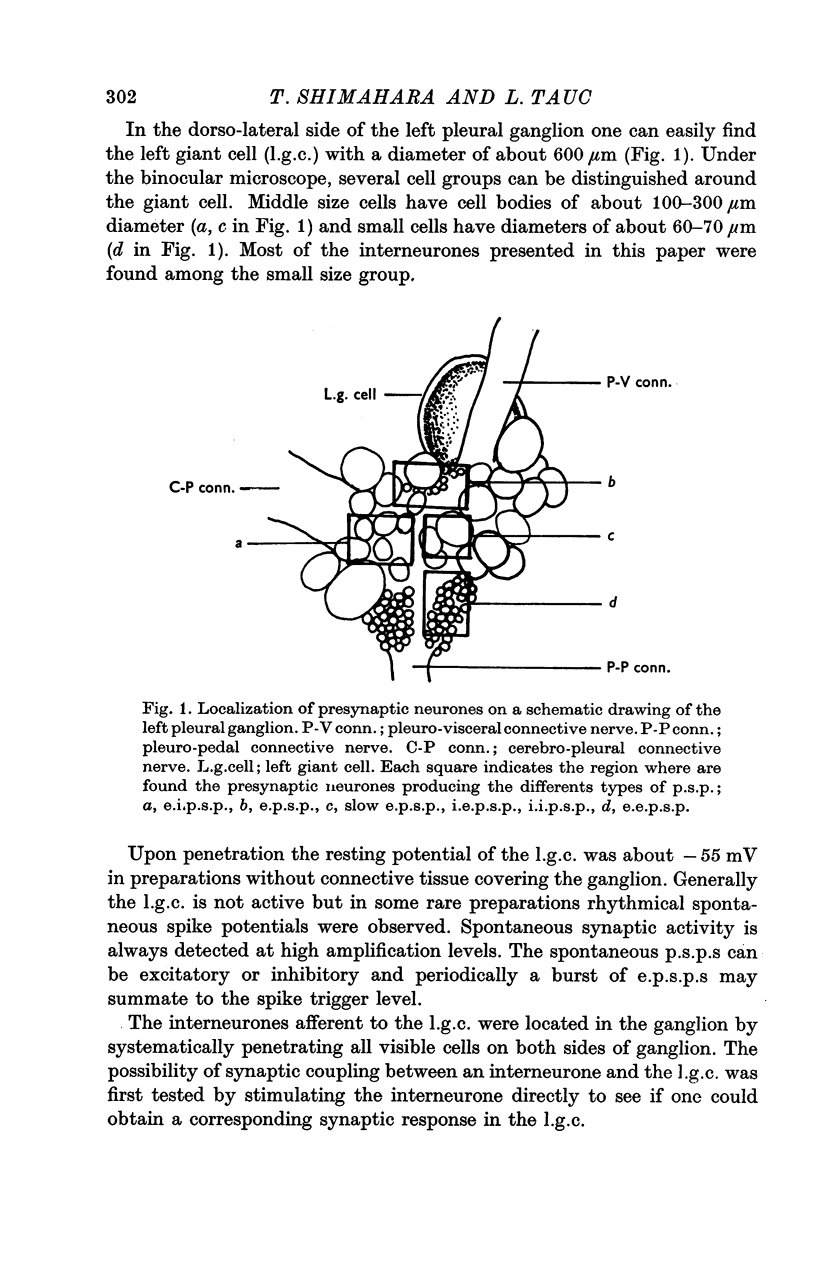
Abstract
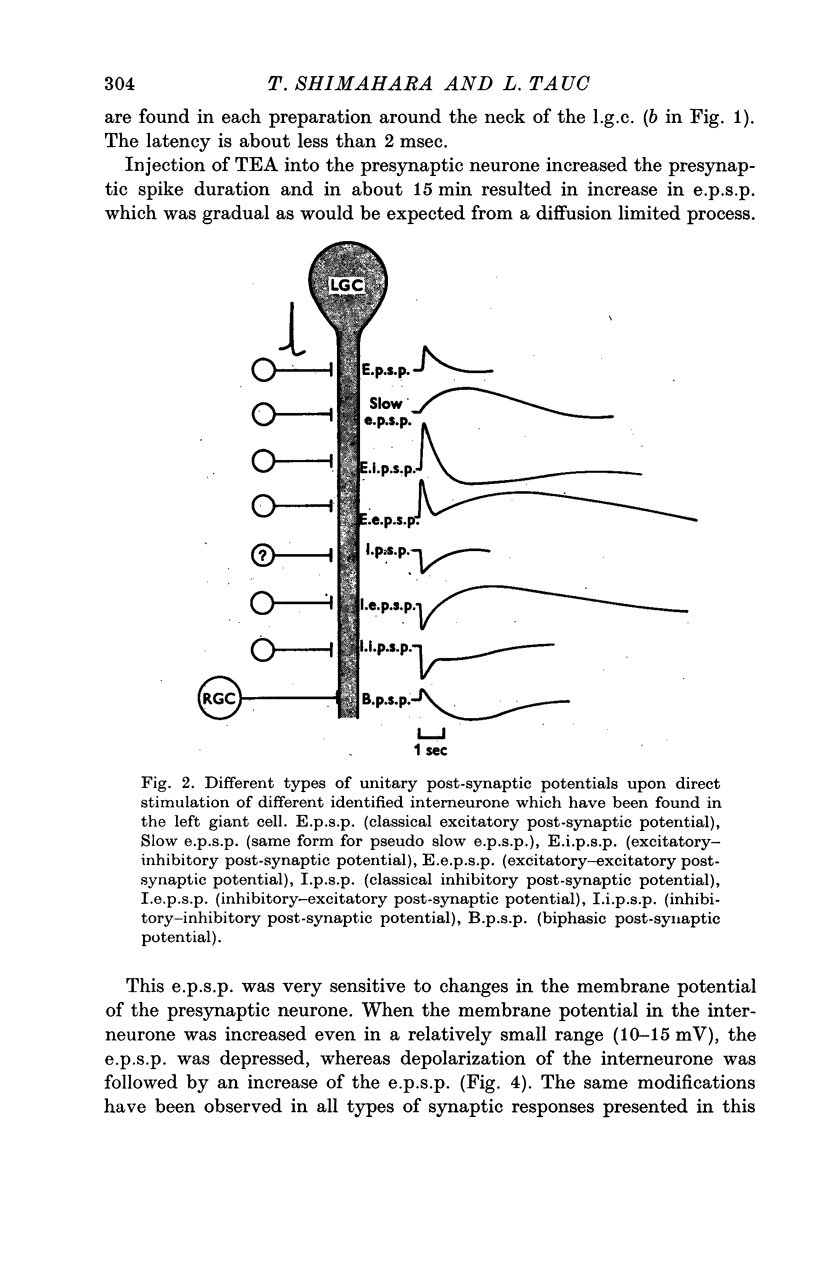
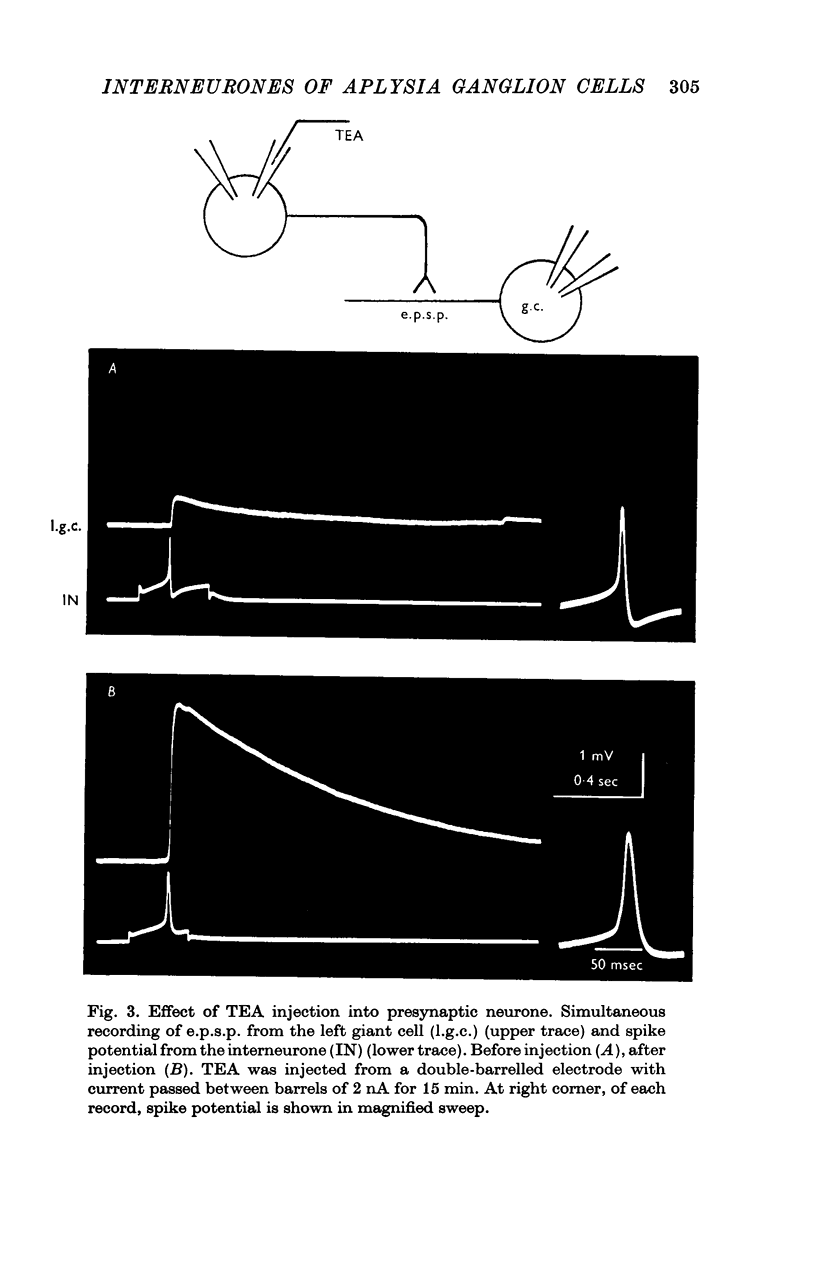
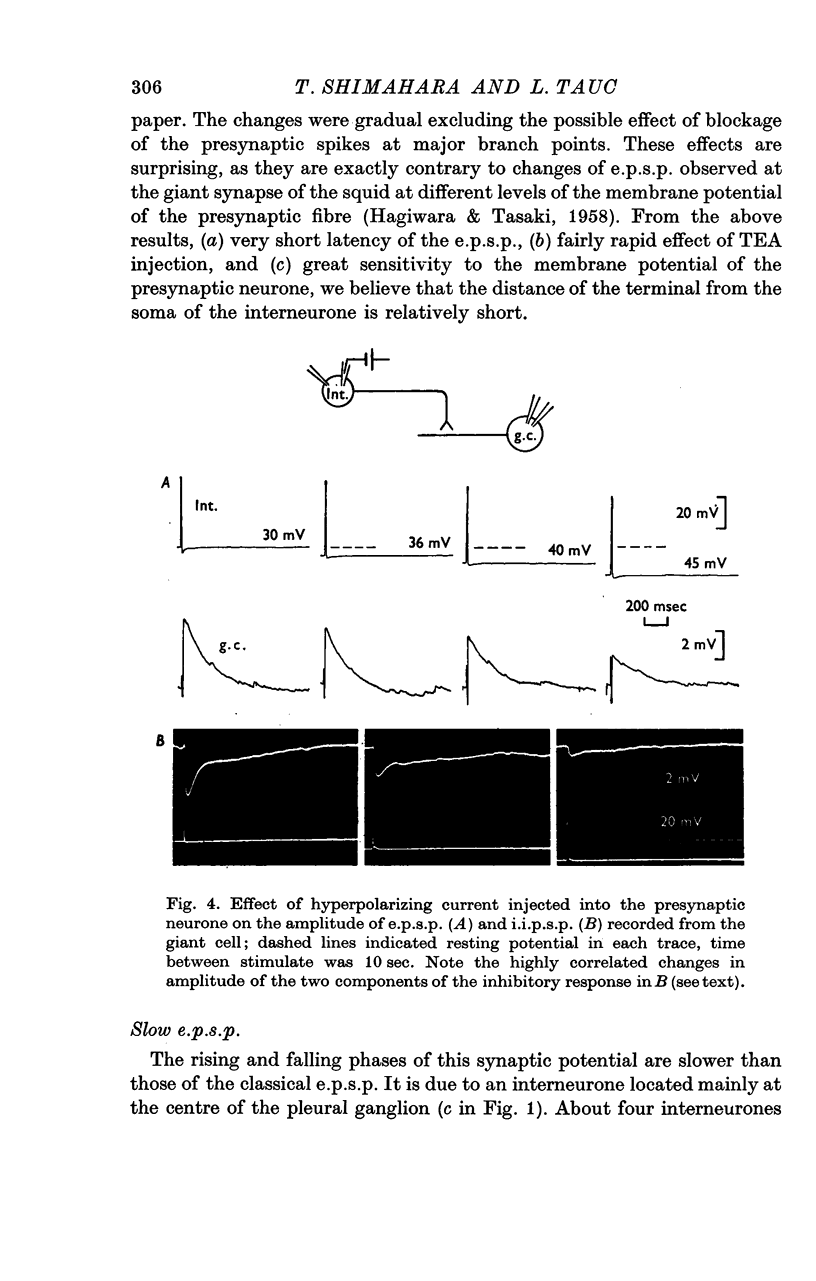
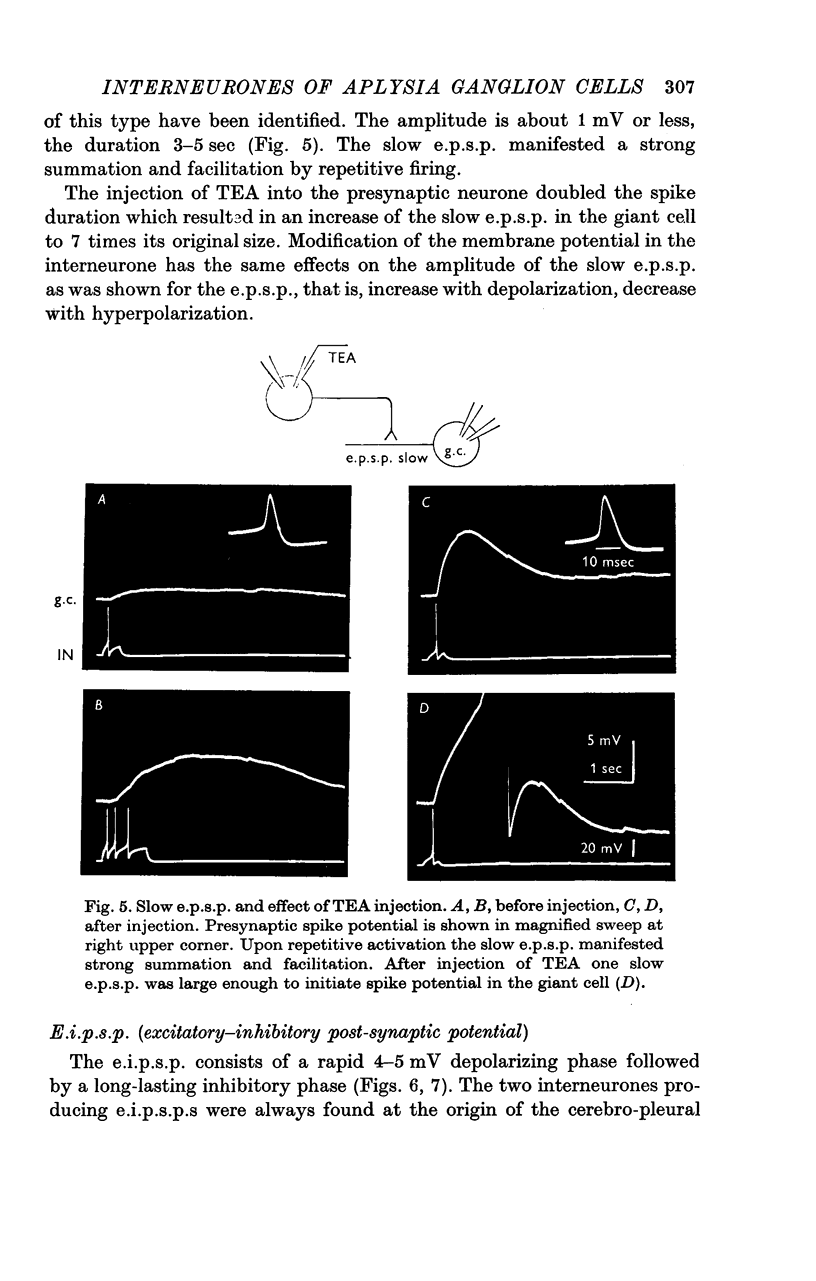
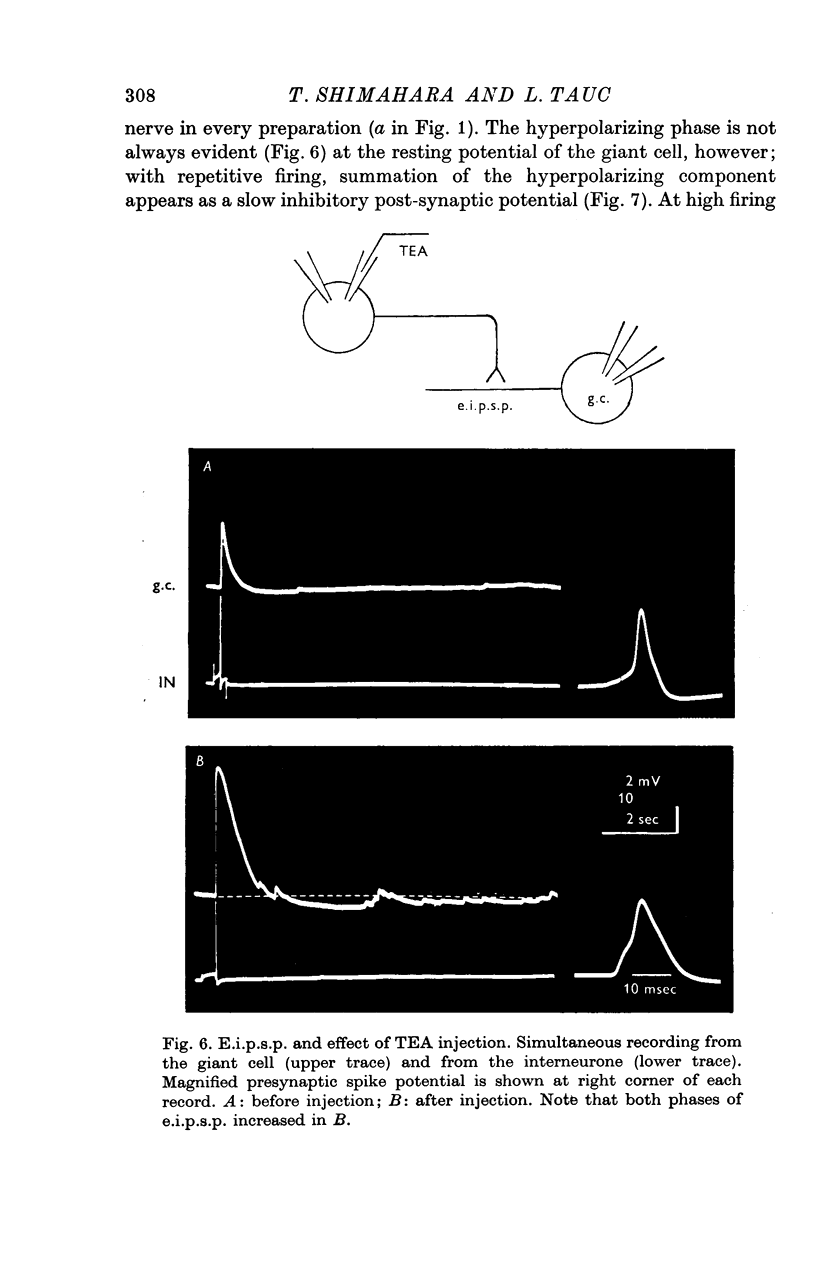
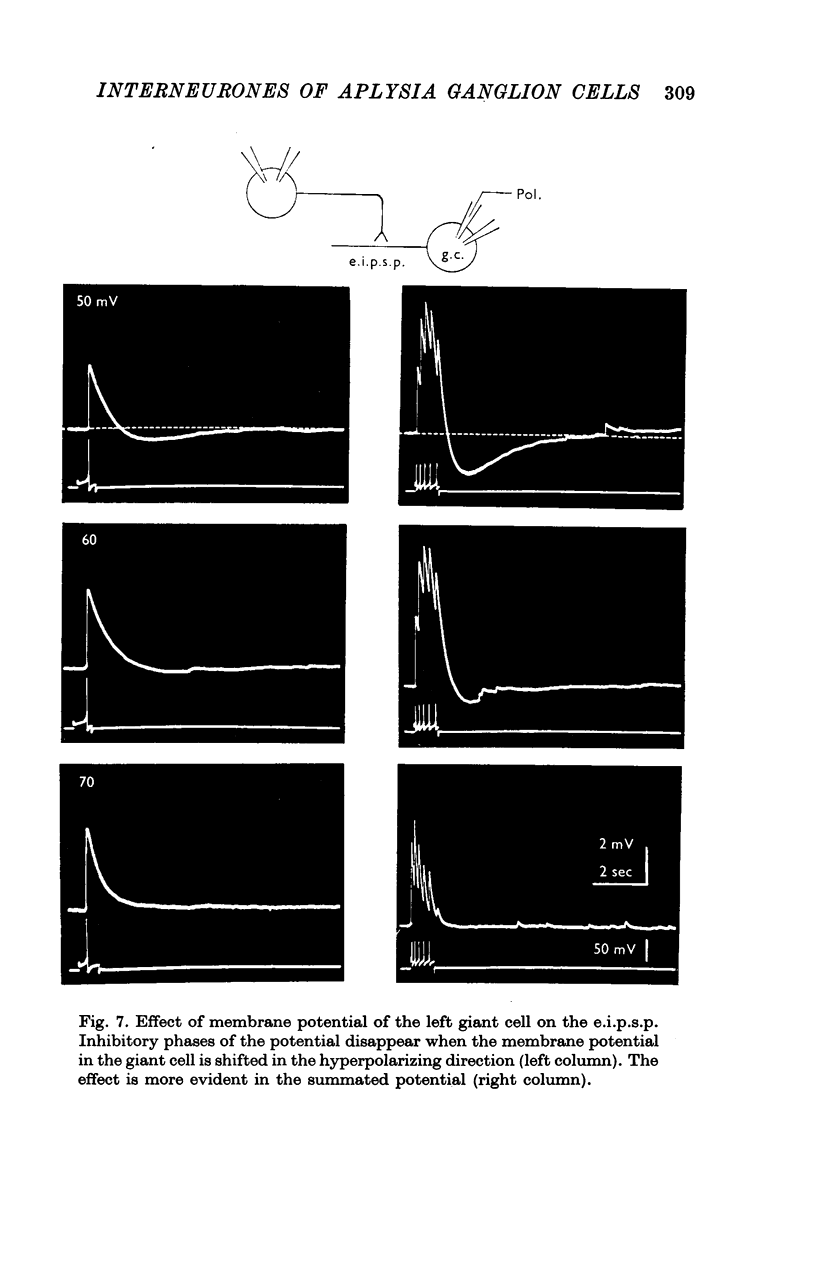
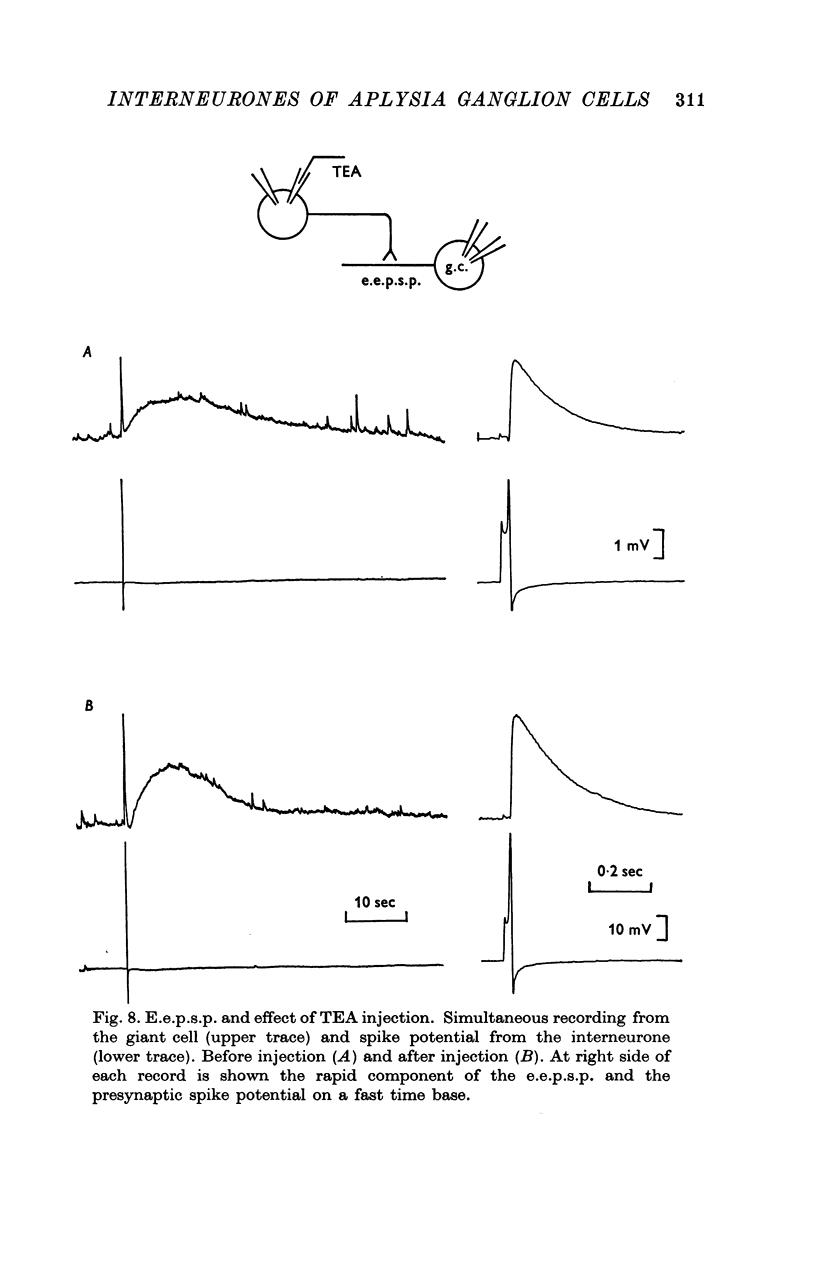
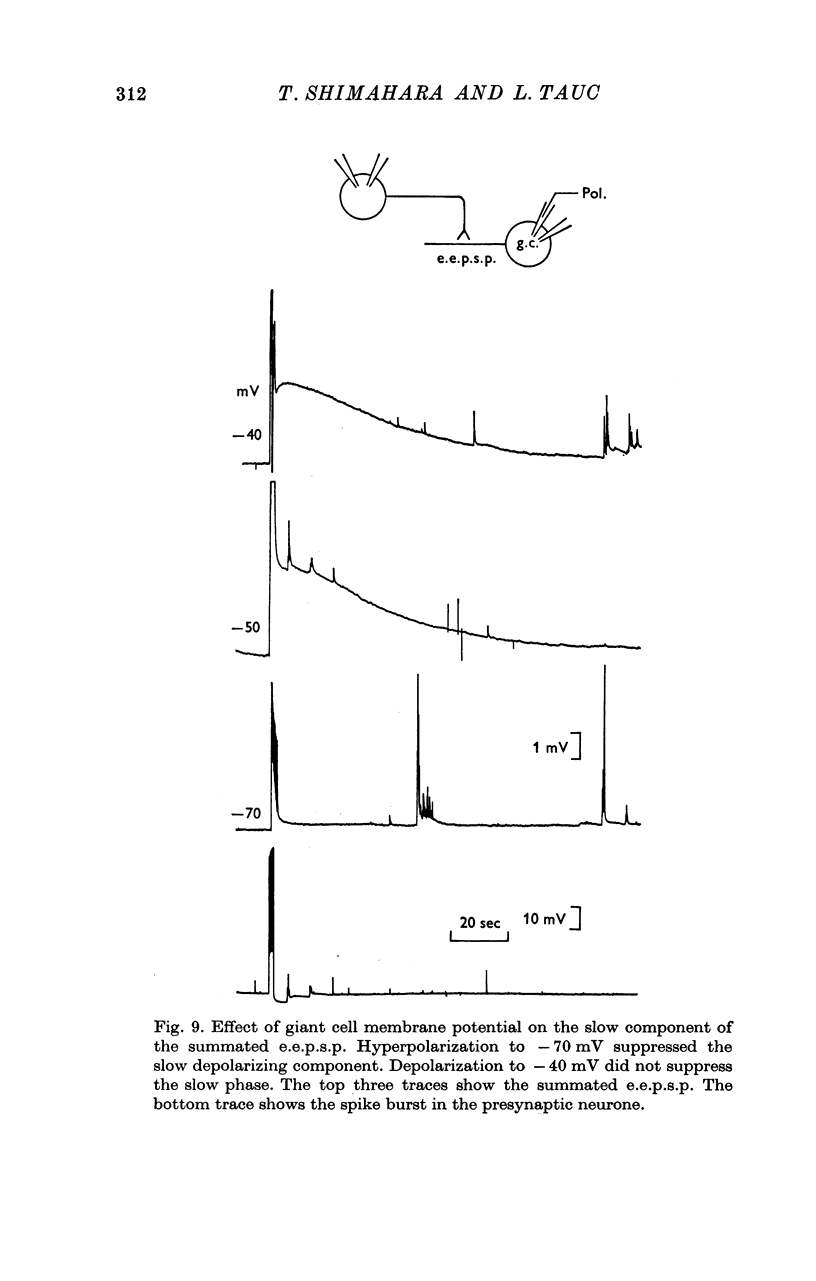
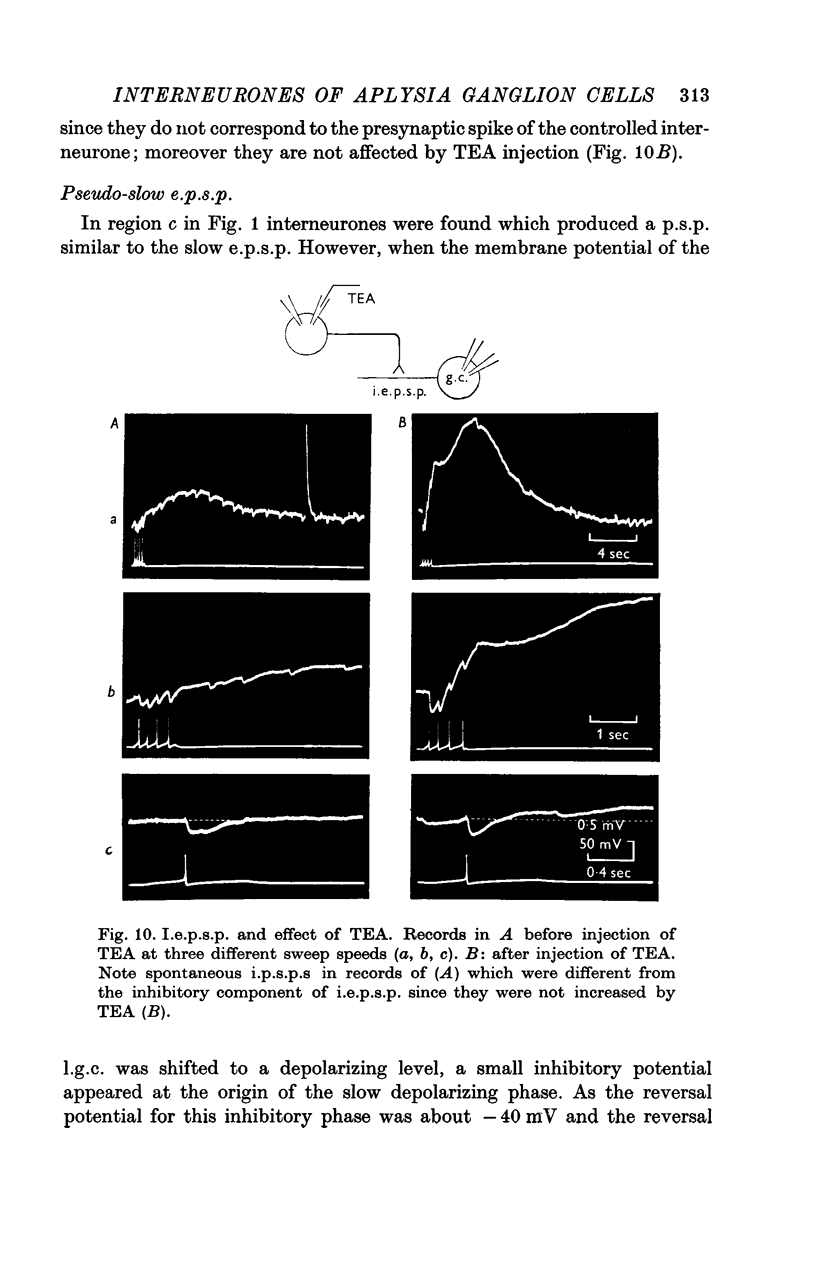
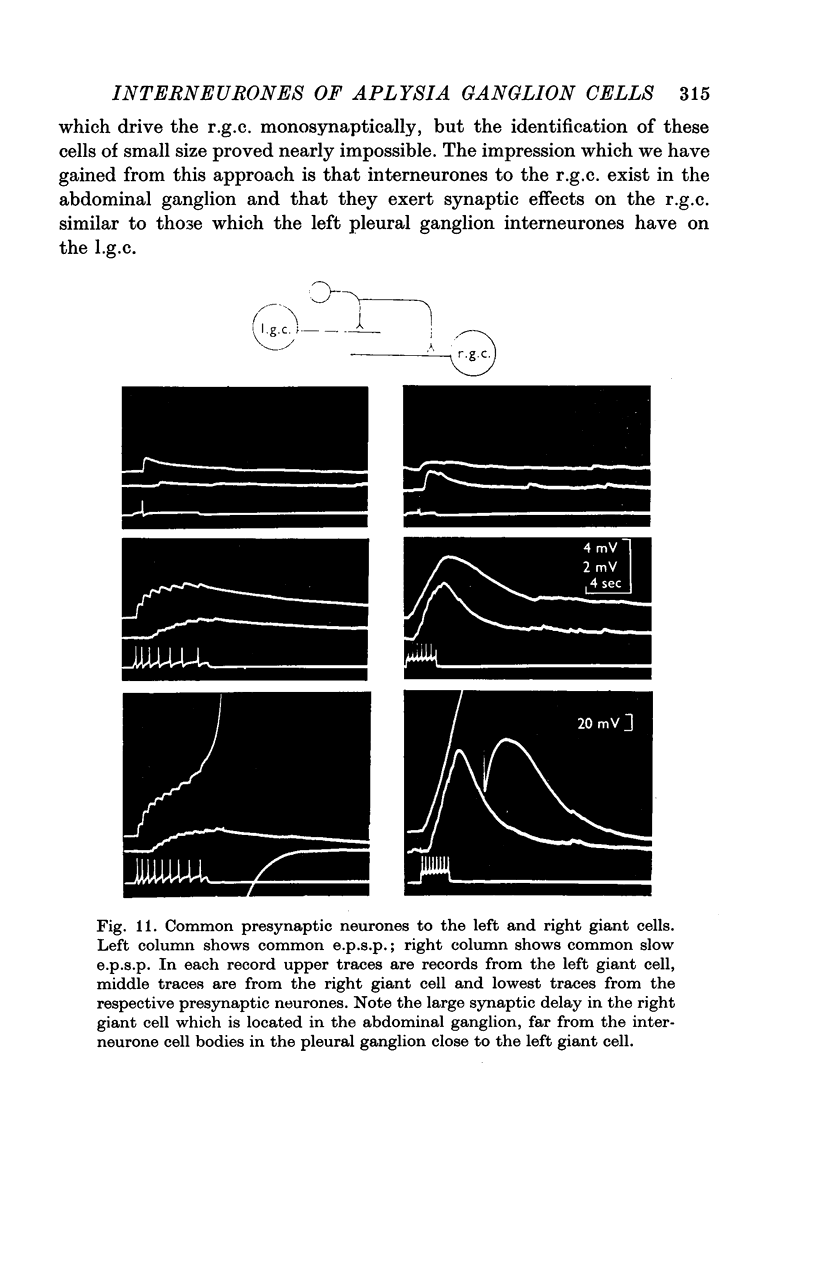
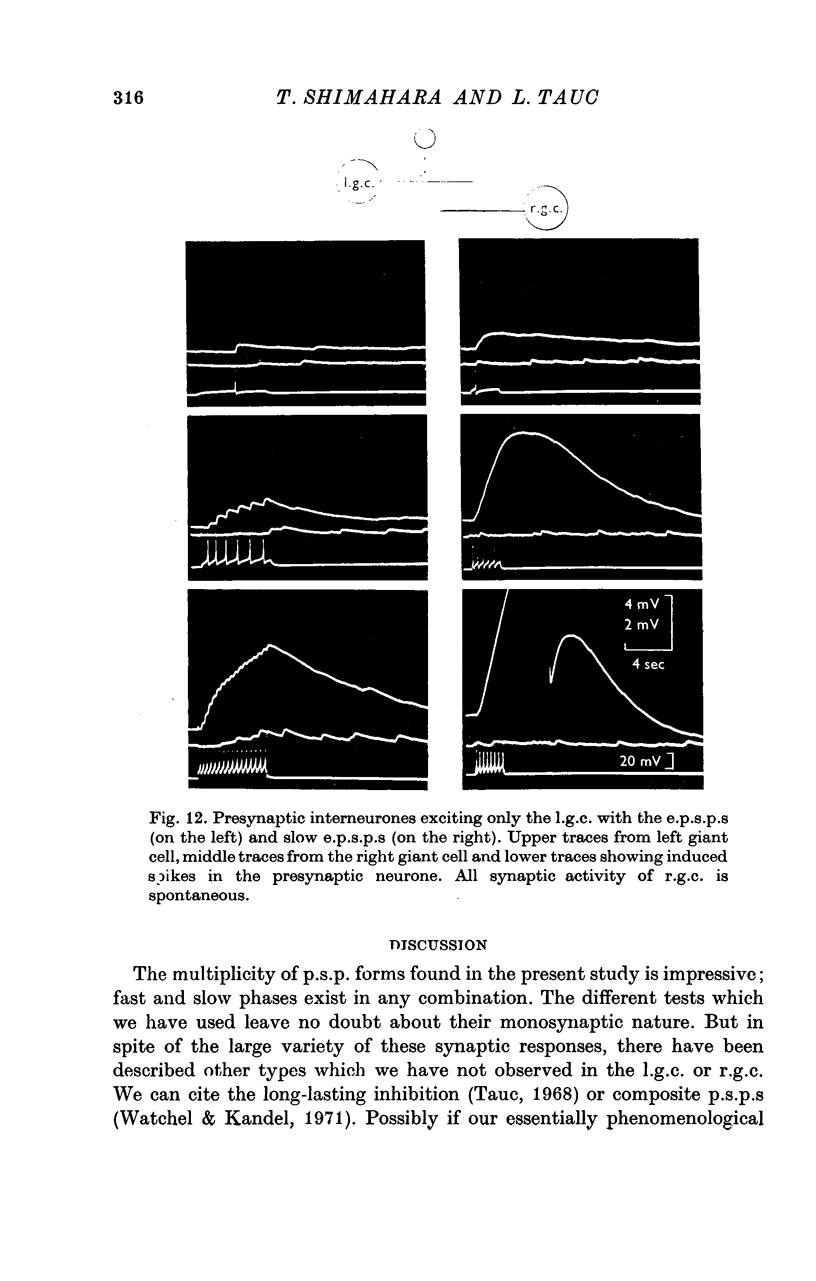
1. Several different types of presynaptic neurones to the giant cells of Aplysia have been found in the pleural ganglion. Some of these presynaptic neurones are common to the left giant cell in the pleural ganglion and to the right giant cell in the abdominal ganglion but others make contact only with one. 2. Interneurones of the left giant cell were studied in detail. They can be identified not only physiologically from the type of post-synapitc potential (p.s.p.) which they produce in the left giant cell, but also by their localization in the ganglion. 3. Direct stimulation of these presynaptic neurones produced not only the classical types of post-synaptic potentials known as e.p.s.p. or i.p.s.p. but also a slow e.p.s.p. and more complex post-synaptic potentials consisting of a rapid depolarizing or hyperpolarizing component (e for excitatory; i for inhibitory). According the p.s.p.s. which have been found were classified as being of eight different types: e.p.s.p., slow e.p.s.p., pseudo-slow e.p.s.p., e.i.p.s.p., i.e.p.s.p., i.i.p.s.p., to which is added the biphasic p.s.p. (b.p.s.p.) of electrical origin. 4. The monosynaptic nature of each of these p.s.p.s. was established by four criteria: (a) ability to follow one to one the presynaptic spike, (b) short and constant latency, (c) change of p.s.p. with the presynaptic spike when the duration is prolonged by iontophoretic injection of TEA, (d) sensitivity of the synaptic efficacy to presynaptic polarization. 5. For all p.s.p.s., the hyperpolarization of the interneurone was followed by a decrease in the corresponding amplitude; on the contrary depolarization produced an increase in p.s.p. amplitude. 6. The physiological role of these p.s.p.s. and their possible mechanism are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. The effect of polarizing current on action potential and transmitter release in crayfish motor nerve terminals. Pflugers Arch. 1971;324(3):227–248. doi: 10.1007/BF00586421. [DOI] [PubMed] [Google Scholar]
- Gardner D. Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia. Science. 1971 Aug 6;173(3996):550–553. doi: 10.1126/science.173.3996.550. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Serotonin: two different inhibitory actions on snail neurons. Science. 1971 Mar 26;171(3977):1252–1254. doi: 10.1126/science.171.3977.1252. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., TASAKI I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol. 1958 Aug 29;143(1):114–137. doi: 10.1113/jphysiol.1958.sp006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. An electrophysiological investigation of mammalian motor nerve terminals. J Physiol. 1963 Apr;166:145–167. doi: 10.1113/jphysiol.1963.sp007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. AN ELECTROPHYSIOLOGICAL STUDY OF THE ANATOMICAL RELATIONS OF TWO GIANT NERVE CELLS IN APLYSIA DEPILANS. J Exp Biol. 1963 Sep;40:469–486. doi: 10.1242/jeb.40.3.469. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I. Microphysiology of vertebrate neuromuscular transmission. Physiol Rev. 1973 Jul;53(3):674–723. doi: 10.1152/physrev.1973.53.3.674. [DOI] [PubMed] [Google Scholar]
- Hughes G. M., Tauc L. A direct synaptic connexion between the left and right giant cells in Aplysia. J Physiol. 1968 Aug;197(3):511–527. doi: 10.1113/jphysiol.1968.sp008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Waziri R., Coggeshall R. E. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967 Nov;30(6):1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. J Physiol. 1966 Mar;183(2):287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Pharmacological characteristics and ionic bases of a 2 component postsynaptic inhibition. Nature. 1967 Sep 30;215(5109):1503–1505. doi: 10.1038/2151503b0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Single presynaptic neurone mediates a two component postsynaptic inhibition. Nature. 1969 Mar 1;221(5183):866–868. doi: 10.1038/221866a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Tetraethylammonium ions: effect of presynaptic injection on synaptic transmission. Science. 1967 Mar 10;155(3767):1257–1259. doi: 10.1126/science.155.3767.1257. [DOI] [PubMed] [Google Scholar]
- LIBET B. SLOW SYNAPTIC RESPONSES AND EXCITATORY CHANGES IN SYMPATHETIC GANGLIA. J Physiol. 1964 Oct;174:1–25. doi: 10.1113/jphysiol.1964.sp007471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan H., Tauc L. Acetylcholine receptors: topographic distribution and pharmacological properties of two receptor types on a single molluscan neurone. J Physiol. 1972 May;222(3):537–558. doi: 10.1113/jphysiol.1972.sp009813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupardin-Tritsch D., Gerschenfeld H. M. Neuronal responses to 5-hydroxytryptamine resulting from membrane permeability decreases. Nat New Biol. 1973 Aug 8;244(136):171–173. doi: 10.1038/newbio244171a0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. Cholinergic transmission mechanisms for both excitation and inhibition in molluscan central synapses. Nature. 1961 Oct 28;192:366–367. doi: 10.1038/192366a0. [DOI] [PubMed] [Google Scholar]
- Tauc L. Transmission in invertebrate and vertebrate ganglia. Physiol Rev. 1967 Jul;47(3):521–593. doi: 10.1152/physrev.1967.47.3.521. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]


