerke AG, Marburg, Germany). Positive samples were reanalyzed by the same ELISA. If the sample was positive by retesting, the result was confirmed by an HIV-1 ELISA (Wellcozyme anti HIV-1 VK 57; Murex Diagnostics Ltd., Dartford, United Kingdom). If the sample was positive by all three ELISAs, the result was considered definite. If the test results were inconsistent by the different ELISAs, the definite result was established at SMI by Western blotting (version 2.2; Diagnostic Biotechnology, Genelabs Diagnostics SA, Geneva, Switzerland).
Drug resistance.
According to recent recommendations (38), drug resistance in patients with new cases of TB refers to drug resistance in patients with no history of treatment for TB. Drug resistance in patients with previously treated cases of TB pertains to drug resistance in patients with a history of treatment for TB of at least 1 month's duration. The term “any resistance” means resistance to any of the primary TB drugs, and the term “multidrug resistance” refers to resistance to at least isoniazid and rifampin.
Definition of a cluster.
A cluster was defined as a group of isolates with identical RFLP patterns. For isolates that apparently belonged to a cluster but that had less than five bands by RFLP analysis of IS6110, an identical spoligotype was required to meet the criterion for a cluster.
Statistical analysis.
JMP software (version 3.2; SAS Institute Inc., Cary, N.C.) and Epi-Info 2000 software (version 1.0.5; Centers for Disease Control and Prevention, Atlanta, Ga.) were used for statistical analysis. The χ2 test was used to assess differences in proportions, and multivariate logistic regression analysis was used to assess the relationship between variables and outcomes. Variables from the univariate analysis were tested in a multivariate model for the analysis of factors associated with clustering, but only significant variables were included in the final regression model. Age was entered a priori. P values of 0.05 or less were regarded as significant.
Ethical clearance.
The patients were included in the study after they verbally provided informed consent. Testing for HIV was done anonymously with coded serum samples that were unlinked to the patient's identity. If the patient's HIV infection status was required on clinical grounds, testing was performed with the patient's approval and outside the protocol of the study. Patients who had initially been dismissed because they were not considered to have TB because of negative sputum smears but whose sputum was later positive by culture were reappointed for TB treatment. The study was approved by the ethical committees of the Faculty of Medicine at Addis Ababa University, the Addis Ababa University Research and Publications Office, and the Karolinska Institute, Stockholm, Sweden.
RESULTS
Patient characteristics and characterization of isolates.
At AHRI, cultures of sputa from 170 of the 509 patients (33.4%) were positive for mycobacteria. Of these, isolates from 123 (72.4%) could later be subcultured at SMI. Of the remaining 47 cultures, 8 were contaminated and the isolates in 39 did not survive transportation.
Four of the 123 isolates subcultured at SMI were subjected to nucleic acid probe analysis due to atypical colonial morphologies. In total, 121 isolates were characterized as M. tuberculosis and 2 isolates were characterized as members of the M. avium complex. The HIV infection status and background data for the 121 patients positive for M. tuberculosis at SMI did not differ from those for the 47 patients whose isolates were lost for further analysis, except that the latter group had a larger proportion of male patients (83.0 versus 64.5%).
Drug resistance patterns.
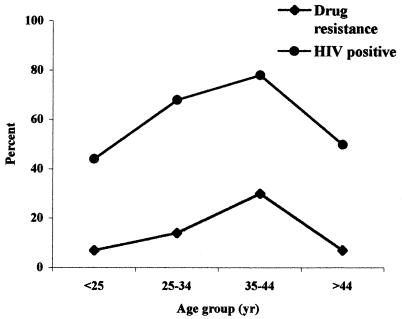
The drug resistance patterns of the isolates are shown in Table 1. The occurrence of resistance was not related to either HIV infection status (with 6 of 49 [12.2%] resistant isolates coming from HIV-negative patients and 11 of 72 [15.3%] coming from HIV-positive patients [P = 0.64]) or to positivity by microscopy of sputum smears, age, sex, residency, or cavitary TB (data not shown). However, the highest rate of resistance (29.6%) and the highest prevalence of HIV infection (77.8%) occurred in the group aged 35 to 44 years (Fig. 1). A significant association between the occurrence of resistance and previous TB treatment was not observed.
TABLE 1.
Drug resistance in M. tuberculosis isolates from Ethiopian patients with new and previously treated pulmonary TB
| Drug resistancea | No. (%) of isolates from patients with:
|
||
|---|---|---|---|
| Total | New cases of TB | Previously treated TB | |
| Total | 121 | 103 | 18 |
| Resistance to any drug | 17 (14.0) | 15 (14.6) | 2 (11.1) |
| Resistance to any of the followinga: | |||
| H | 10 (8.3) | 9 (8.7) | 1 (5.6) |
| R | 3 (2.5) | 2 (1.9) | 1 (5.6) |
| S | 9 (7.4) | 8 (7.8) | 1 (5.6) |
| E | 2 (1.7) | 1 (0.9) | 1 (5.6) |
| Resistance to one drug only | |||
| H | 5 (4.1) | 5 (4.9) | 0 |
| R | 1 (0.8) | 1 (0.9) | 0 |
| S | 5 (4.1) | 5 (4.9) | 0 |
| E | 0 | 0 | 0 |
| Resistance to more than one drug | |||
| HS | 3 (2.5) | 2 (1.9) | 1 (5.6) |
| HE | 1 (0.8) | 1 (0.9) | 0 |
| ER | 1 (0.8) | 0 | 1 (5.6) |
| HRS | 1 (0.8) | 1 (0.9) | 0 |
H, isoniazid; R, rifampin; S, streptomycin; E, ethambutol.
FIG. 1.
Prevalence of HIV infection and overall drug resistance among Ethiopian patients with pulmonary TB stratified by age group.
RFLP patterns and spoligotyping.
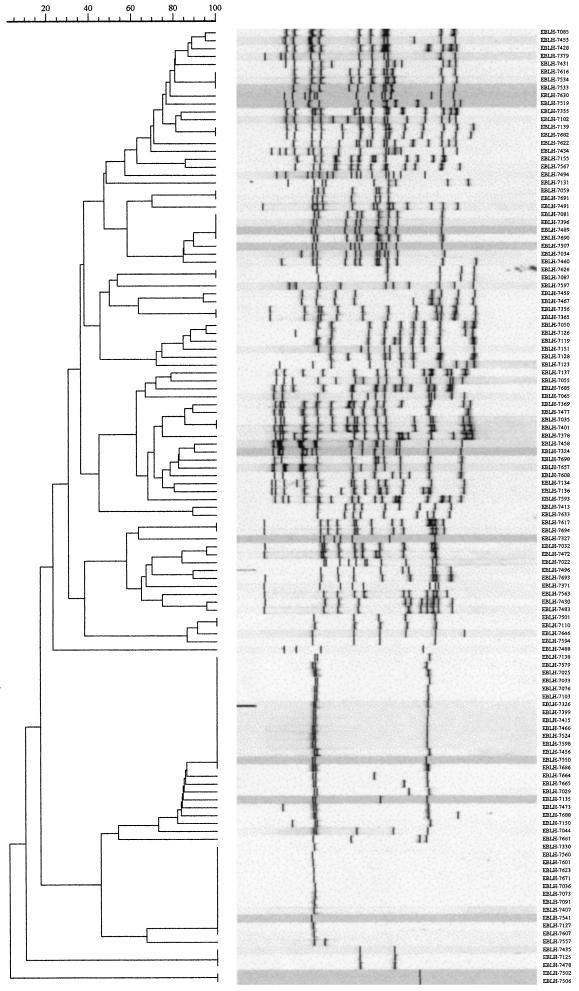
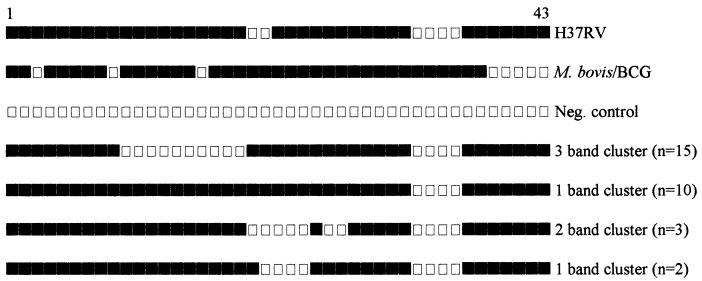
Among the 121 M. tuberculosis isolates, RFLP analysis revealed 81 distinct patterns (1 to 17 IS6110 copies), 68 of which were unique (Fig. 2). The remaining 53 isolates (43.8%) belonged to a total of 13 clusters (Table 2), of which 9 clusters (21 isolates) were identified by RFLP analysis alone. Thirty-two isolates belonged to two major clusters with three bands (n = 15) and one band (n = 12), respectively, and two smaller clusters with two bands (n = 3) and one band (n = 2), respectively. Typing of these isolates showed identical patterns for all but two isolates, which belonged to the larger one-band cluster (Fig. 3). These two isolates were therefore considered nonclustered. The final overall proportion of clustered to nonclustered isolates was 42.1% (95% confidence interval [CI], 33.3 to 50.9%). Among the clustered isolates, the isolates in the two major clusters with three bands and one band, respectively, constituted 49.0% of all isolates and 54.1% of the isolates from Addis Ababa residents. All isolates with less than five bands were niacin positive. None of them exhibited the specific spoligotyping patterns of M. africanum, M. bovis, or M. canetti. Thus, all strains possessed spacers 39 to 43, which are typically lacking in classical M. bovis isolates (25); none of them showed the simultaneous absence of spacers 8, 9, and 39, which is characteristic of M. africanum isolates (24, 41); and they all lacked the typical M. canetti spoligotyping patterns (presence of spacers 30 and 36 and a lack of all others spacers) (39; our unpublished data).
FIG. 2.
IS6110 banding patterns and similarity matrices for 121 M. tuberculosis isolates from Ethiopian patients with pulmonary TB. Banding patterns are ordered by similarity. The corresponding dendrograms are to the left of the panels. The positions of the bands in each lane are adjusted (normalized) so that the band positions for all strains are comparable. The scale depicts similarity coefficients (which are defined elsewhere [37]).
TABLE 2.
Cluster size and number of clusters among M. tuberculosis isolates from 121 Ethiopian patients with pulmonary TB, with special reference to the situation in Addis Ababa
| Cluster size (no. of isolates) | No. of clusters | Total no. (%) of isolates (n = 121) | No. of clusters for Addis Ababa residents | Total no. (%) of isolates for Addis Ababa residents (n = 91) |
|---|---|---|---|---|
| 2 | 8 | 16 | 7 | 14 |
| 3 | 2 | 6 | 1 | 3 |
| 4 | 1 | 4 | 0 | 0 |
| 10 | 1 | 10 | 1 | 8 |
| 15 | 1 | 15 | 1 | 12 |
| Total | 13 | 51 (41.2)a | 10 | 37 (40.7)b |
95% CI, 33.3 to 50.9%.\
95% CI 30.6 to 50.8%.
FIG. 3.
Schematic representation of the spoligotypes of M. tuberculosis isolates belonging to four clusters with less than five bands by RFLP analysis of IS6110 (Fig. 2) compared with the spoligotypes of reference strains H37RV and M. bovis BCG. The filled rectangles depict positive hybridization signals, and empty rectangles represent a lack of hybridization.
Factors associated with clustering.
In a univariate analysis of possible associations between clustering and demographic and clinical parameters, only positivity for HIV was significantly associated with clustering (P = 0.036), with 50.7% of isolates from HIV-positive individuals belonging to clusters and 30.6% of isolates from HIV-negative individuals belonging to clusters. In a multivariate regression analysis after adjustment for age, HIV positivity remained an important predictor of clustering (Table 3).
TABLE 3.
Risk factors associated with clustering in Ethiopian patients with pulmonary TB by multivariate regression analysis
| Variable | All patients
|
Addis Ababa residents
|
||||||
|---|---|---|---|---|---|---|---|---|
| Clustered (n = 51) | Nonclustered (n = 70) | Adjusted ORa (95% CI) | P value | Clustered (n = 37) | Nonclustered (n = 54) | Adjusted ORb (95% CI) | P value | |
| Mean age (yr [median; range]) | 29.3 (29.0;16-50) | 31.1 (28.0;15-62) | 3.69 (0.61-25.48) | 0.17 | 29.7 (30;17-50) | 31.2 (29;15-65) | 2.43 (0.25-27.51) | 0.45 |
| Sex (no. [%] of patients) | ||||||||
| Male | 30 (58.8) | 48 (68.6) | 19 (51.4) | 38 (70.4) | 1.00 | 0.11 | ||
| Female | 21 (41.2) | 22 (31.4) | 18 (48.7) | 16 (29.6) | 2.10 (0.85-5.25) | |||
| No. (%) of patients with: | ||||||||
| Residence in Addis Ababa | 40 (78.4) | 51 (72.9) | ||||||
| Previous TB treatment | 8 (15.7) | 10 (14.3) | 5 (13.5) | 7 (13.0) | ||||
| Isolate resistance with (any drug) | 7 (13.2) | 10 (14.7) | 7 (16.7) | 7 (14.3) | ||||
| Positive for AFB by direct exam- ination of a sputum smear | 28 (54.9) | 39 (55.7) | 19 (51.4) | 29 (53.7) | ||||
| Cavitary TB | 16 (31.4) | 32 (32.9) | 11 (29.7) | 17 (31.5) | ||||
| HIV status (no. [%] of patients) | ||||||||
| Positive | 37 (70.6) | 36 (51.4) | 2.55 (1.17-5.80) | 0.021 | 28 (75.7) | 32 (59.3) | 2.33 (0.90-6.45) | 0.088 |
| Negative | 15 (29.4) | 34 (48.6) | 1.00 | 9 (24.3) | 22 (40.7) | 1.00 | ||
The final model includes age and HIV infection status. The remaining variables are not significant in the multivariate model.\
The final model includes age, sex, and HIV infection status. The remaining variables are not significant in the multivariate model.
For the subgroup of 91 isolates (75.2% of total) recovered from patients who permanently resided in Addis Ababa, univariate analysis showed no significant association between clustering and demographic and clinical parameters. However, HIV positivity was more common among patients infected with clustered isolates (P = 0.11). Furthermore, a larger proportion of female patients than male patients was infected with isolates that belonged to a cluster, with a ratio of clustered versus nonclustered isolates of 1.7 for female TB patients compared to a ratio of clustered versus nonclustered isolates of 0.7 for male TB patients (P = 0.068). When isolates from the subgroup of individuals who permanently resided in Addis Ababa were entered into a multivariate regression model after adjustment for age and sex, the odds ratio (OR) for HIV positivity was 2.33 (95% CI, 0.90 to 6.45), indicating a higher tendency of clustering among isolates from HIV-positive patients (Table 3). Although it did not reach statistical significance, an indication of increased clustering was also observed among isolates recovered from women, with an OR of 2.10 (95% CI, 0.85 to 5.25).
To further analyze possible interactions between female sex and HIV infection status, a subgroup analysis of HIV-positive women aged 15 to 44 years was performed by comparing the proportion of clustered M. tuberculosis isolates among the isolates from this group with the corresponding proportion among the isolates from the remaining patients. The proportion of clustered isolates for HIV-positive women aged 15 to 44 years was 17 of 28 (60.7%), whereas it was 34 of 93 (36.6%) for the remaining patients, giving an OR of 2.68 (95% CI, 1.04 to 7.00) (P = 0.02). The corresponding figures for HIV-positive women in this age group residing in Addis Ababa were 14 of 23 (60.9%) versus 23 of 68 (33.8%), giving an OR of 3.04 (95% CI, 1.04 to 9.08) (P = 0.02).
Of the 17 isolates that showed drug resistance, 7 (41.2%) isolates from previously untreated patients were distributed in four clusters. Of these, three isolates belonged to the cluster with three bands. Two of these three isolates were resistant to isoniazid and streptomycin; the third isolate was resistant to isoniazid and streptomycin as well as to rifampin. Two isolates were in a cluster with five bands, and one of the isolates was resistant to streptomycin and the other was resistant to rifampin. Five of eight patients (62.5%) in the group aged 35 to 44 years were infected with clustered isolates, and these isolates consisted of the isolates that belonged to the clusters with three bands and five bands described above. These patients were all Addis Ababa residents. The two resistant isolates from previously treated TB patients had unique RFLP patterns.
DISCUSSION
To our knowledge, this is the first study from sub-Saharan Africa that has analyzed the molecular epidemiology and drug resistance of M. tuberculosis isolates in relation to HIV infection states in consecutive outpatients of both sexes irrespective of sputum smear status. A large (42.1%) proportion of our M. tuberculosis isolates belonged to clusters, indicating a high prevalence of recent transmission. Similar prevalences of clustering were found in population-based studies from Botswana (42%) (29), South Africa (45%) (44), and Estonia (49%) (27), as well as among randomly sampled patients from Ethiopia in 1992 and 1993 (36%) (21).
Our demonstration of a higher frequency of clustered isolates among HIV-positive TB patients than among HIV-negative TB patients is in agreement with the results of previous studies from urban settings in the United States (2, 36) as well as with the results of a recent study from Brazil (14). Previous population-based studies from sub-Saharan Africa have failed to show an association between clustering and HIV positivity (16, 18, 28, 29, 44). A recruitment bias through exclusion of women (16) or AFB-negative HIV-infected patients (18, 28, 29, 44) may have influenced the outcomes of those studies. With more advanced immunosuppression, HIV-positive patients with culture-verified TB present with an uncharacteristic pulmonary disease resembling primary pulmonary TB and sputum smears that are negative for AFB at higher rates (19). By excluding smear-negative, culture-positive patients in studies of the molecular epidemiology of TB in areas where HIV is highly endemic, there is an obvious risk of excluding a significant proportion of HIV-positive patients at risk of recent infection. In our own study population, even after concentration, only 50% of sputum smears from HIV-positive patients with culture-positive pulmonary TB were positive for AFB, whereas 81% of the corresponding sputum smears from HIV-negative patients were positive for AFB (P < 0.001), as reported previously (3).
Male sex is a risk factor for TB globally (22). Although in our study male TB patients were in the majority, a trend toward increased clustering was observed with strains from female patients residing in Addis Ababa, and this trend was significant in a subgroup analysis of HIV-positive women aged 15 to 44 years in comparison with the remaining patients. An association between clustering and female sex was also noted at one of four study sites in a recent study from Botswana (29). In other studies from sub-Saharan Africa with a predominance of isolates recovered from men, male sex has not been associated with clustering (18, 44). Comparison of infection rates with disease rates in some settings suggests that women of reproductive age may progress more rapidly from TB infection to disease, whereas in men a similar rapid development takes place at older ages (22). Dual HIV infection and TB in young women may add to this risk (22), and in sub-Saharan Africa the prevalence of HIV infection is higher in young female TB patients than in young male TB patients (22). If it is assumed that the clustering of isolates is an indicator of recent transmission, our data suggest an increased likelihood of recent TB among women, which may be linked to a higher prevalence of HIV infection in women of reproductive age. In due time, this may influence notification for TB by sex. In fact, since 1995, among individuals aged 15 to 24 years in Addis Ababa, the number of women notified that they have smear-positive TB has consistently surpassed the number of men (G. Fisha [National Tuberculosis and Leprosy Control Programme], personal communication).
Drug-resistant M. tuberculosis threatens national TB control programs in several countries. The spectrum and prevalence of drug resistance in our study are comparable to those in other countries in sub-Saharan Africa during the period from 1994 to 1997, with resistance to isoniazid and streptomycin being more common than resistance to rifampin and ethambutol (33). However, considering that the overall rates of resistance among isolates from previously untreated patients in seven out of eight countries surveyed in sub-Saharan Africa ranged from 3.4 to 13.4%, the corresponding resistance rate of 14.6% in our study is comparatively high.
Previous data on patterns of resistance among isolates from Ethiopia are scarce, as mycobacterial culture and susceptibility testing are not routinely performed. A health care institution-based study conducted in Addis Ababa in 1994 (9) showed an overall prevalence of drug resistance of 15.6% among isolates from patients with new cases of TB. Resistance to streptomycin (10.2%) and isoniazid (8.4%) was most commonly observed, whereas the rate of resistance to rifampin was low (1.8%) and resistance to ethambutol did not occur. Testing for HIV was not performed in that study. In contrast, a study conducted in Harar, in eastern Ethiopia, in 1995 showed higher overall prevalences of resistance in patients with new and previously treated cases of TB, namely, 32.5 and 51.2%, respectively (31). Whereas drug resistance in patients with new cases was not associated with HIV infection, in previously treated patients, drug resistance was significantly associated with HIV infection and resulted in higher numbers of treatment failures (32). Among 107 M. tuberculosis strains recovered in 1993 and 1994 from retreated pateints in Addis Ababa, the prevalence of resistance to one or more of the first-line drugs was about 50% (1). However, drug resistance was not related to HIV infection status. This lack of an association between HIV infection status and mycobacterial drug resistance is consistent with the results of other studies from sub-Saharan Africa (4, 8, 10, 25, 42) and our own data. However, in our study, a high rate of resistance was found among isolates from patients aged 35 to 44 years. These patients were also characterized by a high prevalence of HIV infection and by a relatively high proportion of clustered M. tuberculosis isolates. Our findings suggest that the increased risk for recent transmission of M. tuberculosis among HIV-positive individuals also implies a risk for transmission of resistant strains.
In studies by Karstead et al. (26) and Churchyard et al. (4), a previous history of TB treatment was clearly associated with the development of drug resistance. In our study no difference in rates of resistance between isolates from previously untreated patients and treated patients was seen. However, the lack of association between resistance and previous treatment could be due to the small number of patients in this group. The low rate of resistance to rifampin is probably a consequence of the late (1997) introduction of this drug in Ethiopia.
Like Hermans et al. (21), we found two dominating families of strains: one with a three-band pattern and one with a one-band pattern. However, in contrast to their findings, further subtyping by spoligotyping in our study showed an almost complete homogeneity of the isolates within these clusters, which may indicate the more efficient transmission of these strains in the population at present. Naturally, larger sample sizes and longer recruitment periods increase the percentage of clusters among recovered M. tuberculosis isolates (16, 40). Considering the comparatively small sample size and the relatively short period of patient recruitment of this study, our results likely underestimate the proportion of isolates that were recently transmitted and also the proportion of resistant isolates transmitted among TB patients in the Addis Ababa area.
In conclusion, our finding of a relatively high proportion of clusters among isolates from HIV-positive individuals suggests that recent transmission within this group may be an important cause of the rising incidence of TB. This underlines the importance of strengthening classical case finding and treatment of smear-positive patients according to the ongoing DOTS program. In addition, the introduction of more active case finding among HIV-positive young adults with TB is necessary to interrupt the chain of transmission. Also, as our data suggest that Ethiopia may soon face a rapid increase in the number of new cases of drug-resistant TB, regular monitoring of drug resistance patterns is essential.
Acknowledgments
We thank nurses Muleta Gerbaba and Zauditu Deressa at the Chest Unit, Black Lion University Hospital, for excellent assistance with patients and samples and research assistants Selamawit Tadesse and Abebech Demissie at AHRI for careful processing of mycobacterial cultures and HIV serology. We thank Gunilla Källenius for constructive criticism, Tuija Koivula at SMI for excellent technical assistance and Jan Kowalski for statistical assistance.
This study was financially supported by the Swedish International Development Cooperation Agency and the Centre for International Health in Bergen, Norway.
REFERENCES
- 1.Abate, G., H. Miörner, O. Ahmed, and S. E. Hoffner. 1998. Drug resistance in Mycobacterium tuberculosis strains isolated from re-treatment cases of pulmonary tuberculosis in Ethiopia: susceptibility to first-line and alternative drugs. Int. J. Tuberc. Lung Dis. 2:580-584. [PubMed] [Google Scholar]
- 2.Alland, D., G. E. Kalkut, A. R. Moss, R. A. McAdam, J. A. Hahn, W. Bosworth, E. Drucker, and B. R. Bloom. 1994. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N. Engl. J. Med. 330:1710-1716. [DOI] [PubMed] [Google Scholar]
- 3.Bruchfeld, J., G. Aderaye, I. Berggren Palme, B. Bjorvatn, G. Källenius, and L. Lindquist. 2000. Sputum concentration improves diagnosis of tuberculosis in a high HIV prevalence setting. Trans. R. Soc. Trop. Med. Hyg. 94:677-680. [DOI] [PubMed] [Google Scholar]
- 4.Churchyard, G. J., E. L. Corbett, I. Kleinschmidt, D. Mulder, and K. M. De Cock. 2000. Drug-resistant tuberculosis in South African gold miners: incidence and associated factors. Int. J. Tuberc. Lung Dis. 4:433-440. [PubMed] [Google Scholar]
- 5.Cohn, D. L., and R. J. O'Brien. 1998. The use of restriction fragment length polymorphism (RFLP) analysis for epidemiological studies of tuberculosis in developing countries. Int. J. Tuberc. Lung Dis. 2:16-26. [PubMed] [Google Scholar]
- 6.Coronado, V. G., C. M. Beck-Sague, M. D. Hutton, B. J. Davies, P. Nicholas, C. Villareal, C. L. Woodely, J. O. Kilburn, J. T. Crawford, T. R. Frieden, R. L. Sinkowitz, and W. R. Jarvis. 1993. Transmission of multi-drug resistant Mycobacterium tuberculosis among persons with human immunodeficiency virus infection in an urban hospital: epidemiologic and restriction fragment length polymorphism analysis. J. Infect. Dis. 168:1052-1055. [DOI] [PubMed] [Google Scholar]
- 7.Daley, C. L., P. M. Small, G. F. Schecter, G. K. Schoolnik, R. A. McAdam, W. R. Jacobs, Jr., and P. C. Hopewell. 1992. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphism. N. Engl. J. Med. 326:231-235. [DOI] [PubMed] [Google Scholar]
- 8.Davies, G. R., M. Pillay, A. W. Sturm, and D. Wilkinson. 1999. Emergence of multidrug-resistant tuberculosis in a community-based directly observed treatment programme in rural South Africa. Int. J. Tuberc. Lung Dis. 3:799-804. [PubMed] [Google Scholar]
- 9.Demissie, M., M. Gebeyehu, and Y. Berhane. 1997. Primary resistance to anti-tuberculosis drugs in Addis Ababa, Ethiopia. Int. J. Tuberc. Lung Dis. 1:64-67. [PubMed] [Google Scholar]
- 10.Dosso, M., D. Bonard, P. Msellati, A. Bamba, C. Doulhourou, V. Vincent, M. Peyre, M. Traore, K. Koofi, and I. M. Coulibaly. 1999. Primary resistance to antituberculosis drugs: a national survey conducted in Cote d'Ivoire in 1995-1996. Int. J. Tuberc. Lung Dis. 3:805-809. [PubMed] [Google Scholar]
- 11.Dye, C., S. Scheele, P. Dolin, V. Pathania, M. C. Raviglione, et al. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 12.Edlin, B. R., J. I. Tokars, M. H. Grieco, J. T. Crawford, J. Williams, E. M. Sordillo, K. R. Ong, J. O. Kilburn, S. W. Dooley, K. G. Castro, W. R. Jarvis, and S. D. Holmberg. 1992. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 326:1514-1521. [DOI] [PubMed] [Google Scholar]
- 13.Epidemiology and AIDS Department, Ministry of Health. 1998. In AIDS in Ethiopia: background, projections, impacts, interventions, 2nd ed., p. 3-4. Epidemiology and AIDS Department, Ministry of Health, Addis Ababa, Ethiopia.
- 14.Ferrazoli, L., M. Palaci, L. R. M. Marques, L. F. Jamal, J. B. Afiune, E. Chimara, M. C. Martins, M. A. da Silva Telles, C. A. F. Oliveira, M. C. Palhares, D. T. A. Spada, and L. W. Riley. 2000. Transmission of tuberculosis in an endemic urban setting in Brazil. Int. J. Tuberc. Lung Dis. 4:18-25. [PubMed] [Google Scholar]
- 15.Girardi, E., M. C. Raviglione, G. Antonucci. P. Godfrey-Fausett, and G. Ippolito. 2000. Impact of the HIV epidemic on the spread of other diseases: the case of tuberculosis. AIDS 14(Suppl. 3):S47-S56. [PubMed] [Google Scholar]
- 16.Godfrey-Faussett, P., P. Sonnenberg, S. C. Shearer, M. C. Bruce, C. Mee, L. Morris, and J. Murray. 2000. Tuberculosis control and molecular epidemiology in a South African gold-mining community. Lancet 356:1066-1071. [DOI] [PubMed] [Google Scholar]
- 17.Groothuis, D. G., and M. D. Yates (ed.). 1991. In: Manual of diagnostic and public health mycobacteriology, 2nd ed., p. 63. Bureau of Hygiene and Tropical Diseases, European Society for Mycobacteriology, London, United Kingdom.
- 18.Haas, W. H., G. Engelmann, B. Amthor, S. Shyamba, F. Mugala, M. Felten, M. Rabbow, M. Leichsenring, O. J. Oosthuizen, and H. J. Bremer. 1999. Transmission dynamics of tuberculosis in a high incidence country: prospective analysis by PCR DNA fingerprinting. J. Clin. Microbiol. 37:3975-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harries, A. D. 1998. The association between HIV and tuberculosis in the developing world, p. 322-323. In P. D. O. Davies (ed.), Clinical tuberculosis. Chapman & Hall, London, United Kingdom.
- 20.Havlir, D. V., and P. F. Barnes. 1999. Tuberculosis in patients with human immunodeficiency virus infection. N. Engl. J. Med. 340:367-373. [DOI] [PubMed] [Google Scholar]
- 21.Hermans, P. W. M., F. Messadi, H. Guebrexabher, D. van Soolingen, P. E. W. de Haas, H. Heersma, H. de Neeling, A. Ayoub, F. Portaels, D. Frommel, M. Zribi, and J. D. A. van Embden. 1995. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia and the Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J. Infect. Dis. 171:1504-1513. [DOI] [PubMed] [Google Scholar]
- 22.Holmes, C. B., H. Hausler, and P. Nunn. 1998. A review of sex differences in the epidemiology of tuberculosis. Int. J. Tuberc. Lung Dis. 2:96-104. [PubMed] [Google Scholar]
- 23.Kaeto-Maeda, M., and P. M. Small. 2000. How molecular epidemiology has changed what we know about tuberculosis. West. J. Med. 172:256-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Källenius, G., T. Koivula, S. Ghebremichael, S. E. Hoffner, R. Norberg, E. Svensson, F. Dias, B.-I. Marklund, and S. B. Svenson. 1999. Evolution of clonal traits of Mycobacterium tuberculosis complex in Guinea Bissau. J. Clin. Microbiol. 37:3872-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamerbeek, J., L. Shouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karstead, A. S., N. Jones, M. Khoosal, and H. H. Crewe-Brown. 1998. The bacteriology of pulmonary tuberculosis in a population with high human immunodeficiency virus seroprevalence. Int. J. Tuberc. Lung Dis. 4:312-316. [PubMed] [Google Scholar]
- 27.Kruuner, A., S. E. Hoffner, H. Sillastu, M. Danilovits, K. Levina, S. B. Svenson, S. Ghebremichael, T. Koivula, and G. Källenius. 2001. Spread of drug-resistant pulmonary tuberculosis in Estonia. J. Clin. Microbiol. 9:3339-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lockman, S., J. D. Sheppard, M. Mwasekaga, T. A. Kenyon, N. J. Binkin, C. R. Braden, C. L. Woodley, D. W. Rumisha, and J. W. Tappero. 2000. DNA fingerprinting of a national sample of Mycobacterium tuberculosis isolates, Botswana 1995-1996. Int. J. Tuberc. Lung Dis. 4:584-587. [PubMed] [Google Scholar]
- 29.Lockman, S., J. D. Sheppard, C. R. Braden, M. J. Mwasekaga, C. L. Woodley, T. A. Kenyon, N. J. Binkin, M. Steinman, F. Montsho, M. Kesupile-Reed, C. Hirschfeldt, M. Notha, T. Moeti, and J. W. Tappero. 2001. Molecular and conventional epidemiology of Mycobacterium tuberculosis in Botswana: a population-based prospective study of 301 pulmonary tuberculosis patients. J. Clin. Microbiol. 39:1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ministry of Health. 1997. In Manual, National Tuberculosis and Leprosy Control Programme, p. 17-20. Ministry of Health, Addis Ababa, Ethiopia.
- 31.Mitike, G., D. Kebede and H. Yeneneh. 1997. Prevalence of antituberculosis drug resistance in Harar tuberculosis centre, Ethiopia. East Afr. Med. J. 74:158-161. [PubMed] [Google Scholar]
- 32.Mitike, G., D. Kebede, and H. Yeneneh. 1997. HIV infection and antituberculosis drug resistance among pulmonary tuberculosis patients in Harar tuberculosis centre, Ethiopia. East Afr. Med. J. 74:154-157. [PubMed] [Google Scholar]
- 33.Pablos-Mendez, A., M. C. Raviglione, A. Laszlo, N. Binkin, H. L. Rieder, F. Bustreo, D. Cohn, C. S. B. Lambregts-van Weezenbeek, S. J. Kim, P. Chaulet, and P. Nunn. 1998. Global surveillance for antituberculosis drug resistance, 1994-1997. N. Engl. J. Med. 338:1641-1649. [DOI] [PubMed] [Google Scholar]
- 34.Selwyn, P. A., D. Hartel, V. A. Lewis, E. E. Schoenbaum, S. H. Vermund, R. S. Klein, A. T. Walker, and G. H. Friedland. 1989. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Engl. J. Med. 320:545-550. [DOI] [PubMed] [Google Scholar]
- 35.Siddiqi, S. H., J. P. Libonati, and G. Middlebrook. 1981. Evaluation of a rapid radiometric method for drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 13:908-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Small, P. M., P. C. Hopewell, S. P. Singh, A. Paz, J. Parsonnet, D. C. Ruston, G. F. Schecter, C. L. Daley, and G. K. Schoolnik. 1994. The epidemiology of tuberculosis in San Francisco. A population based study using conventional and molecular methods. N. Engl. J. Med. 330:1703-1709. [DOI] [PubMed] [Google Scholar]
- 37.Van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, and T. M. Shinnik. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Rie, A., R. Warren, M. Richardson, R. P. Gie, D. Enarson, N. Beyers, and P. D. van Heiden. 2000. Classification of drug-resistant tuberculosis in an epidemic area. Lancet 356:22-25. [DOI] [PubMed] [Google Scholar]
- 39.Van Soolingen, D., T. Hoogenboezem, P. E. W. de Haas, P. W. M. Hermans, M. A. Koedam, K. S. Teppema, P. J. Brennan, G. S. Besra, F. Portaels, J. Top, L. M. Schouls, and J. D. A. van Embden. 1997. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of exceptional isolates from Africa. Int. J. Syst. Bacteriol. 47:1236-1245. [DOI] [PubMed] [Google Scholar]
- 40.Van Soolingen, D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J. Int. Med. 249:1-26. [DOI] [PubMed] [Google Scholar]
- 41.Viana-Niero, C., C. Guiterres, C. Sola, I. Filliol, F. Boulahbal, V. Vincent, and N. Rastogi. 2001. Genetic diversity of Mycobacterium africanum clinical isolates based on IS6110-restriction fragment length polymorphism analysis, spoligotyping, and variable tandem DNA repeats. J. Clin. Microbiol. 39:57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warndorff, D. K., M. Yates, B. Ngwira, S. Chagaluka, P. A. Jenkins, F. Drobniewski, J. M. Pönnighaus, J. R. Glynn, and P. E. M. Fine. 2000. Trends in anti-tuberculosis drug resistance in Karonga District, Malawi, 1986-1998. Int. J. Tuberc. Lung Dis. 4:752-757. [PubMed] [Google Scholar]
- 43.Wayne, L. G. 1985. The “atypical”mycobacteria: recognition and disease association. Crit. Rev. Microbiol. 12:185-222. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson, D., M. Pillay, J. Crump, C. Lombard, G. R. Davies, and A. W. Sturm. 1997. Molecular epidemiology and transmission dynamics of Mycobacterium tuberculosis in rural Africa. Trop. Med. Int. Health 2:747-753. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. 2001. Global tuberculosis control. WHO report WHO/CDS/TB/2001.287. World Health Organization, Geneva, Switzerland.