Abstract
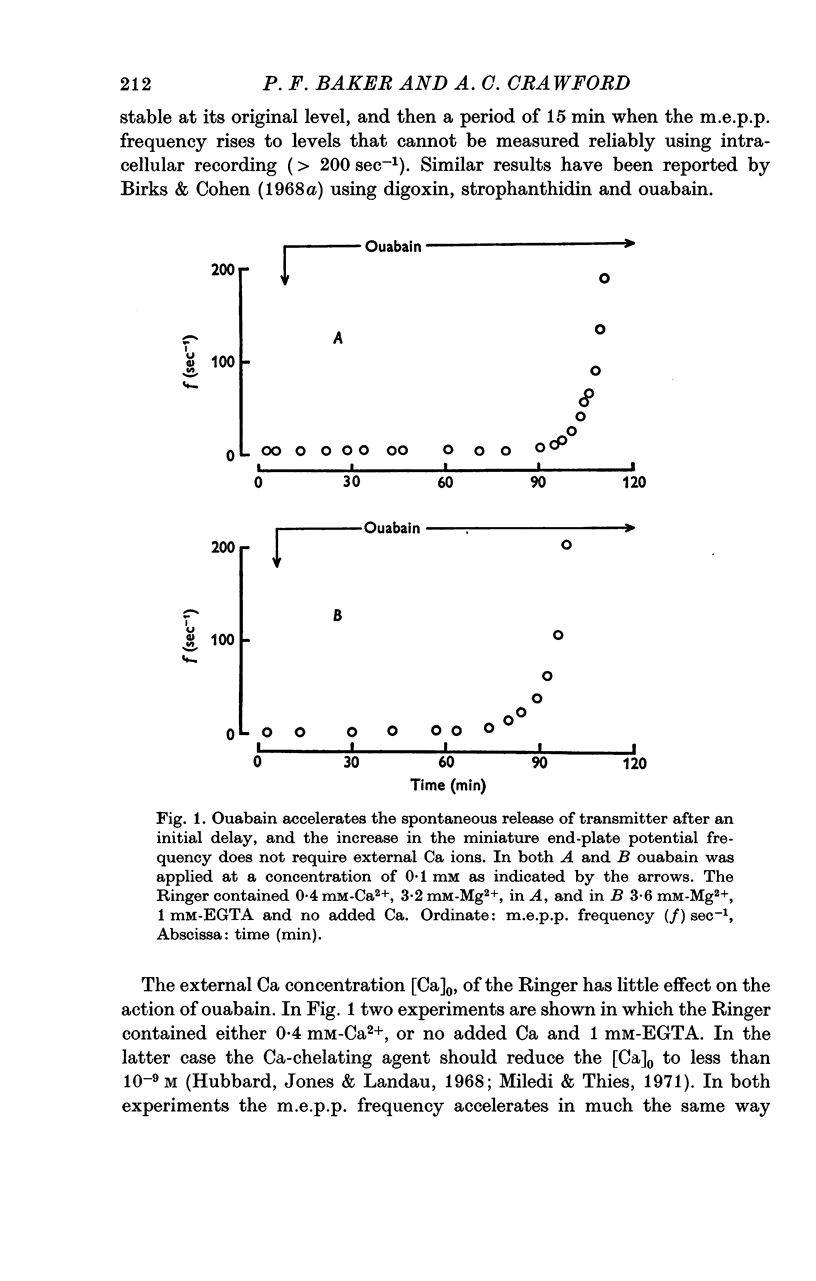
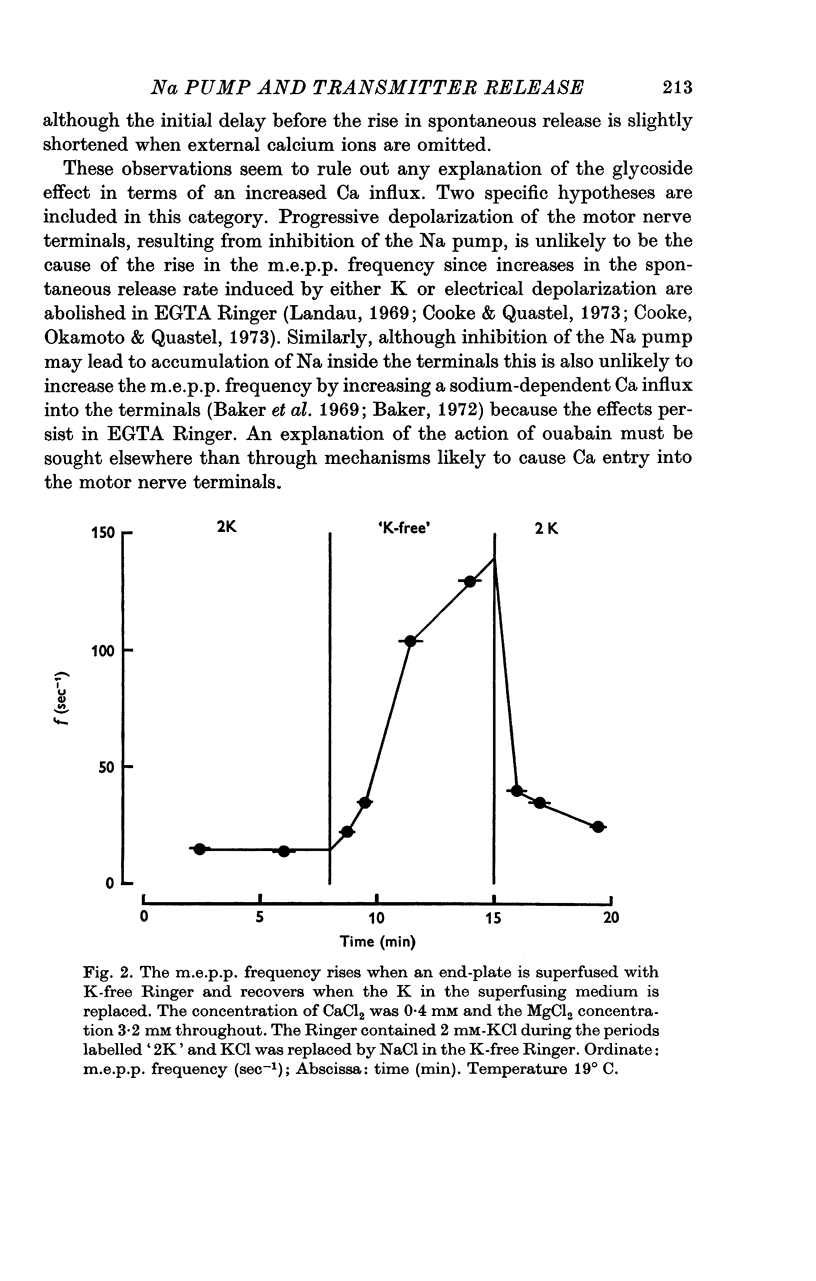
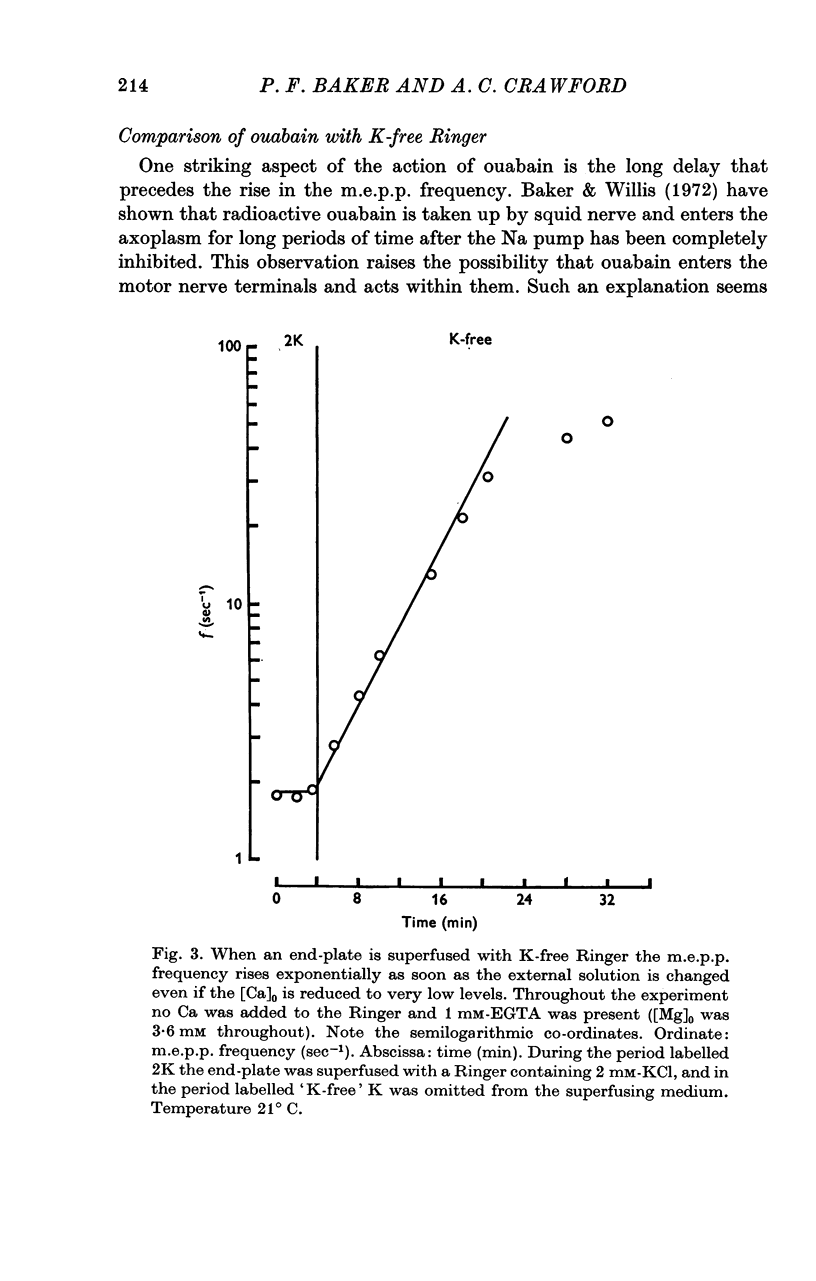
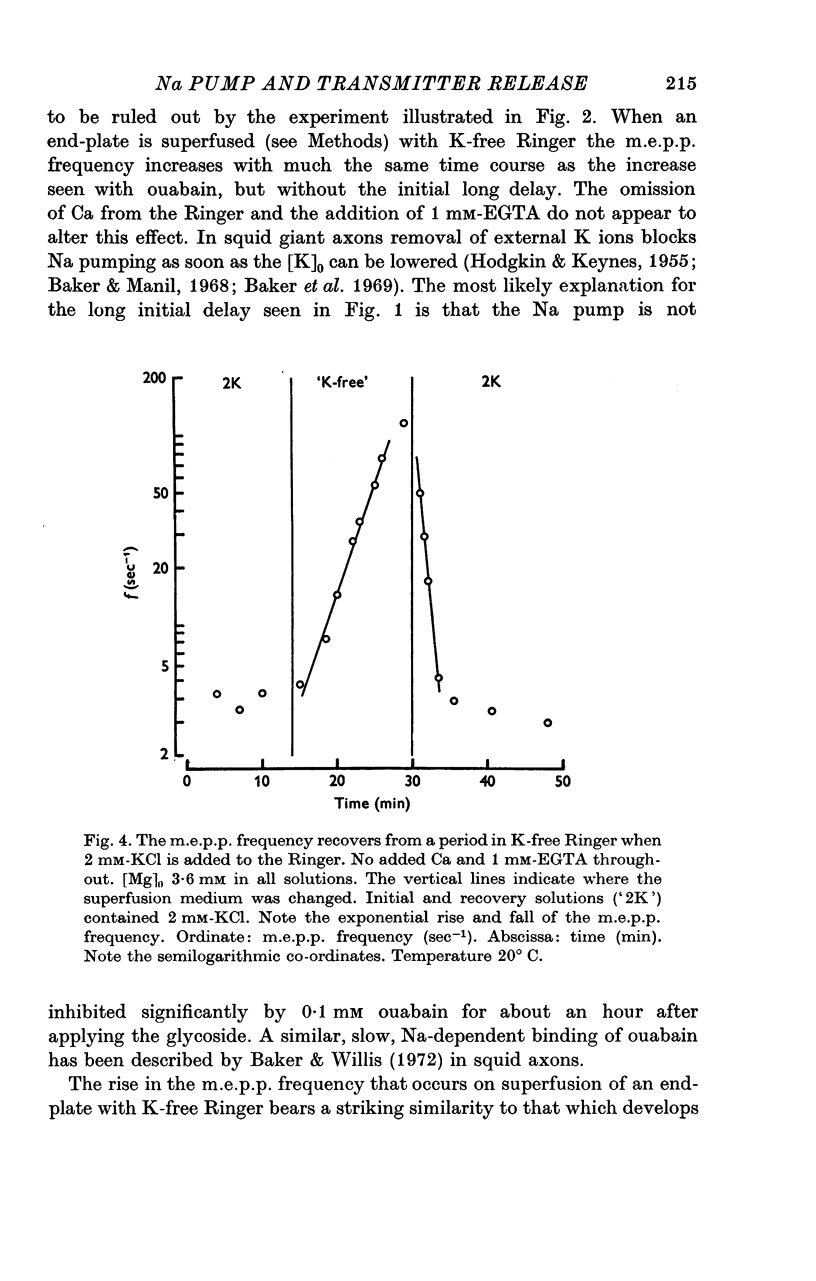
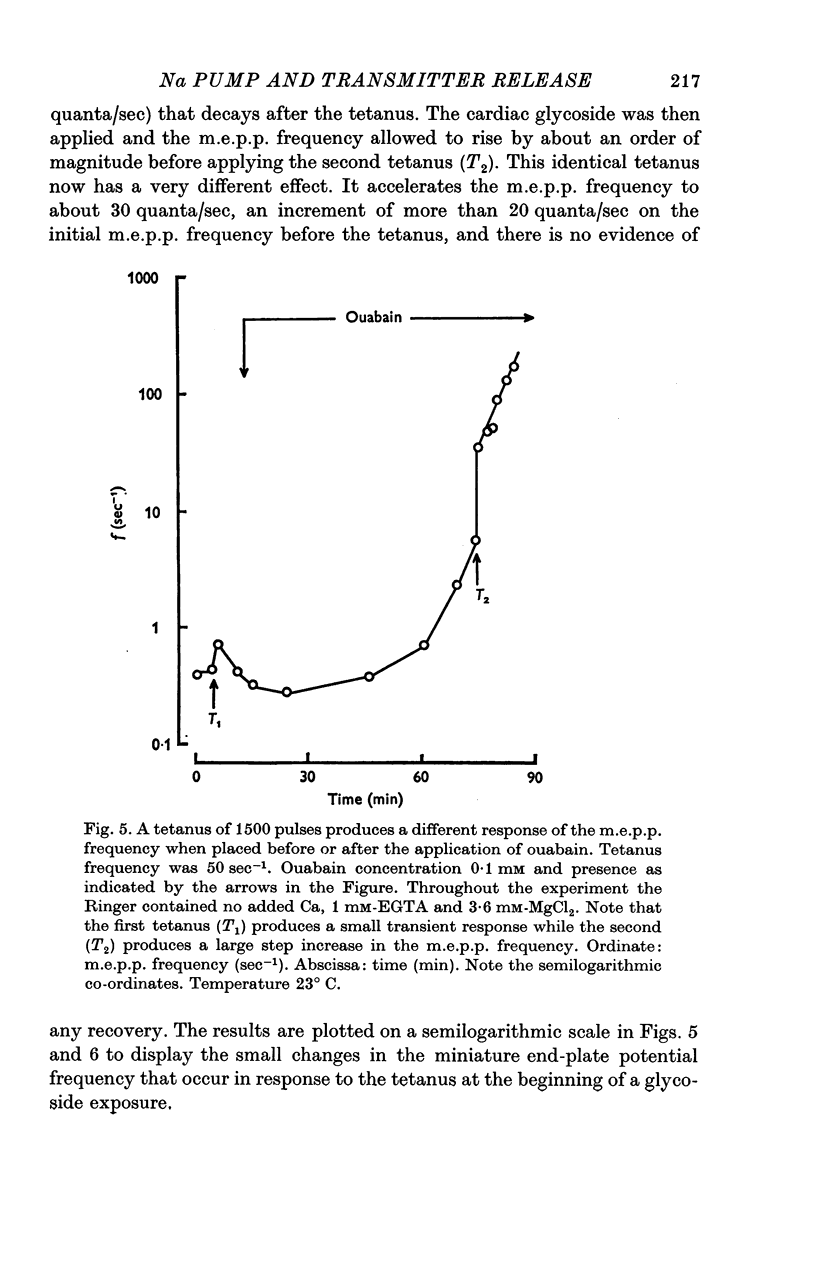
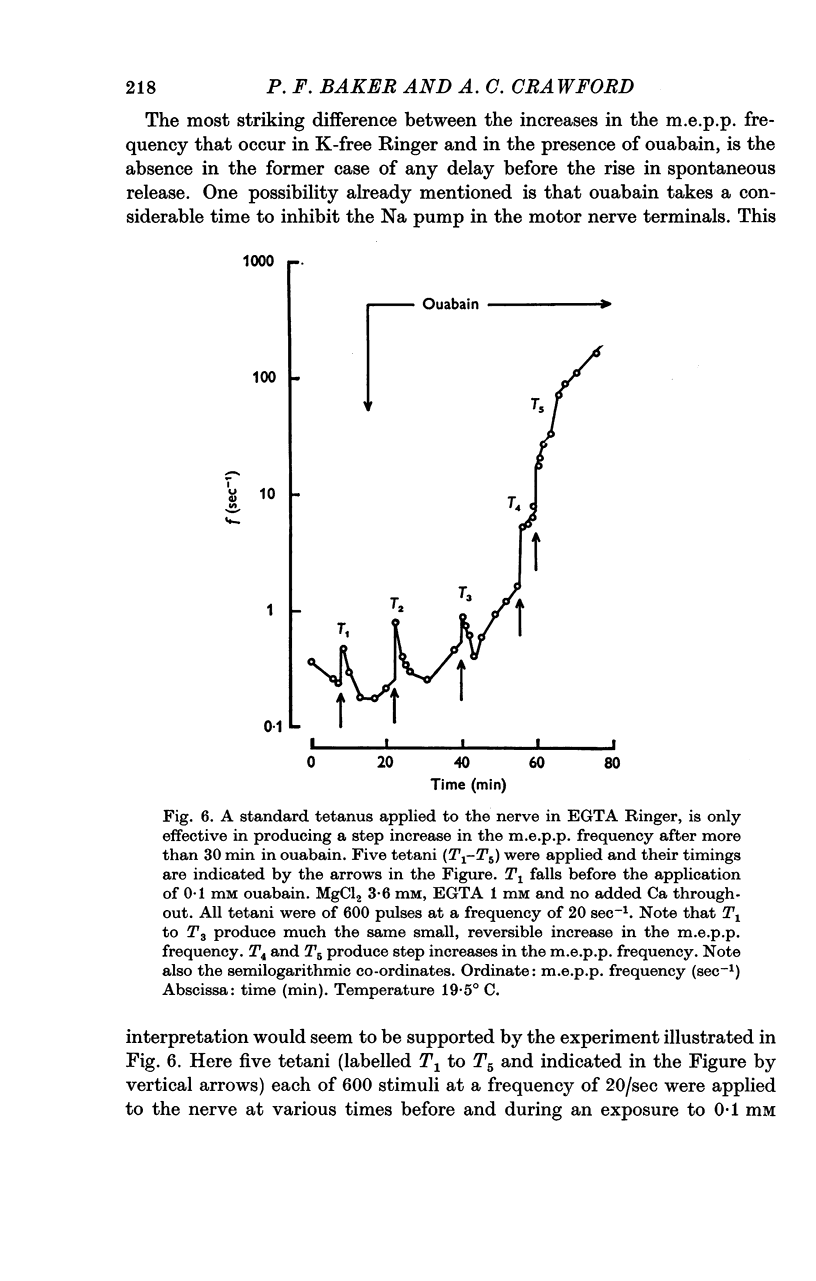
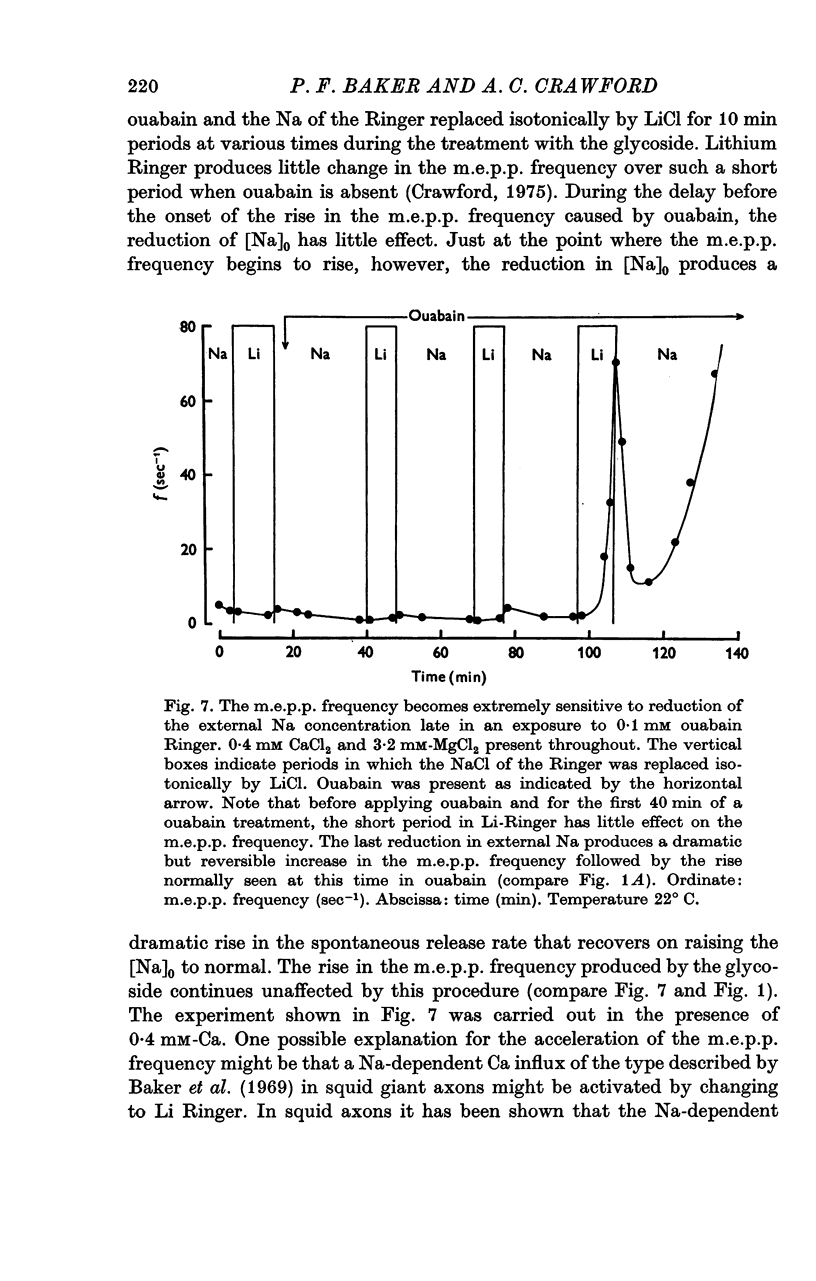
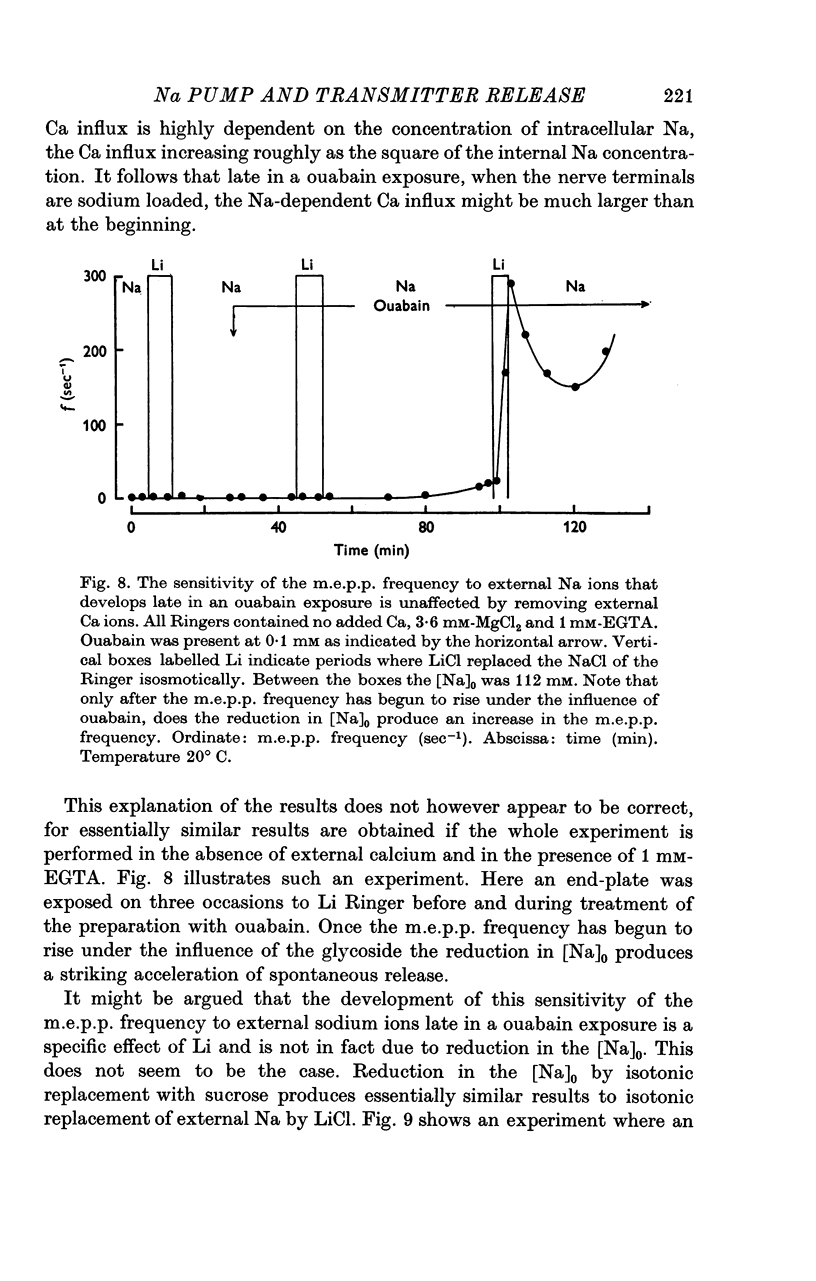
1. The actions of 0-1 mM ouabain and of K-free Ringer have been examined at the frog neuromuscular junction. 2. After a delay of more than 30 min, ouabain produces an increase in the miniature end-plate potential (m.e.p.p.) frequency. This increase occurs unchanged in Ca-free Ringer containing 1 mM-EGTA and is therefore unlikely to be due to an entry of Ca into the motor nerve terminals. 3. If the nerve to the preparation is stimulated repetitively in Ca-free Ringer containing 0-1 mM ouabain and 1 mM-EGTA the response of the m.e.p.p. frequency depends on the timing of the tetanus relative to the beginning of the ouabain treatment. 4. During the first 30 min of exposure to ouabain, the tetanus produces a small, transient increase in the m.e.p.p. frequency similar to that which occurs before ouabain is present. After about 30 min the same tetanus produces large, irreversible increases in the m.e.p.p. frequency. 5. Superfusion of an end-plate with K-free Ringer causes an immediate exponential rise in the m.e.p.p. frequency that is unaffected by the presence of external Ca ions. On replacing the normal K of the Ringer (2 mM) the m.e.p.p. frequency recovers quickly to its original value. 6. Late in an exposure to 0-1 mM ouabain the m.e.p.p. frequency becomes extremely sensitive to changes in the external Na concentration, [Na]o. Reducing [Na]o increases the m.e.p.p. frequency. The sensitivity to [Na]o is independent of external Ca ions or whether the isotonic substitute for NaCl is LiCl or sucrose. 7. It is suggested that the spontaneous release of transmitter is facilitated, in some way, by the changes in the monovalent cation content of the nerve terminals that result from blocking the Na-K exchange pump. The Na sensitivity of the m.e.p.p. frequency that develops simultaneously can be explained if a Na-dependent Ca efflux system is present in the membrane of the presynaptic terminals.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. THE RUBIDIUM AND POTASSIUM PERMEABILITY OF FROG MUSCLE MEMBRANE. J Physiol. 1964 Dec;175:134–159. doi: 10.1113/jphysiol.1964.sp007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Manil J. The rates of action of K+ and ouabain on the sodium pump in squid axons. Biochim Biophys Acta. 1968 Mar 1;150(2):328–330. doi: 10.1016/0005-2736(68)90181-8. [DOI] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Inhibition of the sodium pump in squid giant axons by cardiac glycosides: dependence on extracellular ions and metabolism. J Physiol. 1972 Jul;224(2):463–475. doi: 10.1113/jphysiol.1972.sp009905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Burstyn P. G., Firth D. R. The form of sodium-calcium competition at the frog myoneural junction. J Gen Physiol. 1968 Dec;52(6):887–907. doi: 10.1085/jgp.52.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C. Lithium ions and the release of transmitter at the frog neuromuscular junction. J Physiol. 1975 Mar;246(1):109–142. doi: 10.1113/jphysiol.1975.sp010882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C. The dependence of evoked transmitter release on external calcium ions at very low mean quantal contents. J Physiol. 1974 Jul;240(2):255–278. doi: 10.1113/jphysiol.1974.sp010609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Effects of sodium pump inhibitors on spontaneous acetylcholine release at the neuromuscular junction. J Physiol. 1965 Dec;181(3):498–505. doi: 10.1113/jphysiol.1965.sp007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Competition between sodium and calcium ions in transmitter release at mammalian neuromuscular junctions. J Physiol. 1966 Jul;185(1):95–123. doi: 10.1113/jphysiol.1966.sp007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY J. S. ANTAGONISM BETWEEN NA+ AND CA2+ AT THE NEUROMUSCULAR JUNCTION. Nature. 1965 Jan 16;205:296–297. doi: 10.1038/205296a0. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- Kelly J. S. The antagonism of Ca2+ by Na+ and other monovalent ions at the frog neuromuscular junction. Q J Exp Physiol Cogn Med Sci. 1968 Jul;53(3):239–249. doi: 10.1113/expphysiol.1968.sp001967. [DOI] [PubMed] [Google Scholar]
- Landau E. M. The interaction of presynaptic polarization with calcium and magnesium in modifying spontaneous transmitter release from mammalian motor nerve terminals. J Physiol. 1969 Aug;203(2):281–299. doi: 10.1113/jphysiol.1969.sp008864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., ENDO M. The effect of guanidine on neuromuscular transmission. J Pharmacol Exp Ther. 1960 Mar;128:273–282. [PubMed] [Google Scholar]
- Onodera K., Yamakawa K. The effects of lithium on the neuromuscular junction of the frog. Jpn J Physiol. 1966 Oct 15;16(5):541–550. doi: 10.2170/jjphysiol.16.541. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S., Zar M. A. The mechanism of acetylcholine release from parasympathetic nerves. J Physiol. 1971 Jul;215(3):819–848. doi: 10.1113/jphysiol.1971.sp009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLOMON A. K. The permeability of the human erythrocyte to sodium and potassium. J Gen Physiol. 1952 May;36(1):57–110. doi: 10.1085/jgp.36.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi E. S. Stimulation, by inhibition of (Na + -K + -Mg 2+ )-activated ATP-ase, of acetylcholine release in cortical slices from rat brain. J Physiol. 1972 Oct;226(1):95–117. doi: 10.1113/jphysiol.1972.sp009975. [DOI] [PMC free article] [PubMed] [Google Scholar]


