Abstract
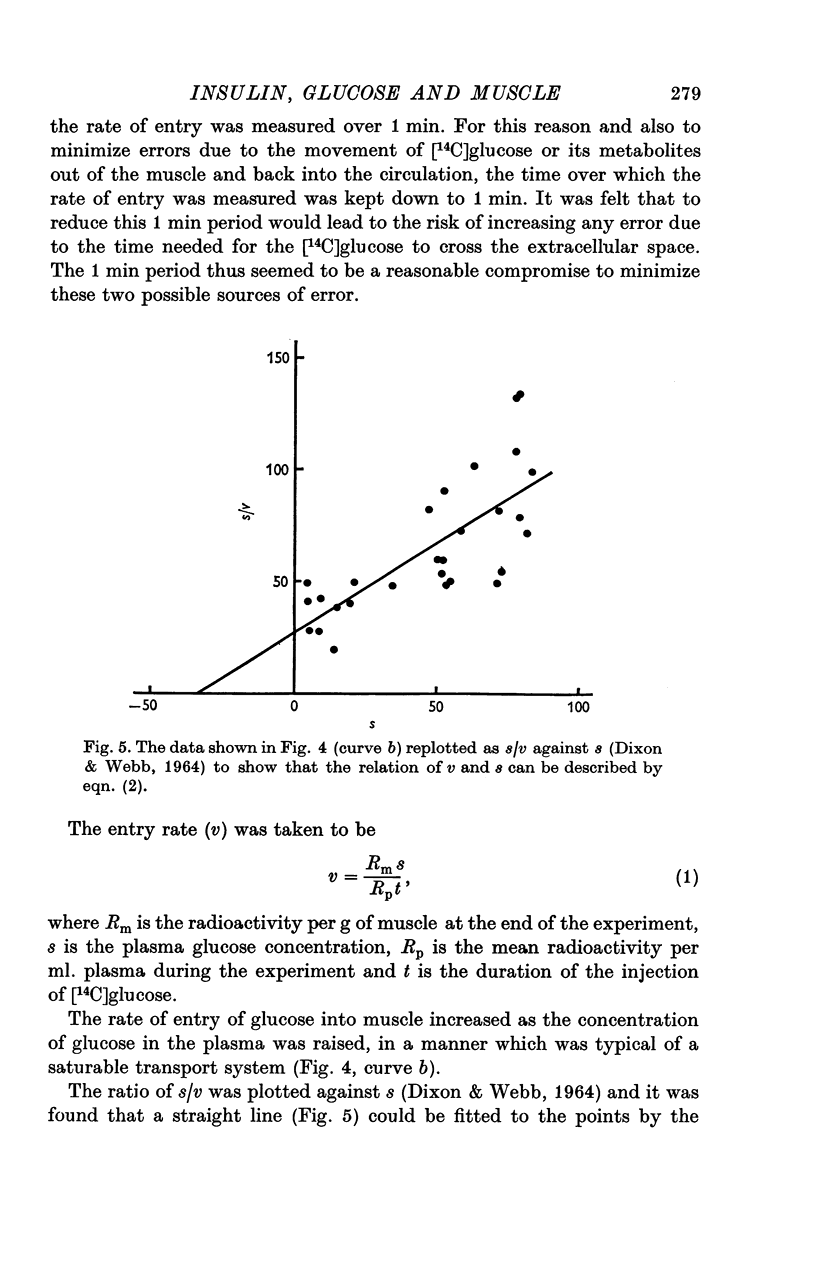
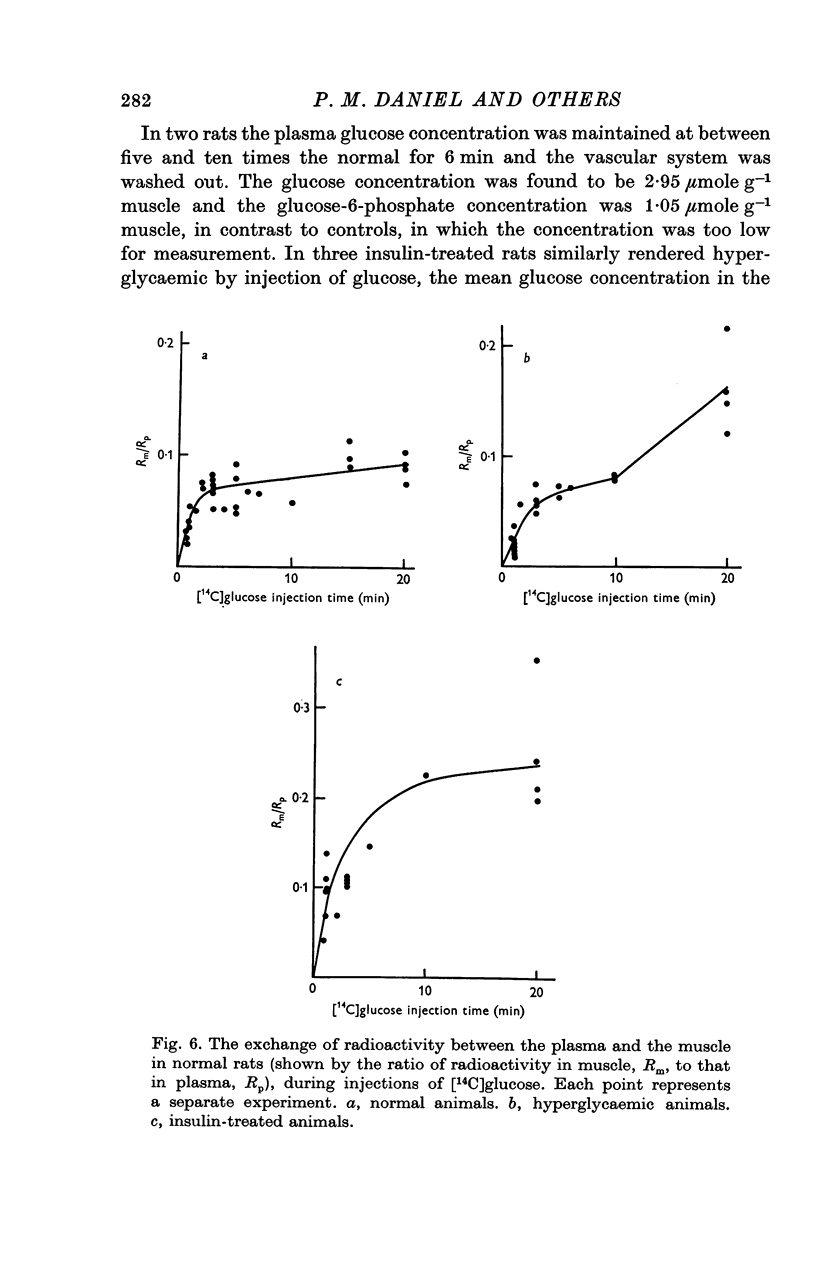
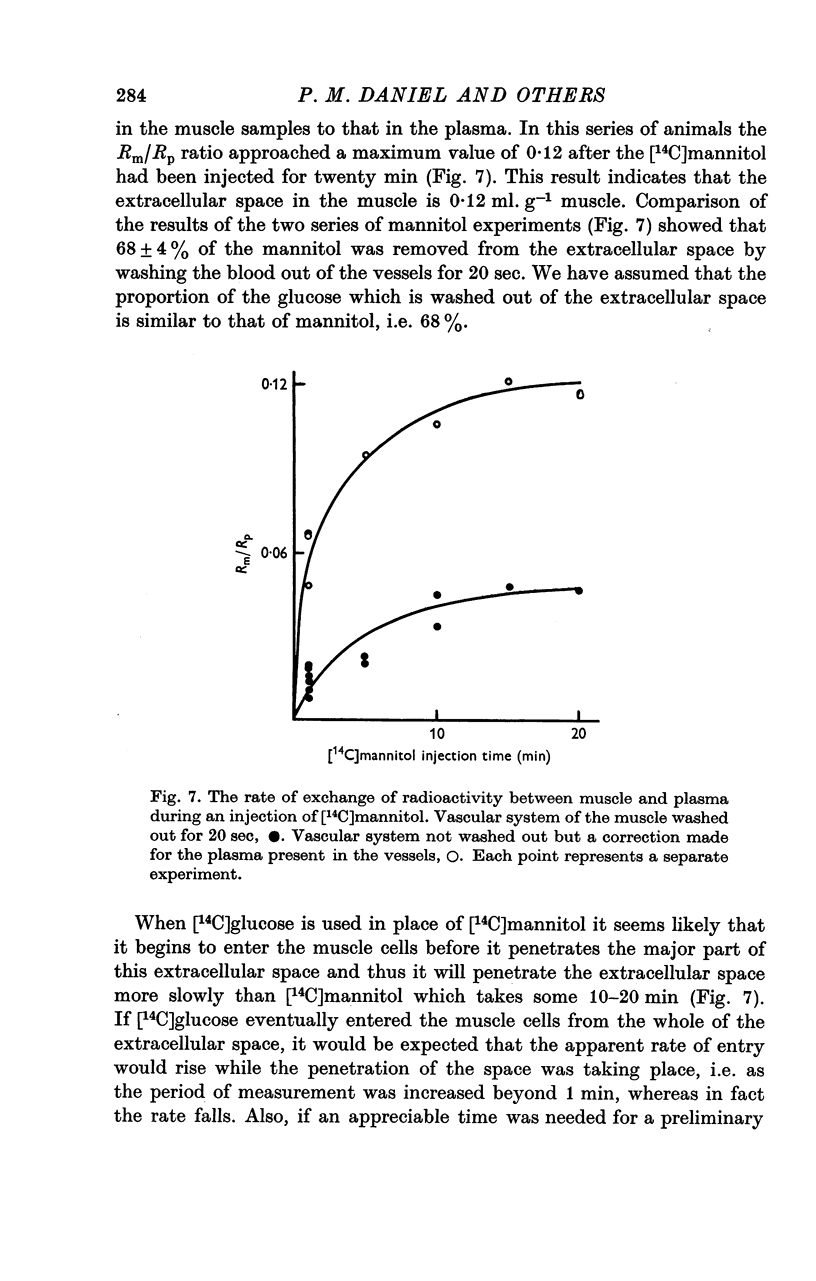
1. The entry of glucose into the pectoralis major muscle of living rats was measured over a wide range of plasma glucose concentrations. A technique was used by which steady concentrations of substances are maintained in the circulation throughout the experiments. 2. Raising the concentration of glucose in the plasma caused saturation of the mechanism by which it is transported into muscle. Estimates of the values of the kinetic constants for this transport system were: Kt, 34 mumole ml-1 and V, 1-2 mumole min-1-g-1 muscle. 3. When the plasma glucose concentration was raised up to at least twelve times normal, there was no sign of saturation of the transport system in insulin-treated animals. This finding could be explained if insulin increased greatly both V and Kt for glucose transport. 4. Insulin increased the rate of entry of glucose into muscle over the entire range of plasma glucose concentrations studied (4-8 mumole ml.-1). There was evidence that endogenous insulin produced a similar increase in entry rate some 10 min after the injection of glucose. Fasting, which is associated with a decrease in insulin level, depressed the rate of entry. In hyperglycaemia insulin caused a rise in the concentration of glucose within the muscle cells. 5. The insulin-induced increase in the rate of glucose entry into muscle ensured that approximately 25% of an I.V. dose of glucose entered the muscle cells of insulin-treated animals within one minute. This illustrates the quantitatively important regulatory role that skeletal muscle plays in these circumstances in limiting the extent of a rise in circulating glucose.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATTAGLIA F. C., RANDLE P. J. Regulation of glucose uptake by muscle. 4. The specificity of monosaccharide-transport systems in rat-diaphragm muscle. Biochem J. 1960 May;75:408–416. doi: 10.1042/bj0750408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudry I. H., Gould M. K. Kinetics of glucose uptake in isolated soleus muscle. Biochim Biophys Acta. 1969 May 6;177(3):527–536. doi: 10.1016/0304-4165(69)90315-8. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Donaldson J., Pratt O. E. The rapid achievement and maintenance of a steady level of an injected substance in the blood plasma. J Physiol. 1974 Mar;237(2):8P–9P. [PubMed] [Google Scholar]
- FISHER R. B., LINDSAY D. B. The action of insulin on the penetration of sugars into the perfused heart. J Physiol. 1956 Mar 28;131(3):526–541. doi: 10.1113/jphysiol.1956.sp005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock E. V., Tyce G., Owen C. A., Jr Regulatory effects of insulin and liver on brain glucose metabolism. Endocrinology. 1969 Feb;84(2):392–406. doi: 10.1210/endo-84-2-392. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIPNIS D. M., HELMREICH E., CORI C. F. Studies of tissue permeability. IV. The distribution of glucose between plasma and muscle. J Biol Chem. 1959 Jan;234(1):165–170. [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- MORGAN H. E., REGEN D. M., PARK C. R. IDENTIFICATION OF A MOBILE CARRIER-MEDIATED SUGAR TRANSPORT SYSTEM IN MUSCLE. J Biol Chem. 1964 Feb;239:369–374. [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P., CORI C. F. Studies of tissue permeability. VII. The effect of insulin on glucose penetration and phosphorylation in frog muscle. J Biol Chem. 1960 Dec;235:3370–3378. [PubMed] [Google Scholar]
- Pratt O. E. An electronically controlled syringe drive for giving an injection at a variable rate according to a preset programme. J Physiol. 1974 Mar;237(2):5P–6P. [PubMed] [Google Scholar]
- Randle P. J., Garland P. B., Hales C. N., Newsholme E. A., Denton R. M., Pogson C. I. Interactions of metabolism and the physiological role of insulin. Recent Prog Horm Res. 1966;22:1–48. doi: 10.1016/b978-1-4831-9825-5.50004-x. [DOI] [PubMed] [Google Scholar]
- Strang R. H., Bachelard H. S. Rapid enzymic methods for determination of the specific radioactivity of metabolic intermediates in unpurified brain tissue extracts. Anal Biochem. 1971 Jun;41(2):533–542. doi: 10.1016/0003-2697(71)90176-x. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


