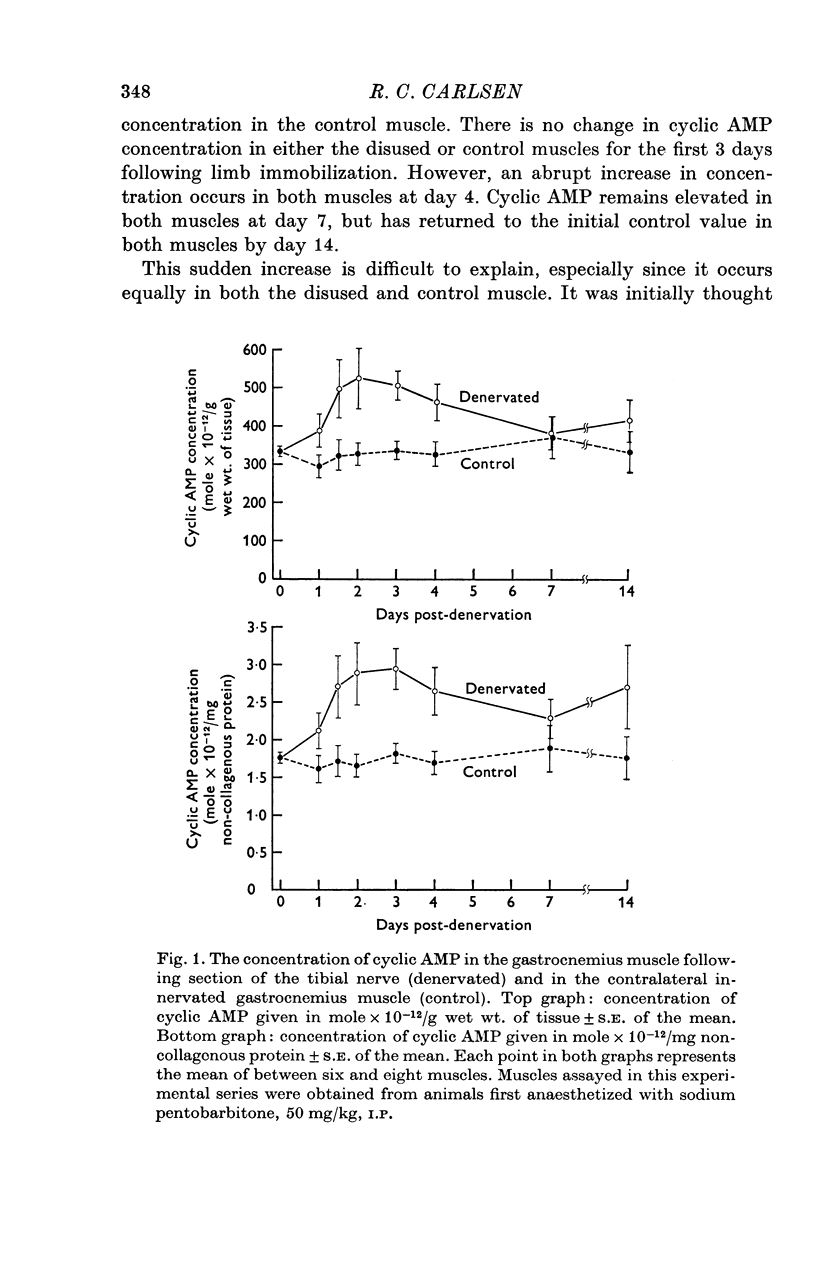
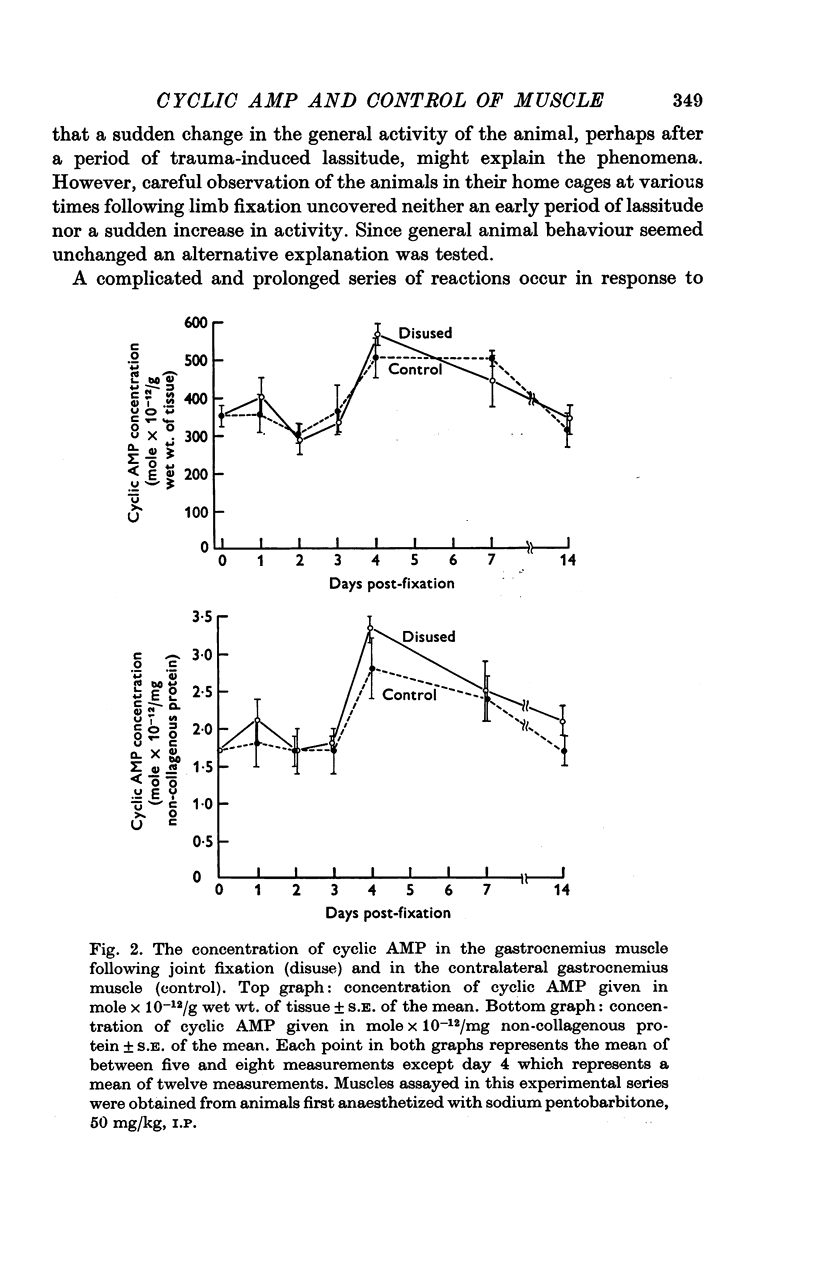
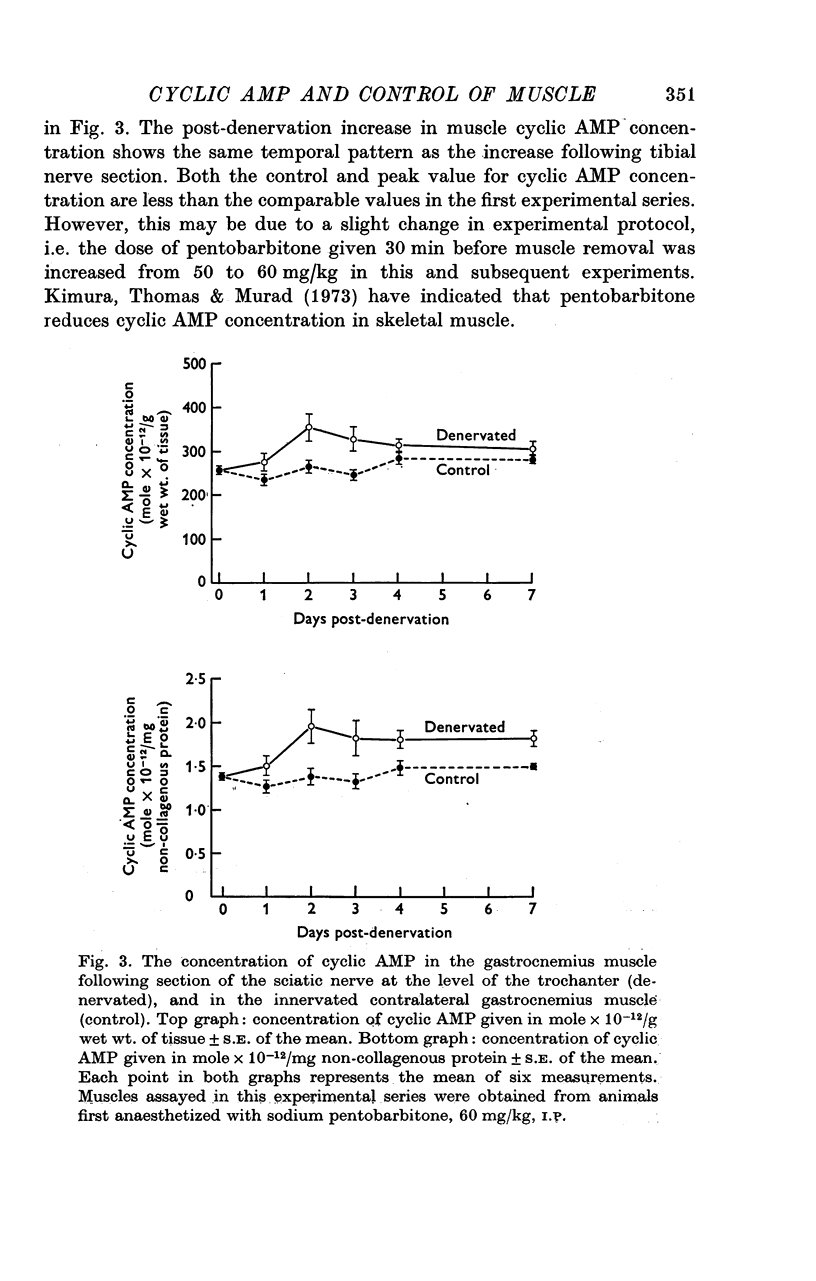
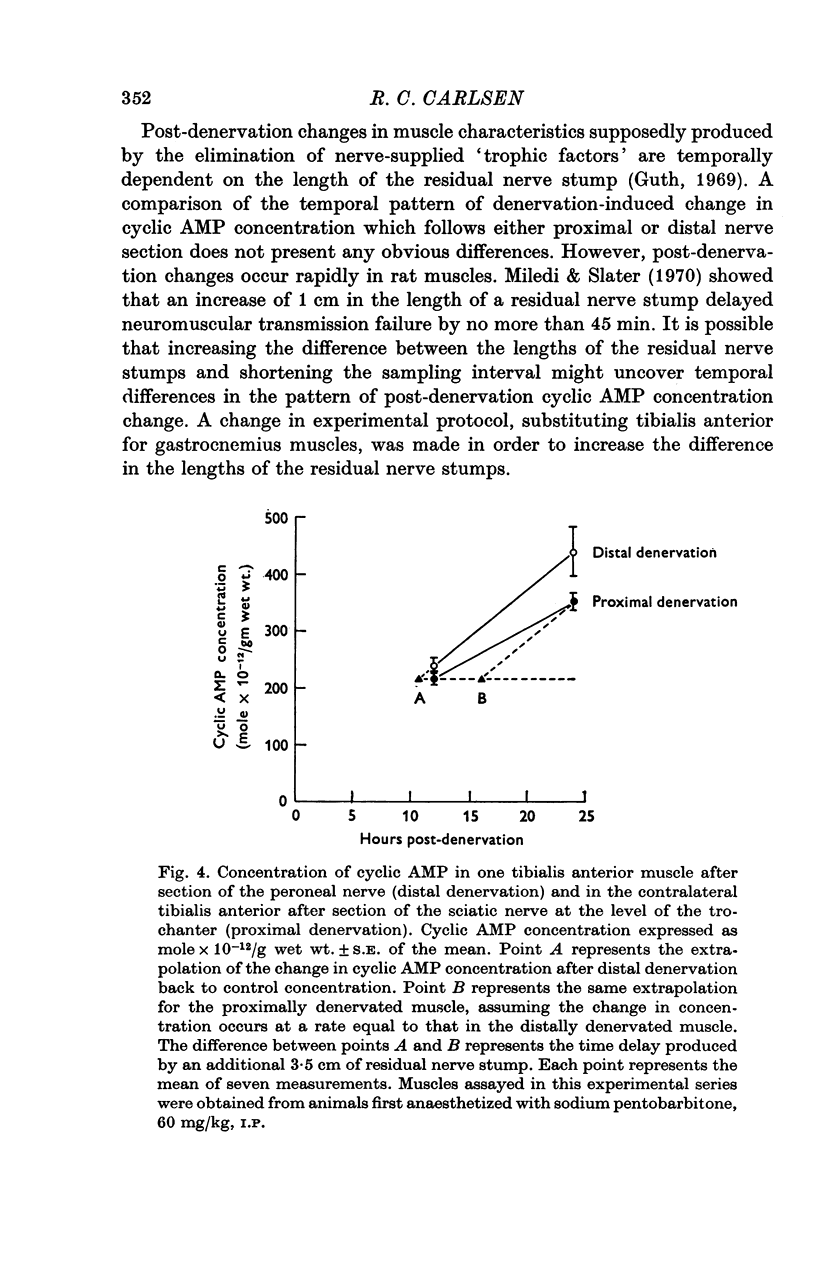
Abstract
1. Motoneurones provide trophic control of some of the functional characteristics of skeletal muscle fibres. This study has been designed to test whether the adenylate cyclase: cyclic AMP system may offer one potential mechanism for the mediation of neurotrophic regulation. 2. The concentration of cyclic AMP was measured at various intervals after muscle denervation. Muscle cyclic AMP concentration increases for the first 2 days after nerve section. It reaches a maximum value at 48 h and subsequently returns to the control value at 7 days. 3. Cyclic AMP concentration is unchanged by muscle disuse for the first 3 days following limb immobilization. Four days after immobilization, however, cyclic AMP increases in both the disused and contralateral control muscles. This phenomenon has been tentatively ascribed to some aspect of the inflammatory response. 4. Changing the level of nerve section, and therefore the length of the residual nerve stump, changes the temporal pattern of the increase in muscle cyclic AMP concentration. 5. Reinnervation of a denervated muscle produces a decrease in muscle cyclic AMP concentration. 6. It is concluded from the results that some aspect of nerve function provides trophic regulation of the muscle adenylate cyclase: cyclic AMP system. The mechanisms by which this regulation may be applied are considered in the Discussion.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E., Tasse J. R., Sansone F. M. Effects of vinblastine and colchicine on neural regulation of the fast and slow skeletal muscles of the rat. Exp Neurol. 1972 Dec;37(3):607–634. doi: 10.1016/0014-4886(72)90103-3. [DOI] [PubMed] [Google Scholar]
- BEATTY C. H., PETERSON R. D., BOCEK R. M., CRAIG N. C., WELEBER R. EFFECT OF GLUCAGON ON INCORPORATION OF GLYCINE-C14 INTO PROTEIN OF VOLUNTARY SKELETAL MUSCLE. Endocrinology. 1963 Dec;73:721–726. doi: 10.1210/endo-73-6-721. [DOI] [PubMed] [Google Scholar]
- BOWMAN W. C., RAPER C. THE EFFECTS OF SYMPATHOMIMETIC AMINES ON CHRONICALLY DENERVATED SKELETAL MUSCLES. Br J Pharmacol Chemother. 1965 Feb;24:98–109. doi: 10.1111/j.1476-5381.1965.tb02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmar J., Eyzaguirre C. Pacemaker site of fibrillation potentials in denervated mammmalian muscle. J Neurophysiol. 1966 May;29(3):425–441. doi: 10.1152/jn.1966.29.3.425. [DOI] [PubMed] [Google Scholar]
- Drummond G. I., Harwood J. P., Powell C. A. Studies on the activation of phosphorylase in skeletal muscle by contraction and by epinephrine. J Biol Chem. 1969 Aug 10;244(15):4235–4240. [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine sensitivity of muscle fiber membranes: mechanism of regulation by motoneurons. Science. 1970 Apr 17;168(3929):372–373. doi: 10.1126/science.168.3929.372. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Robbins N. Changes in contractile properties of disused soleus muscles. J Physiol. 1969 Apr;201(2):305–320. doi: 10.1113/jphysiol.1969.sp008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Robbins N. Effect of chronic disuse of rat soleus neuromuscular junctions on postsynaptic membrane. J Neurophysiol. 1971 Jul;34(4):562–569. doi: 10.1152/jn.1971.34.4.562. [DOI] [PubMed] [Google Scholar]
- GUTMANN E. NEUROTROPHIC RELATIONS IN THE REGENERATION PROCESS. Prog Brain Res. 1964;13:72–114. doi: 10.1016/s0079-6123(08)60140-5. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grampp W., Harris J. B., Thesleff S. Inhibition of denervation changes in skeletal muscle by blockers of protein synthesis. J Physiol. 1972 Mar;221(3):743–754. doi: 10.1113/jphysiol.1972.sp009780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L. "Trophic" influences of nerve on muscle. Physiol Rev. 1968 Oct;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- Jones R., Vrbová G. Two factors responsible for the development of denervation hypersensitivity. J Physiol. 1974 Feb;236(3):517–538. doi: 10.1113/jphysiol.1974.sp010450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE DEVELOPMENT OF ACETYLCHOLINE SENSITIVITY IN NERVE-FREE SEGMENTS OF SKELETAL MUSCLE. J Physiol. 1964 Mar;170:389–396. doi: 10.1113/jphysiol.1964.sp007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. 8. An assay method for the measurement of adenosine 3',5'-monophosphate in various tissues and a study of agents influencing its level in adipose cells. J Biol Chem. 1970 Aug 25;245(16):4067–4073. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lentz T. L. A role of cyclic AMP in a neurotrophic process. Nat New Biol. 1972 Aug 2;238(83):154–155. doi: 10.1038/newbio238154a0. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSNER J. B., STERN R., KREBS E. G. EFFECTS OF ELECTRICAL STIMULATION AND EPINEPHRINE ON MUSCLE PHOSPHORYLASE, PHOSPHORYLASE B KINASE, AND ADENOSINE 3',5'-PHOSPHATE. J Biol Chem. 1965 Mar;240:982–985. [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- STEWART D. M. Protein composition of denervated muscle. Am J Physiol. 1962 Feb;202:281–284. doi: 10.1152/ajplegacy.1962.202.2.281. [DOI] [PubMed] [Google Scholar]
- Salafsky B., Bell J., Prewitt M. A. Development of fibrillation potentials in denervated fast and slow skeletal muscle. Am J Physiol. 1968 Sep;215(3):637–643. doi: 10.1152/ajplegacy.1968.215.3.637. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]


