Abstract
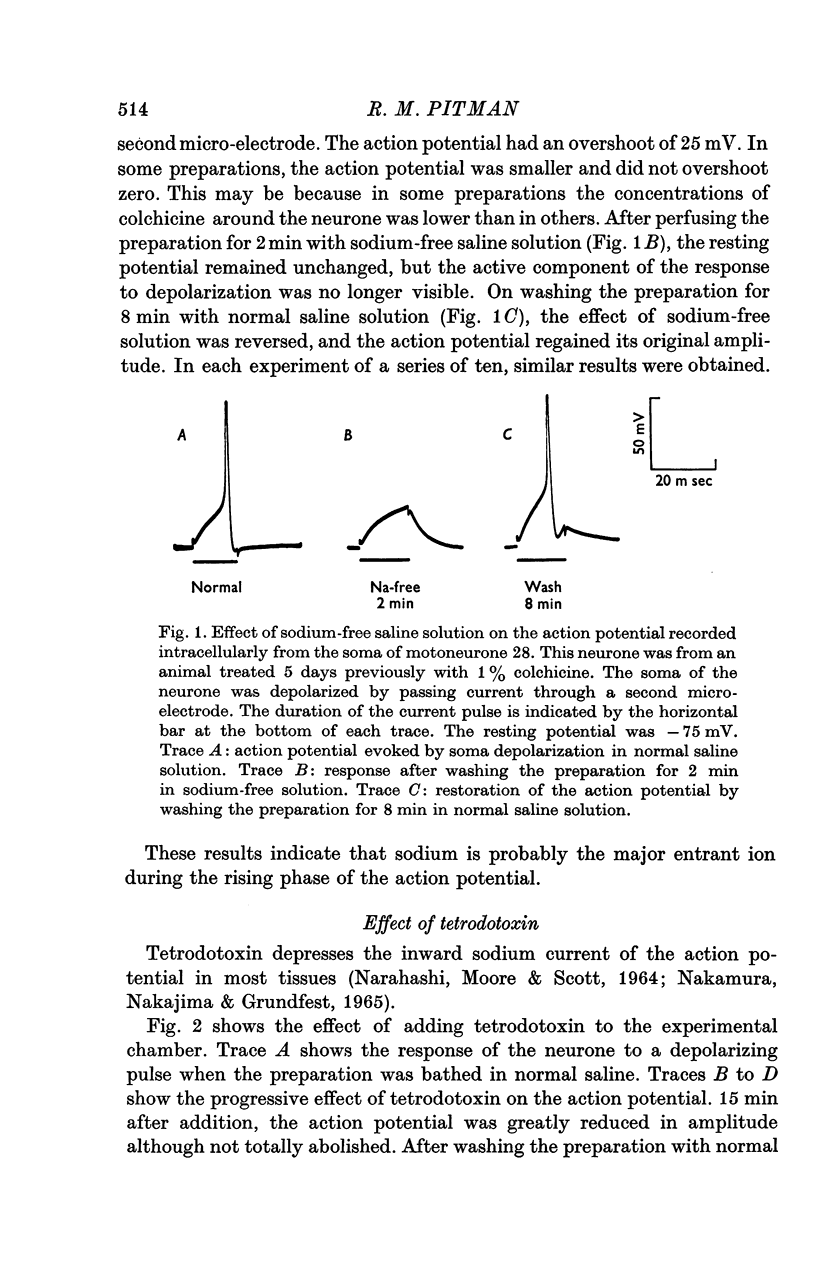
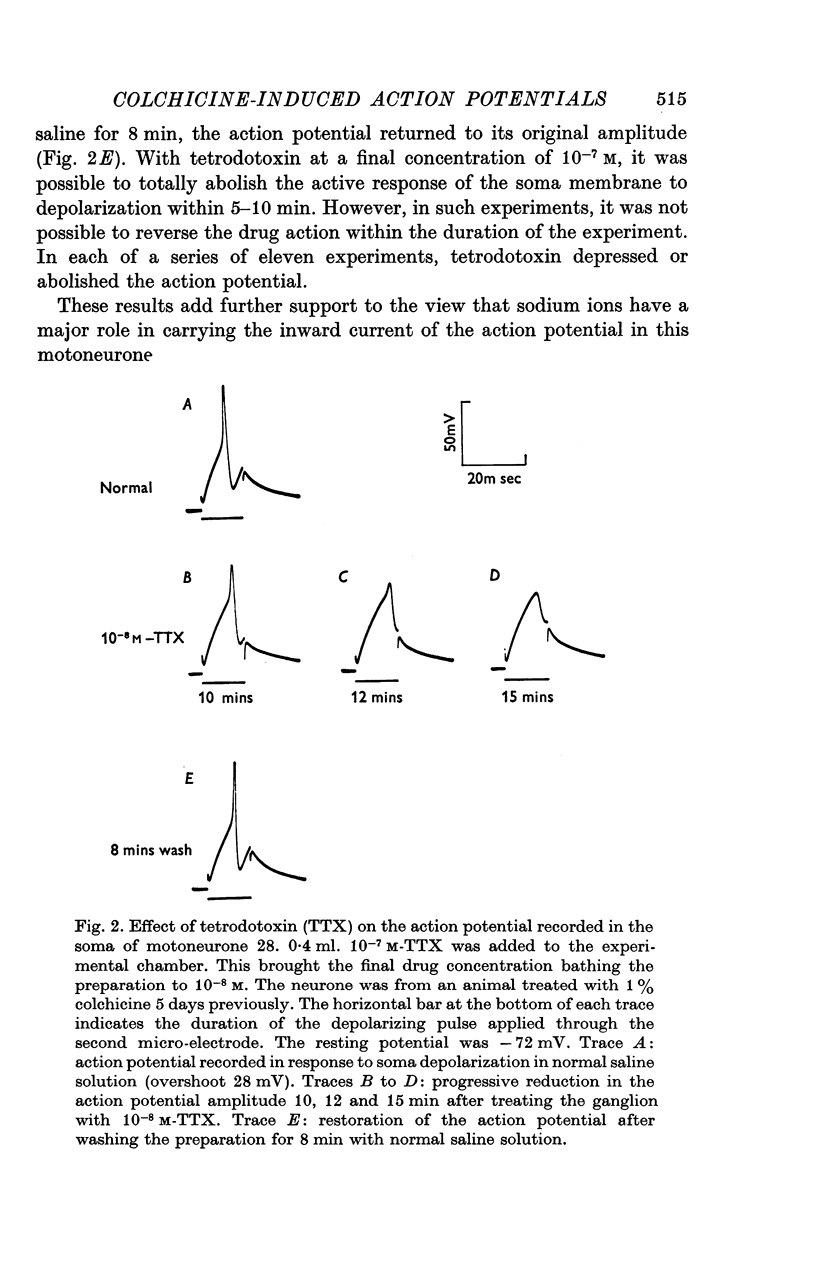
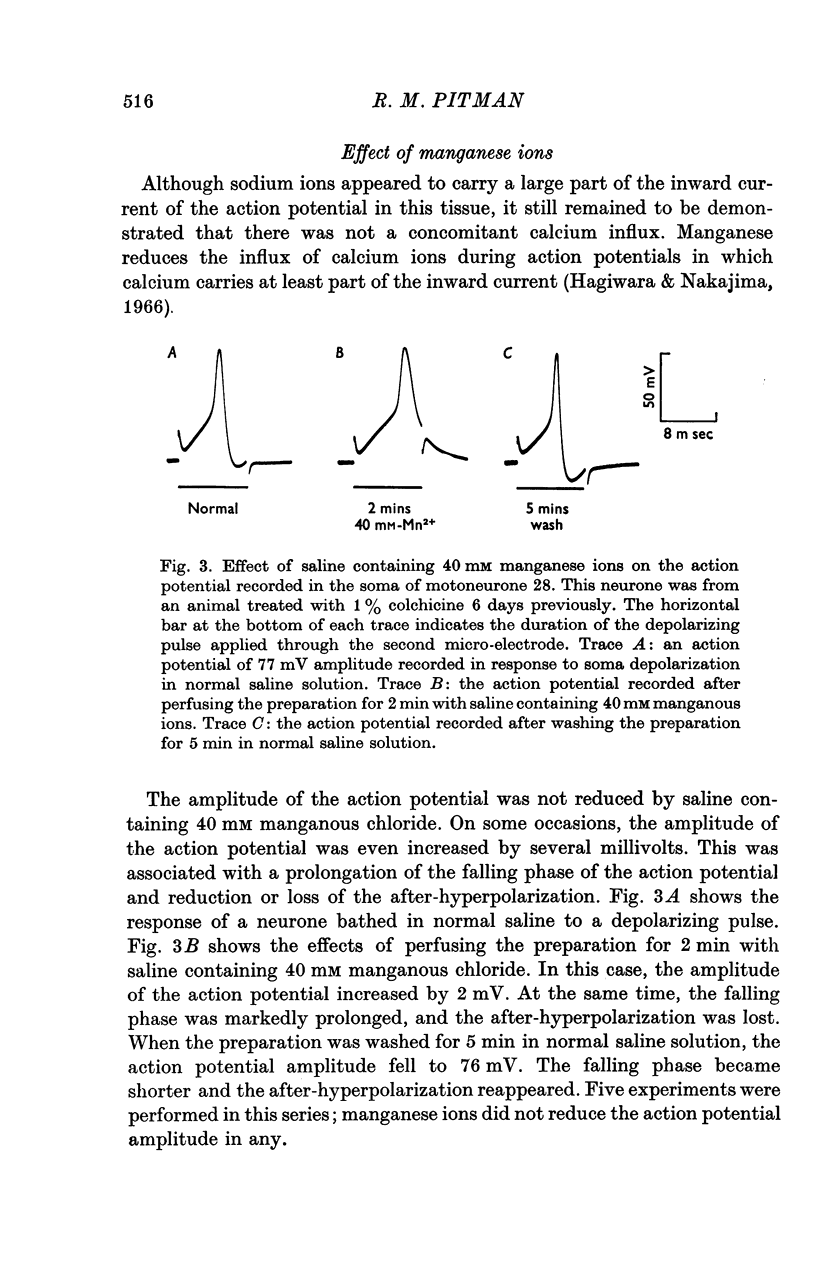
1. The ionic requirements of the action potential recorded in the cell of an identified cockroach (Periplaneta americana) motoneurone following pre-treatment of the animal with colchicine have been studied. 2. Small cubes of gelatin containing 1% colchicine were implanted into one metathoracic leg near to the nerve trunk containing the axon of the identified motoneurone. 3. Electrophysiological experiments were performed 4--10 days after this treatment, when action potentials which frequently overshoot zero potential can be recorded from the cell body. Such action potentials are not normally seen in untreated animals. 4. Sodium-free solution reversibly abolished the action potential within 5 min. 5. Tetrodotoxin (10(-8)M) reversibly depressed the action potential. It was totally abolished by 10(-7)M tetrodotoxin, but this effect was not reversible. 6. Saline solution containing 40 mM manganous chloride either had no effect on the action potential amplitude, or caused a slight increase. It also caused prolongation of the falling phase and loss of the after-hyperpolarization. These effects were all reversible. 7. It is concluded that sodium carries a major proportion of the inward current of the action potential in this neurone. Some calcium probably enters also, and may, at least in part, be responsible for triggering the delayed rise in potassium conductance during the action potential.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., PARNAS I. ELECTRICAL AND MECHANICAL RESPONSES IN DEEP ABDOMINAL EXTENSOR MUSCLES OF CRAYFISH AND LOBSTER. J Gen Physiol. 1965 May;48:919–931. doi: 10.1085/jgp.48.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S. G., Kerkut G. A. Voltage clamp studies on snail (Helix aspersa) neurons. Nature. 1967 Oct 7;216(5110):89–89. doi: 10.1038/216089a0. [DOI] [PubMed] [Google Scholar]
- Dahlström A. Effect of colchicine on transport of amine storage granules in sympathetic nerves of rat. Eur J Pharmacol. 1968 Dec;5(1):111–113. doi: 10.1016/0014-2999(68)90165-9. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., LIBET B., YOUNG R. R. The behaviour of chromatolysed motoneurones studied by intracellular recording. J Physiol. 1958 Aug 29;143(1):11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Junge D. Sodium and calcium components of action potentials in the Aplysia giant neurone. J Physiol. 1968 Dec;199(2):347–365. doi: 10.1113/jphysiol.1968.sp008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., STAMPFLI R. Effect of potassium and sodium on resting and action potentials of single myelinated nerve fibers. J Physiol. 1951 Feb;112(3-4):496–508. doi: 10.1113/jphysiol.1951.sp004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Studies on tetrodotoxin resistant action potentials in denervated skeletal muscle. Acta Physiol Scand. 1971 Nov;83(3):382–388. doi: 10.1111/j.1748-1716.1971.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Karlsson J. O., Hansson H. A., Sjöstrand J. Effect of colchicine on axonal transport and morphology of retinal ganglion cells. Z Zellforsch Mikrosk Anat. 1971;115(2):265–283. doi: 10.1007/BF00391128. [DOI] [PubMed] [Google Scholar]
- Karlsson J. O., Sjöstrand J. The effect of colchicine on the axonal transport of protein in the optic nerve and tract of the rabbit. Brain Res. 1969 May;13(3):617–619. doi: 10.1016/0006-8993(69)90274-1. [DOI] [PubMed] [Google Scholar]
- Kreutzberg G. W. Neuronal dynamics and axonal flow. IV. Blockage of intra-axonal enzyme transport by colchicine. Proc Natl Acad Sci U S A. 1969 Mar;62(3):722–728. doi: 10.1073/pnas.62.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Magura I. S. Calcium ions as inward current carriers in mollusc neurones. Comp Biochem Physiol. 1970 Aug 15;35(4):857–866. doi: 10.1016/0010-406x(70)90080-0. [DOI] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McINTYRE A. K., BRADLEY K., BROCK L. G. Responses of motoneurons undergoing chromatolysis. J Gen Physiol. 1959 May 20;42(5):931–958. doi: 10.1085/jgp.42.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. The ionic requirements for the production of action potentials in helix pomatia neurones. Pflugers Arch. 1968;304(3):215–241. doi: 10.1007/BF00592126. [DOI] [PubMed] [Google Scholar]
- Miledi R., Stefani E., Steinbach A. B. Induction of the action potential mechanism in slow muscle fibres of the frog. J Physiol. 1971 Sep;217(3):737–754. doi: 10.1113/jphysiol.1971.sp009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreton R. B. Ionic mechanism of the action potentials of giant neurones of Helix aspersa. Nature. 1968 Jul 6;219(5149):70–71. doi: 10.1038/219070a0. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nakajima S., Grundfest H. The action of tetrodotoxin on electrogenic components of squid giant axons. J Gen Physiol. 1965 Jul;48(6):975–996. [PubMed] [Google Scholar]
- Norström A., Hansson H. A., Sjöstrand J. Effects of colchicine on axonal transport and ultrastructure of the hypothalamo-neurohypophyseal system of the rat. Z Zellforsch Mikrosk Anat. 1971;113(2):271–293. doi: 10.1007/BF00339421. [DOI] [PubMed] [Google Scholar]
- Pearson K. G., Iles J. F. Discharge patterns of coxal levator and depressor motoneurones of the cockroach, Periplaneta americana. J Exp Biol. 1970 Feb;52(1):139–165. doi: 10.1242/jeb.52.1.139. [DOI] [PubMed] [Google Scholar]
- Pichon Y., Treherne J. E. Extraneuronal potentials and potassium depolarization in cockroach giant axons. J Exp Biol. 1970 Oct;53(2):485–493. doi: 10.1242/jeb.53.2.485. [DOI] [PubMed] [Google Scholar]
- Pitman R. M., Tweedle C. D., Cohen M. J. Electrical responses of insect central neurons: augmentation by nerve section or colchicine. Science. 1972 Nov 3;178(4060):507–509. doi: 10.1126/science.178.4060.507. [DOI] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Treherne J. E., Lane N. J., Moreton R. B., Pichon Y. A quantitative study of potassium movements in the central nervous system of Periplaneta americana. J Exp Biol. 1970 Aug;53(1):109–136. doi: 10.1242/jeb.53.1.109. [DOI] [PubMed] [Google Scholar]
- Wald F. Ionic differences between somatic and axonal action potentials in snail giant neurones. J Physiol. 1972 Jan;220(2):267–281. doi: 10.1113/jphysiol.1972.sp009706. [DOI] [PMC free article] [PubMed] [Google Scholar]


