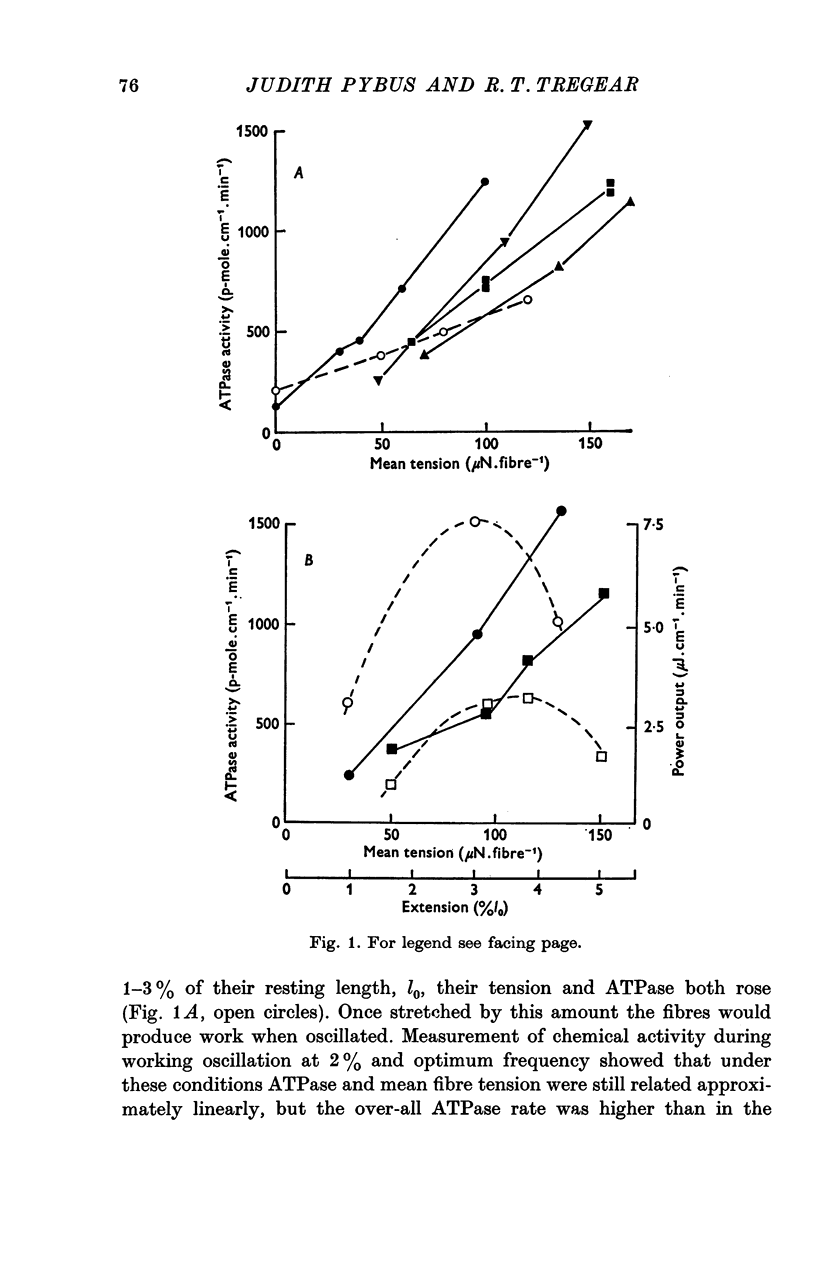
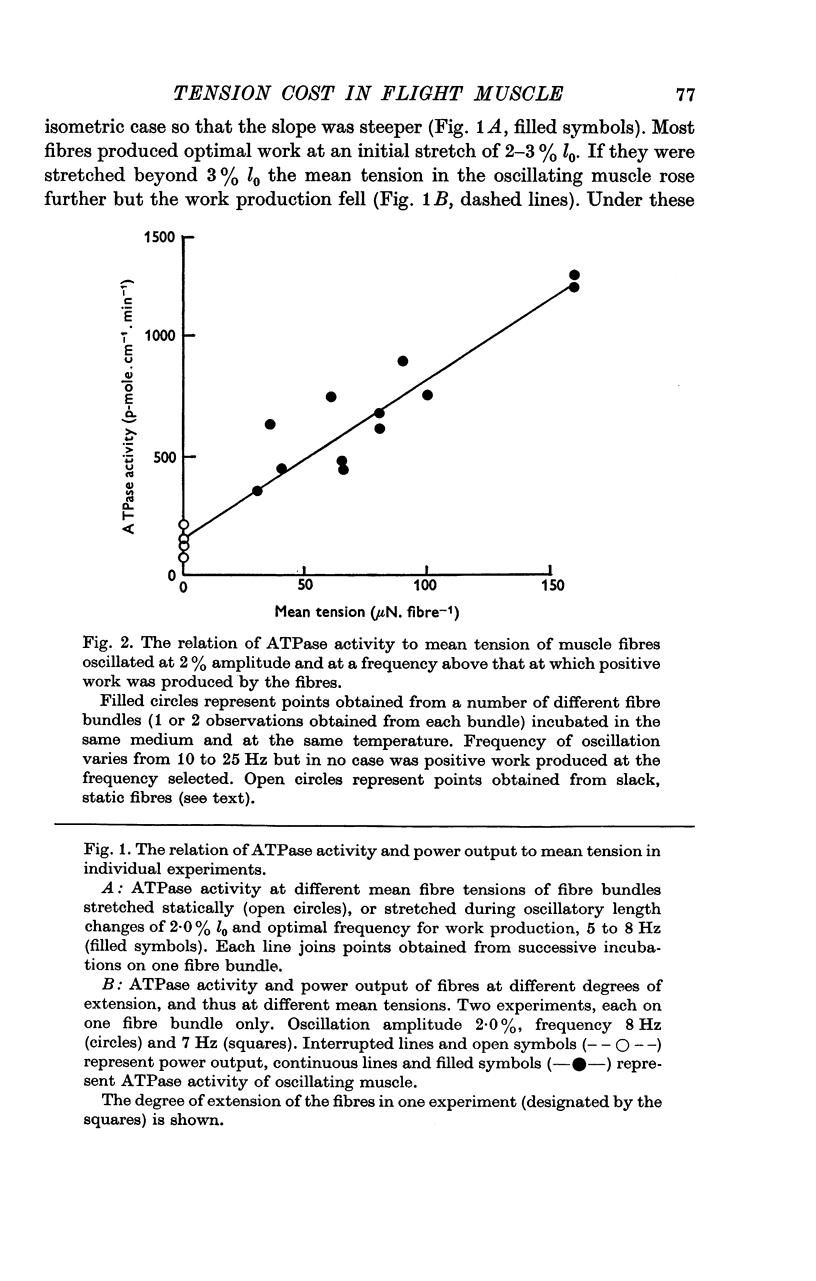
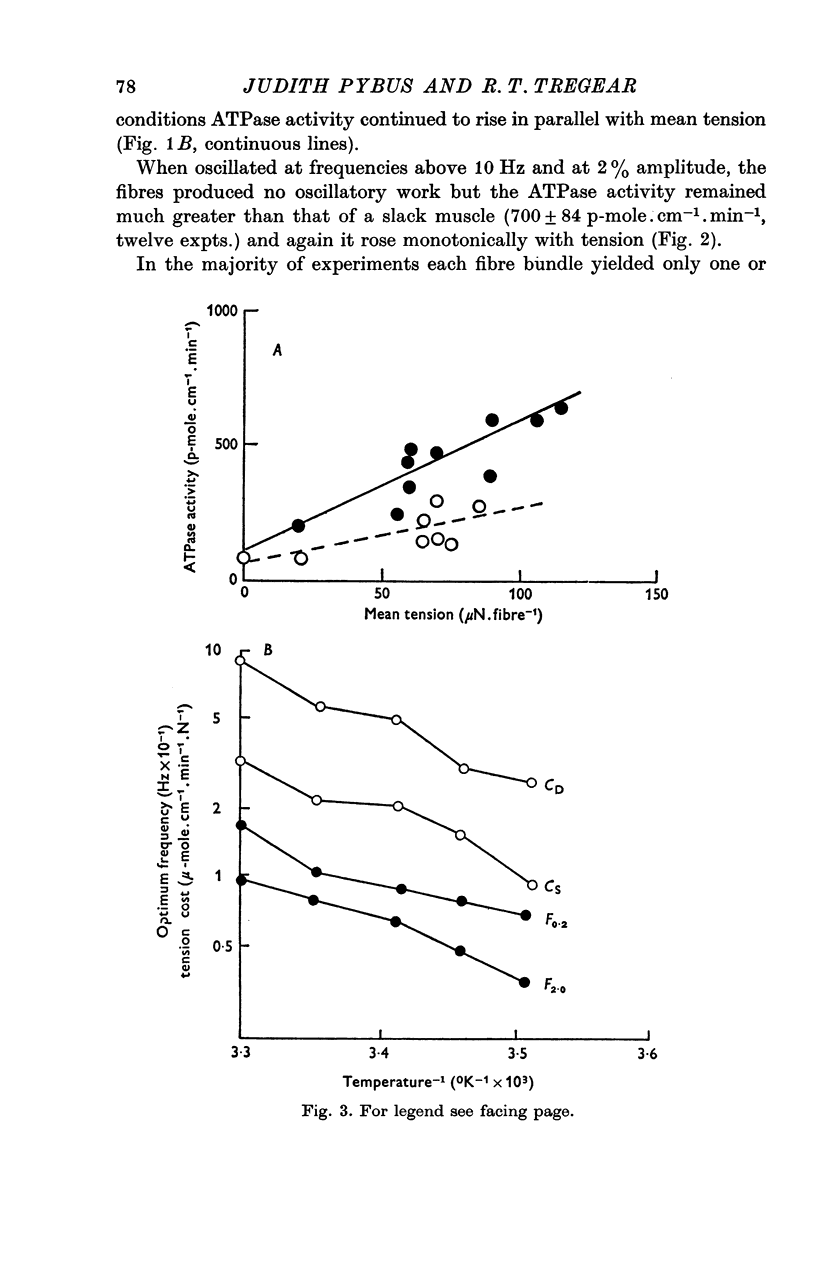
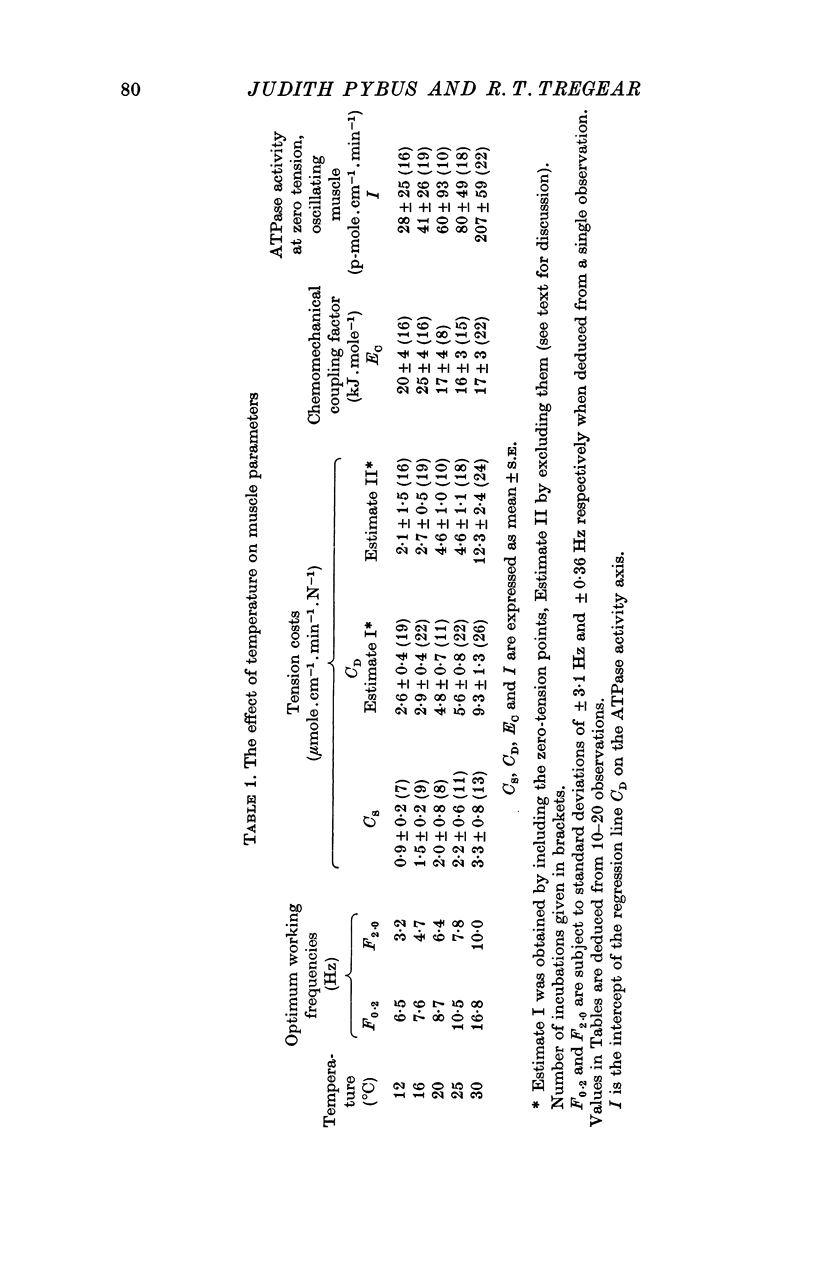
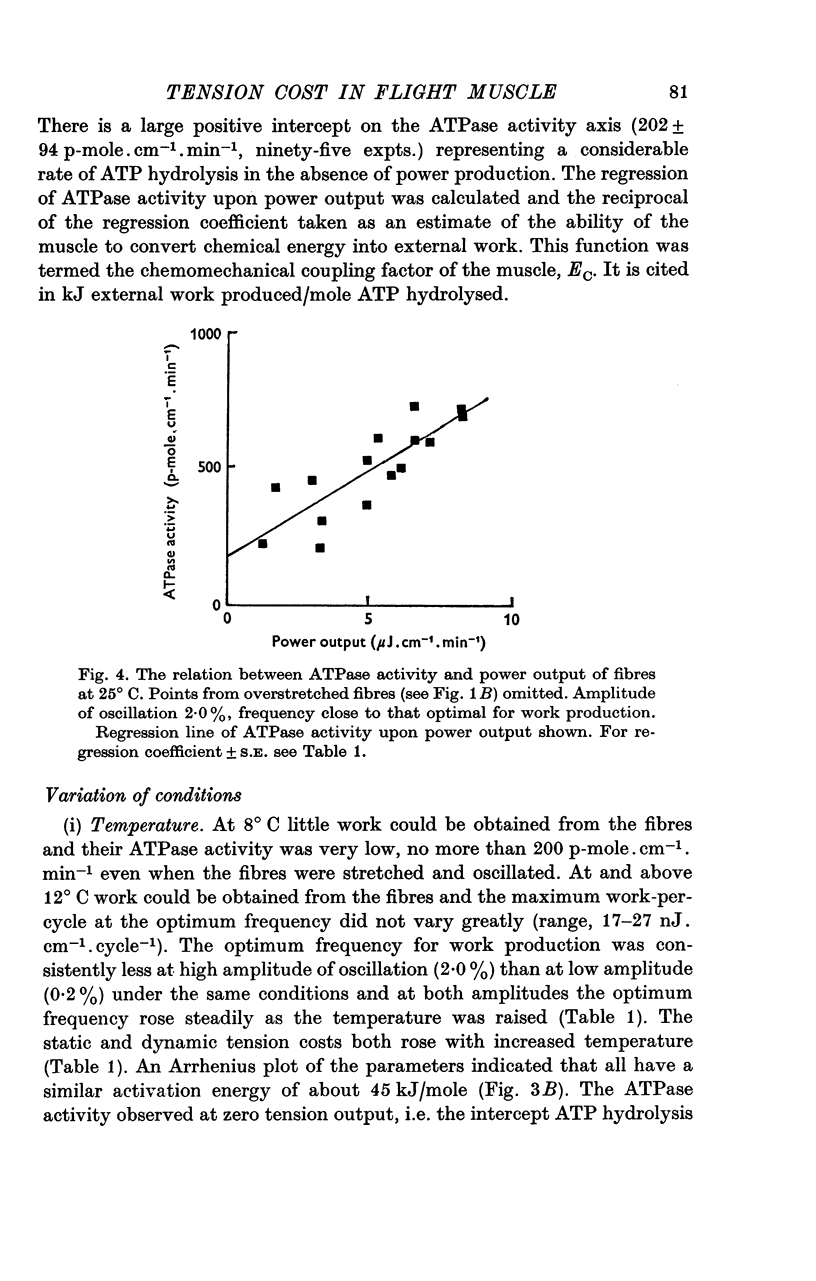
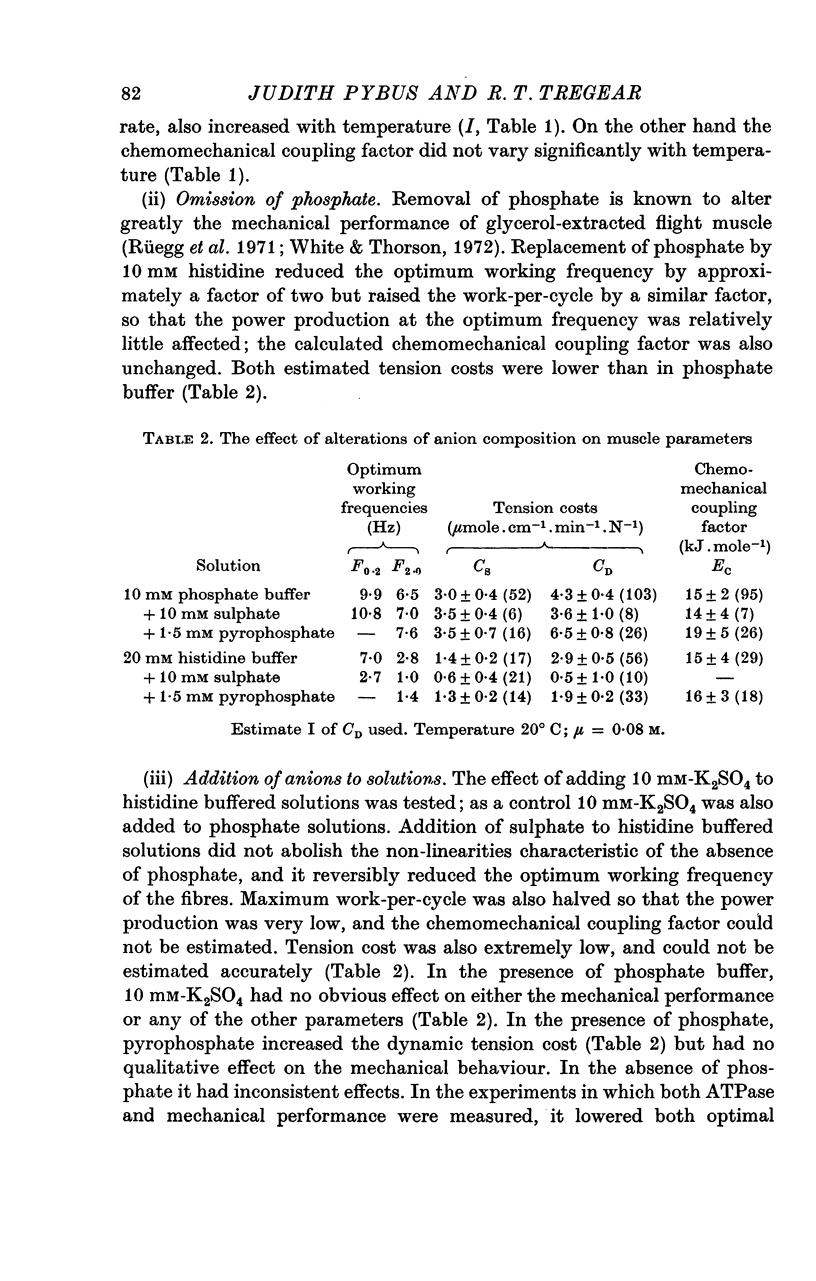
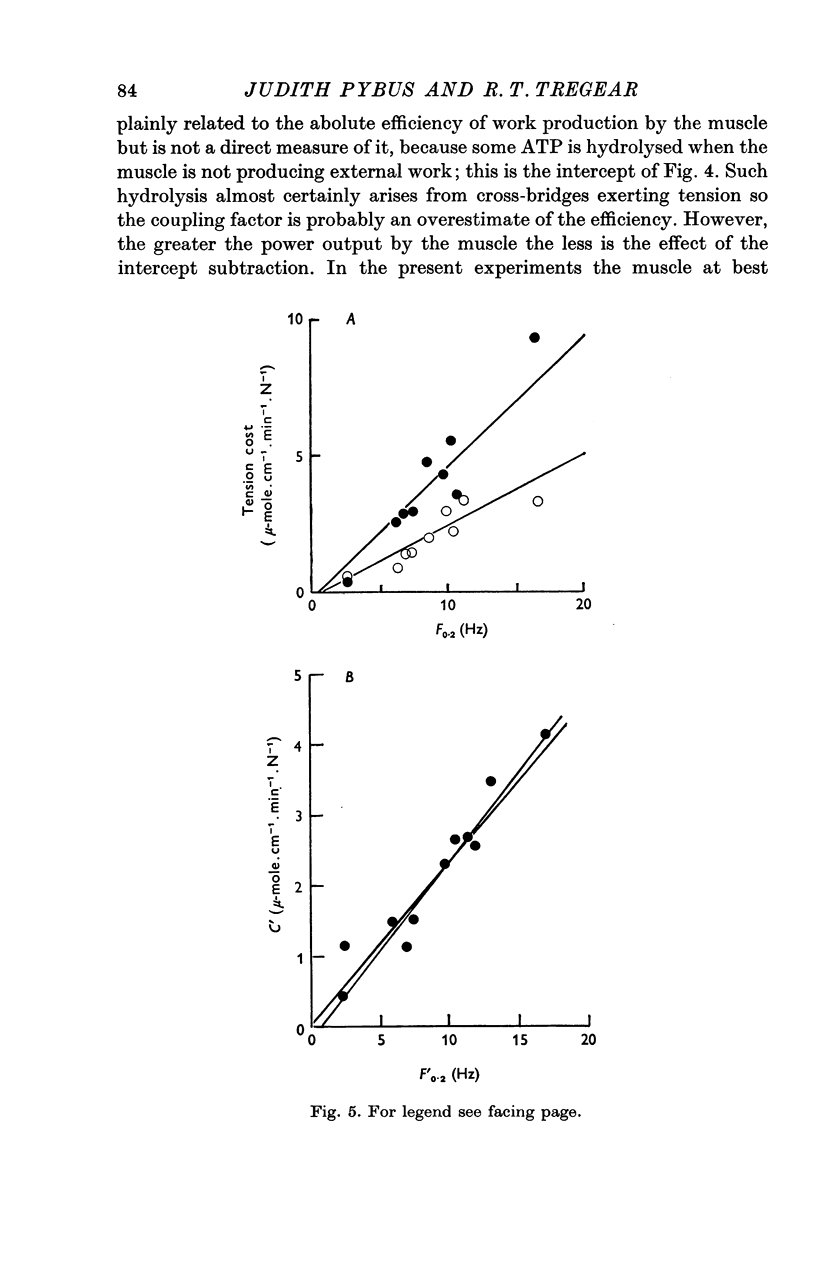
Abstract
1. On a simple model of actomyosin interaction, the tension cost (ATP hydrolysed/unit tension) and the frequency of low amplitude oscillation optimum for work production are both determined by the rate of detachment from the actin filament of the myosin crossbridge. To test this model, the two parameters were measured under different conditions using glycerol-extracted Lethocerus cordofanus dorsal longitudinal flight muscle fibres. 2. The ATPase activity of the static muscle rose by an amount approximately proportional to the rise in tension as the muscle was stretched. 3. When the muscle fibres were sinusoidally oscillated at 5-10 Hz by 2% of their resting length they produced a large amount of mechanical power and hydrolysed approximately twice as much ATP per unit mean tension as they did when static. The ATPase activity was linearly related to the mean tension during oscillation. 4. The experiments were repeated at temperatures between 12 and 30 degrees C and the tension cost and the optimal frequency of oscillation of the fibres were found to rise with temperature. 5. Removal of phosphate from the incubating medium reduced both the tension cost and the optimal working frequency. Addition of pyrophosphate or sulphate reduced both parameters still further. 6. From these results the tension cost of static muscle was shown to be proportional to its optimal working frequency. 7. ATPase activity rose monotonically with power production at work-producing frequencies and at moderate degrees of stretch. A high absolute efficiency was found under a wide range of conditions. 8. The proportionality between tension cost and optimal frequency is evidence for the proposed model of actomyosin interaction.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. H. Computer control of mechanical experiments on muscle. Biochem J. 1971 Jan;121(1):3P–4P. doi: 10.1042/bj1210003pc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott R. H., Leech A. R. Persistence of adenylate kinase and other enzymes in glycerol extracted muscle. Pflugers Arch. 1973 Nov 28;344(3):233–243. doi: 10.1007/BF00588463. [DOI] [PubMed] [Google Scholar]
- Abbott R. H. The effects of fibre length and calcium ion concentration on the dynamic response of glycerol extracted insect fibrillar muscle. J Physiol. 1973 Jun;231(2):195–208. doi: 10.1113/jphysiol.1973.sp010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aidley D. J., White D. C. Mechanical properties of glycerinated fibres from the tymbal muscles of a Brazilian cicada. J Physiol. 1969 Nov;205(1):179–192. doi: 10.1113/jphysiol.1969.sp008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breull W. Myofibrillar ATP-splitting in the elementary contractile cycle of an insect flight muscle. Experientia. 1971 Jul;27(7):779–781. doi: 10.1007/BF02136861. [DOI] [PubMed] [Google Scholar]
- CAIN D. F., INFANTE A. A., DAVIES R. E. Chemistry of muscle contraction. Adenosine triphosphate and phosphorylcreatine as energy supplies for single contractions of working muscle. Nature. 1962 Oct 20;196:214–217. doi: 10.1038/196214a0. [DOI] [PubMed] [Google Scholar]
- CARLSON F. D., HARDY D. J., WILKIE D. R. Total energy production and phosphocreatine hydrolysis in the isotonic twitch. J Gen Physiol. 1963 May;46:851–882. doi: 10.1085/jgp.46.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn W. O. A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J Physiol. 1923 Dec 28;58(2-3):175–203. doi: 10.1113/jphysiol.1923.sp002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Appendix. Free energy and enthalpy of atp hydrolysis in the sarcoplasm. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):315–353. doi: 10.1098/rspb.1969.0096. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Perrin D. D., Sayce I. G. Computer calculation of equilibrium concentrations in mixtures of metal ions and complexing species. Talanta. 1967 Jul;14(7):833–842. doi: 10.1016/0039-9140(67)80105-x. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., Nolan A. C., Zaveler S. A. Cross-bridge properties derived from muscle isotonic velocity transients. Proc Natl Acad Sci U S A. 1969 Oct;64(2):504–511. doi: 10.1073/pnas.64.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. W., Tregear R. T. Mechanical properties of insect fibrillar muscle at large amplitudes of oscillation. Proc R Soc Lond B Biol Sci. 1969 Oct 7;174(1034):33–50. doi: 10.1098/rspb.1969.0079. [DOI] [PubMed] [Google Scholar]
- Rüegg J. C., Schädler M., Steiger G. J., Müller G. Effects of inorganic phosphate on the contractile mechanism. Pflugers Arch. 1971;325(4):359–364. doi: 10.1007/BF00592176. [DOI] [PubMed] [Google Scholar]
- Rüegg J. C., Tregear R. T. Mechanical factors affecting the ATPase activity of glycerol-extracted insect fibrillar flight muscle. Proc R Soc Lond B Biol Sci. 1966 Oct 11;165(1001):497–512. doi: 10.1098/rspb.1966.0080. [DOI] [PubMed] [Google Scholar]
- Schädler M. Proportional Aktivierung von ATPase-Aktivität und Kontraktionsspannung durch Calciumionen in isolierten contractilen Strukturen verschiedener Muskelarten. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;296(1):70–90. [PubMed] [Google Scholar]
- Schädler M., Steiger G. J., Rüegg J. C. Mechanical activation and isometric oscillation in insect fibrillar muscle. Pflugers Arch. 1971;330(3):217–229. doi: 10.1007/BF00588613. [DOI] [PubMed] [Google Scholar]
- Steiger G. J., Rüegg J. C. Energetics and "efficiency" in the isolated contractile machinery of an insect fibrillar muscle at various frequencies of oscillation. Pflugers Arch. 1969;307(1):1–21. doi: 10.1007/BF00589455. [DOI] [PubMed] [Google Scholar]
- Thorson J., White D. C. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J. 1969 Mar;9(3):360–390. doi: 10.1016/S0006-3495(69)86392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Thorson J. Phosphate starvation and the nonlinear dynamics of insect fibrillar flight muscle. J Gen Physiol. 1972 Sep;60(3):307–336. doi: 10.1085/jgp.60.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]


