Abstract
1. Conventional electrophysiological techniques were used to record from isolated rat phrenic nerve-hemidiaphragm preparations. After periods of rest (20 min) or nerve stimulation (7/sec for 20 min) the bathing medium of the preparation was removed and assayed for adenosine triphosphate (ATP) and adenosine diphosphate (ADP) using a sensitive modification of the firefly luciferase method (Silinsky, 1974). 2. In the presence of tubocurarine and normal (2 mM) calcium, fourteen periods of nerve stimulation (eight preparations) caused the appearance of ATP and/or ADP in amounts ranging from 28 to 641 p-mole. Experiments using carbachol (30 muM or 1 mM) suggested that this nucleotide efflux was not produced by a secondary action of released acetylcholine (ACh). 3. Stimulation of isolate phrenic nerve trunks at 7/sec for 20 min did not cause the efflux of ATP or ADP. 4. In solutions of normal osmotic pressure and reduced calcium concentrations (0-1 mM or 'calcium-free'), stimulation failed to release adenine nucleotide from non-contracting preparations. 5. Diaphragms were bathed in normal calcium and indirectly stimulated at 11/sec for 80-90 min in the presence of 5 times 10-minus 5 M hemicholinium-3. After all detectable signs of ACh release were eliminated, nerve stimulation failed to release ATP or ADP. 6. These results in conjunction with experiments on the hydrolysis of exogenous ATP suggest that ATP is released from the motor nerve ending and is subsequently degraded by enzymatic activity. It is also suggested that the released nucleotide may be derived from the cholinergic vesicle.
Full text
PDF






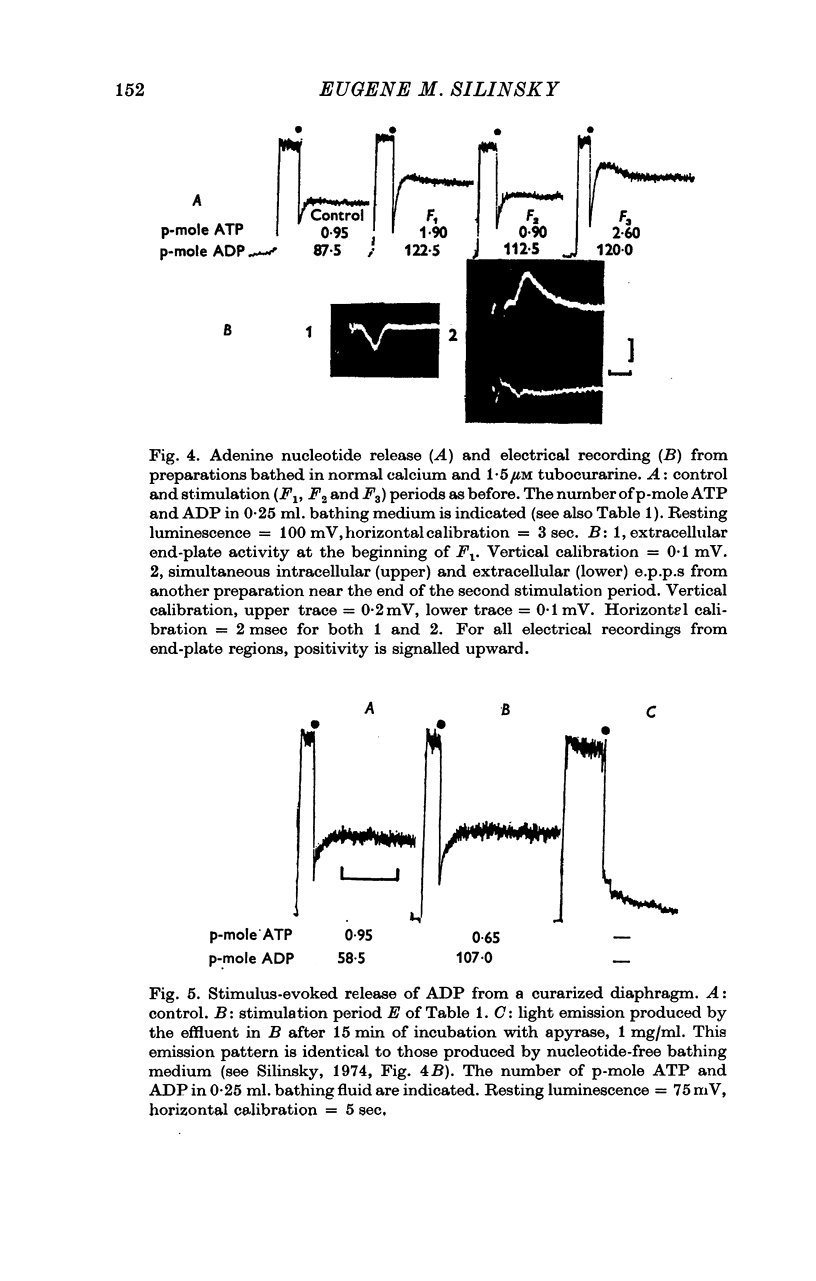




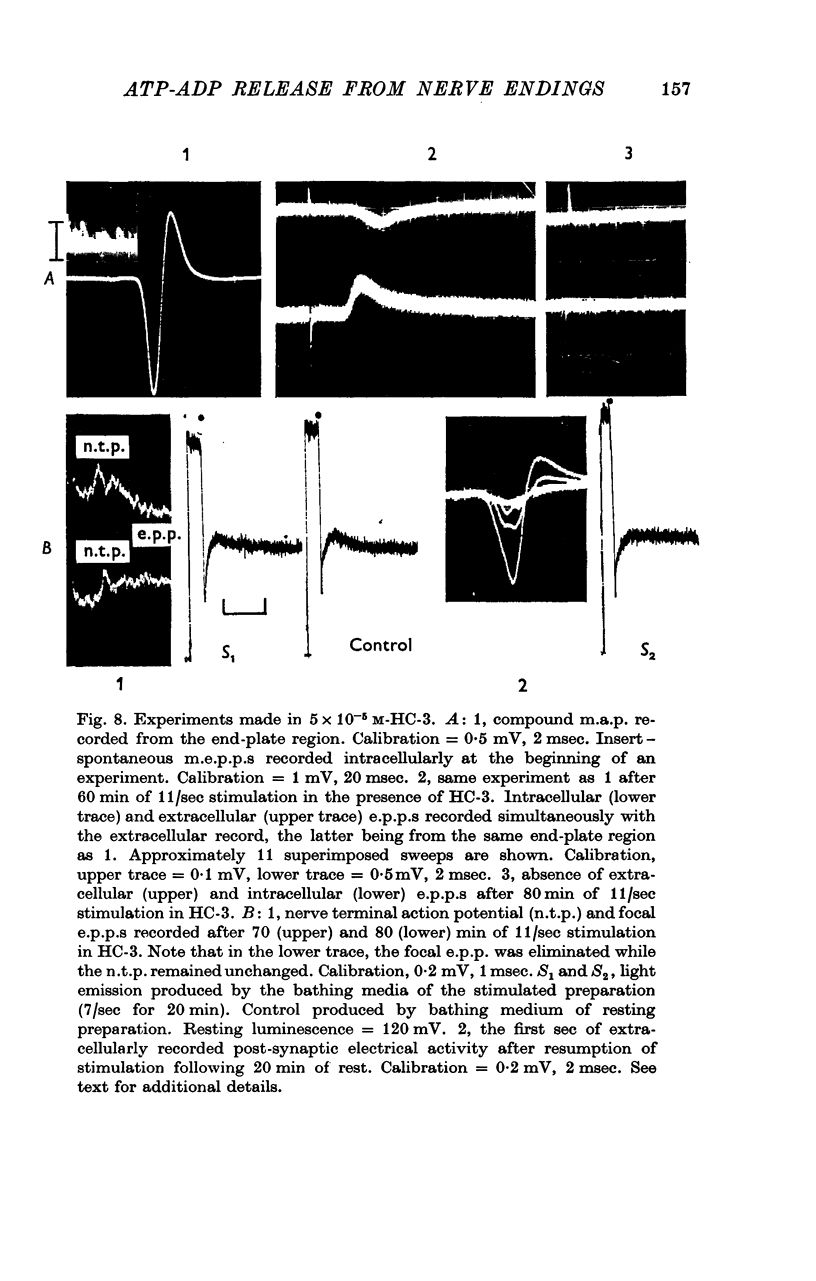





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABOOD L. G., KOKETSU K., MIYAMOTO S. Outflux of various phosphates during membrane depolarization of excitable tissues. Am J Physiol. 1962 Mar;202:469–474. doi: 10.1152/ajplegacy.1962.202.3.469. [DOI] [PubMed] [Google Scholar]
- Abood L. G., Matsubara A. Properties of an ATP-binding protein isolated from membranes of nerve endings. Biochim Biophys Acta. 1968 Dec 10;163(4):539–549. doi: 10.1016/0005-2736(68)90083-7. [DOI] [PubMed] [Google Scholar]
- Betz W., Sakmann B. Effects of proteolytic enzymes on function and structure of frog neuromuscular junctions. J Physiol. 1973 May;230(3):673–688. doi: 10.1113/jphysiol.1973.sp010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- CREESE R. Measurement of cation fluxes in rat diaphragm. Proc R Soc Lond B Biol Sci. 1954 Sep 27;142(909):497–513. doi: 10.1098/rspb.1954.0039. [DOI] [PubMed] [Google Scholar]
- Dowdall M. J., Boyne A. F., Whittaker V. P. Adenosine triphosphate. A constituent of cholinergic synaptic vesicles. Biochem J. 1974 Apr;140(1):1–12. doi: 10.1042/bj1400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B. Neuromuscular transmission of trophic effects. Ann N Y Acad Sci. 1971 Sep 15;183:158–170. doi: 10.1111/j.1749-6632.1971.tb30748.x. [DOI] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T. An estimate of adenosine triphosphate release into the venous effluent from exercising human forearm muscle. J Physiol. 1972 Aug;224(3):611–628. doi: 10.1113/jphysiol.1972.sp009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T., Lind A. R. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol. 1969 Oct;204(2):347–364. doi: 10.1113/jphysiol.1969.sp008917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier D. T. Effect of hemicholinium no. 3 on amphibian nerve. Exp Neurol. 1968 Feb;20(2):245–254. doi: 10.1016/0014-4886(68)90098-8. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. The origin of the post-tetanic hyperpolarization of mammalian motor nerve terminals. J Physiol. 1966 May;184(2):335–352. doi: 10.1113/jphysiol.1966.sp007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. An electrophysiological investigation of mammalian motor nerve terminals. J Physiol. 1963 Apr;166:145–167. doi: 10.1113/jphysiol.1963.sp007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu S., Knox J. A. The end-plate potentials of the rat diaphragm preparation. J Physiol. 1950 Apr 15;111(1-2):43–49. doi: 10.1113/jphysiol.1950.sp004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Kelly R. B. Enzymatic detachment of endplate acetylcholinesterase from muscle. Nat New Biol. 1971 Jul 14;232(28):62–63. doi: 10.1038/newbio232062a0. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I. Mechanism of transmitter release. Prog Biophys Mol Biol. 1970;21:33–124. [PubMed] [Google Scholar]
- Jones S. F., Kwanbunbumpen S. Some effects of nerve stimulation andhemicholinium on quantal transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Mar;207(1):51–61. doi: 10.1113/jphysiol.1970.sp009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. The release of acetylcholine in the isolated rat diaphragm. J Physiol. 1961 Feb;155:246–262. doi: 10.1113/jphysiol.1961.sp006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A. C., Katz H. S., Collier B. Absence of adenine nucleotide release from autonomic ganglion. Nature. 1974 Jun 7;249(457):576–577. doi: 10.1038/249576a0. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A., Walker J. Action of adenosine triphosphate on endplate potentials recorded from muscle fibres of the rat-diaphragm and frog sartorius. Br J Pharmacol. 1973 Dec;49(4):724–725. doi: 10.1111/j.1476-5381.1973.tb08555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Elderfrawi M. E. Sizes of end plate compartments, densities of acetylcholine receptor and other quantitative aspects of neuromuscular transmission. J Histochem Cytochem. 1973 Sep;21(9):769–778. doi: 10.1177/21.9.769. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. A simple, rapid method for detecting the efflux of small quantities of adenosine triphosphate from biological tissues. Comp Biochem Physiol A Comp Physiol. 1974 Jul 1;48(3):561–571. doi: 10.1016/0300-9629(74)90739-7. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M., Hubbard J. I. Thermal synthesis of amino acids from a simulated primitive atmosphere. Nature. 1973 Jun 15;243(5407):404–405. doi: 10.1038/243404a0. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P., Dowdall M. J., Boyne A. F. The storage and release of acetylcholine by cholinergic nerve terminals: recent results with non-mammalian preparations. Biochem Soc Symp. 1972;(36):49–68. [PubMed] [Google Scholar]
- Yellin H. Changes in fiber types of the hypertrophying denervated hemidiaphragm. Exp Neurol. 1974 Feb;42(2):412–428. doi: 10.1016/0014-4886(74)90035-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann H., Whittaker V. P. Effect of electrical stimulation on the yield and composition of synaptic vesicles from the cholinergic synapses of the electric organ of Torpedo: a combined biochemical, electrophysiological and morphological study. J Neurochem. 1974 Mar;22(3):435–450. doi: 10.1111/j.1471-4159.1974.tb07610.x. [DOI] [PubMed] [Google Scholar]






