Abstract
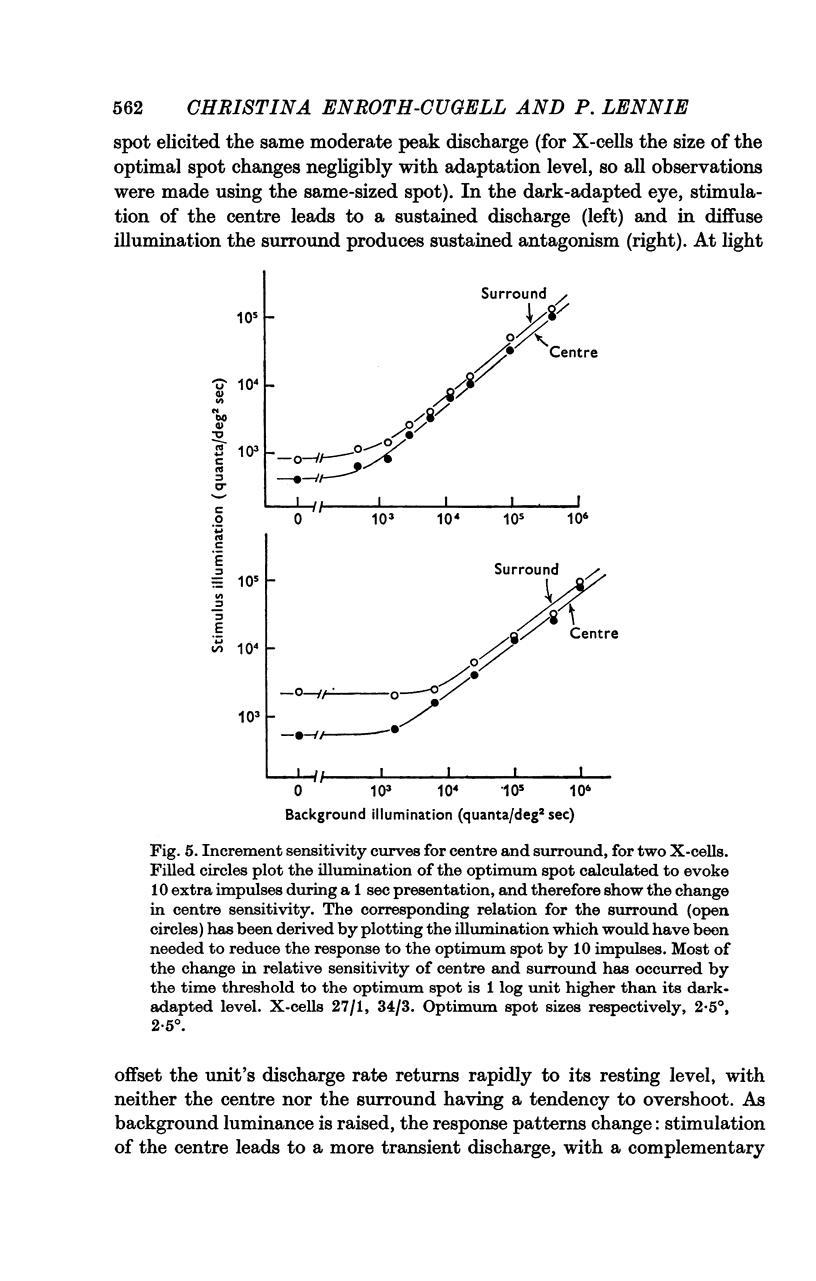
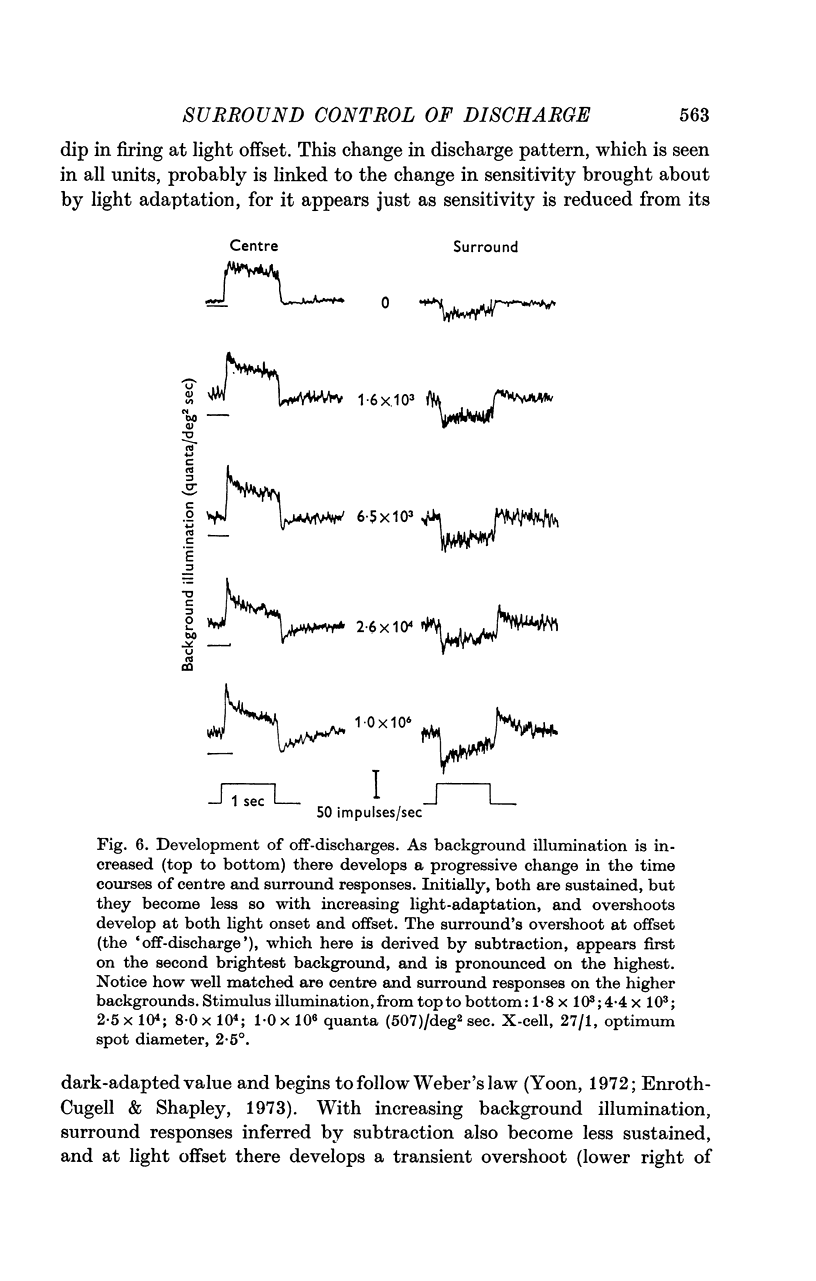
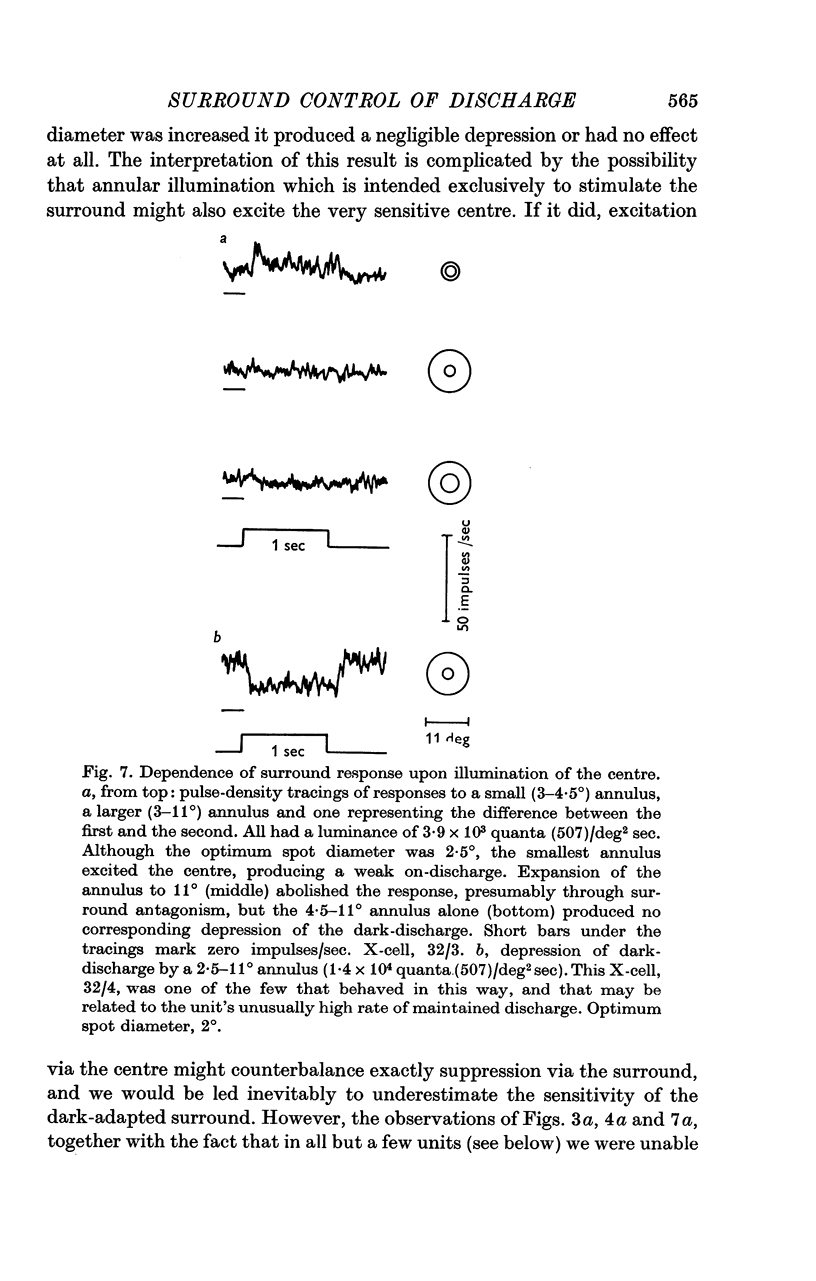
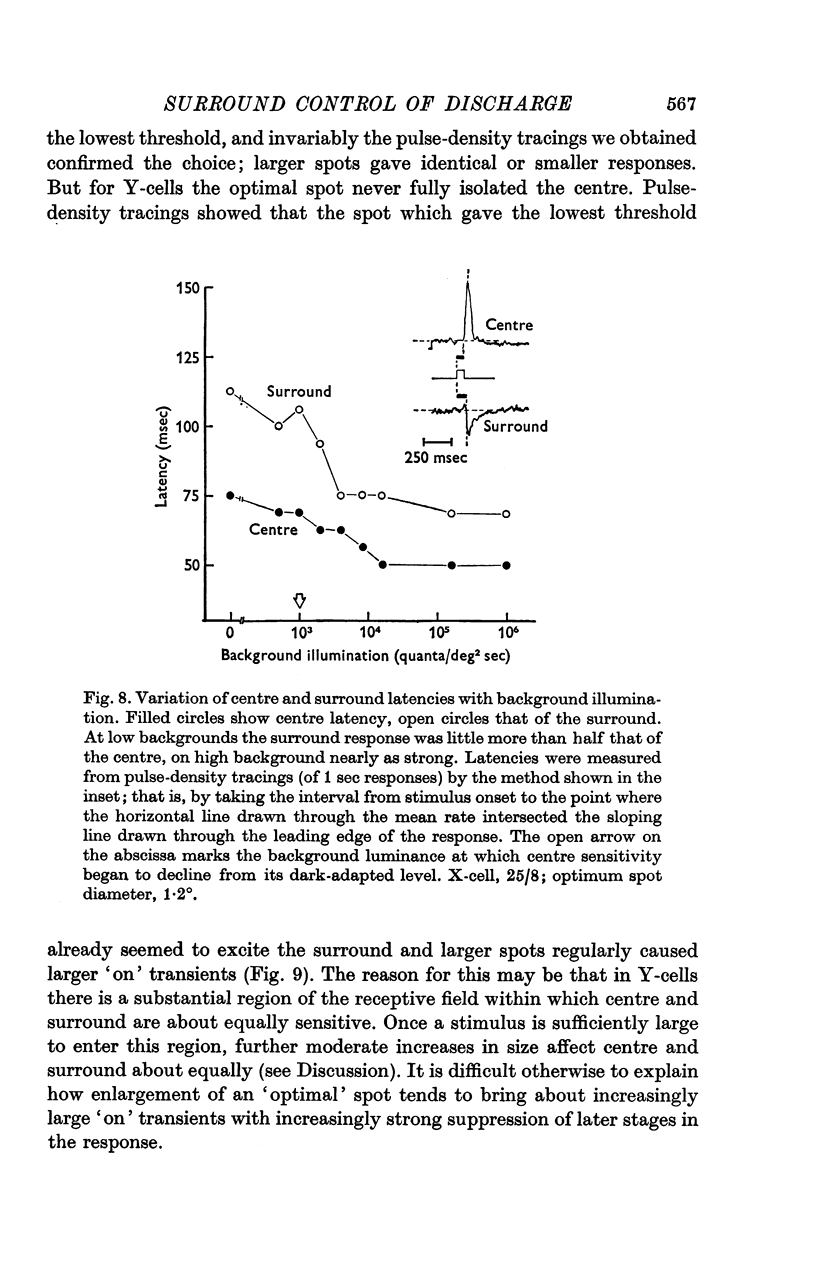
1. This paper describes the behaviour of the receptive field surround, and how surround signals combine with those from the centre to generate the discharge of the retinal ganglion cells of the cat. 2. A small test spot is flashed upon the middle of the receptive field of an on-centre X-cell, alone, or together with a concentric annulus of fixed luminance. The reduction in discharge brought about by the annulus is independent of spot luminance. From this it is inferred that centre and surround signals combine additively. 3. Knowing that the combination of signals is additive, the surround signal can be estimated by comparing the ganglion cell's response to diffuse illumination of its receptive field with that to an equiluminous spot which optimally stimulates the centre while encroaching minimally upon the periphery. 4. Application of this technique to X-cells shows that although the surround seems to have a threshold, it is at its most sensitive in the dark-adapted eye, and typically is only 0.3-0.5 log units less sensitive than the centre. 5. Centre and surround sensitivities are decreased from their dark-adapted levels by increasing background illumination, but the decline of surround sensitivity is initially less rapid than that of the centre. Thus with increasing light-adaptation the surround becomes relatively more sensitive. In the light-adapted eye centre and surround are about equally sensitive to diffuse illumination. 6. Although, in the dark-adapted eye, illumination of the receptive field periphery of an on-centre unit depresses firing, removal of that illumination produces no off-discharge. Off-discharges appear only when background illumination exceeds about 104 quanta (507)/deg 2 sec. This confirms Barlow & Levick (1969b). 7. In the dark-adapted eye surround latency is longer than that of the centre. With increasing background illumination the latency difference is reduced. 8. For X-cells, the rate of the maintained discharge depends to some extent on the balance of centre-surround antagonism. But this antagonism is not the major factor accounting for the relative constancy of mean rate at high background luminances, for the rate then can be almost independent of the size of a steady pot. 9. The mean rate of discharge of Y-cells seems to depend even less upon the balance of centre-surround antagonism. 10. Y-cell surrounds could not properly be isolated with the optimal spot-diffuse illumination technique, so detailed measurements of their behaviour were not made. However, the dark-adapted surround appear to be as sensitive as those of X-cells.
Full text
PDF








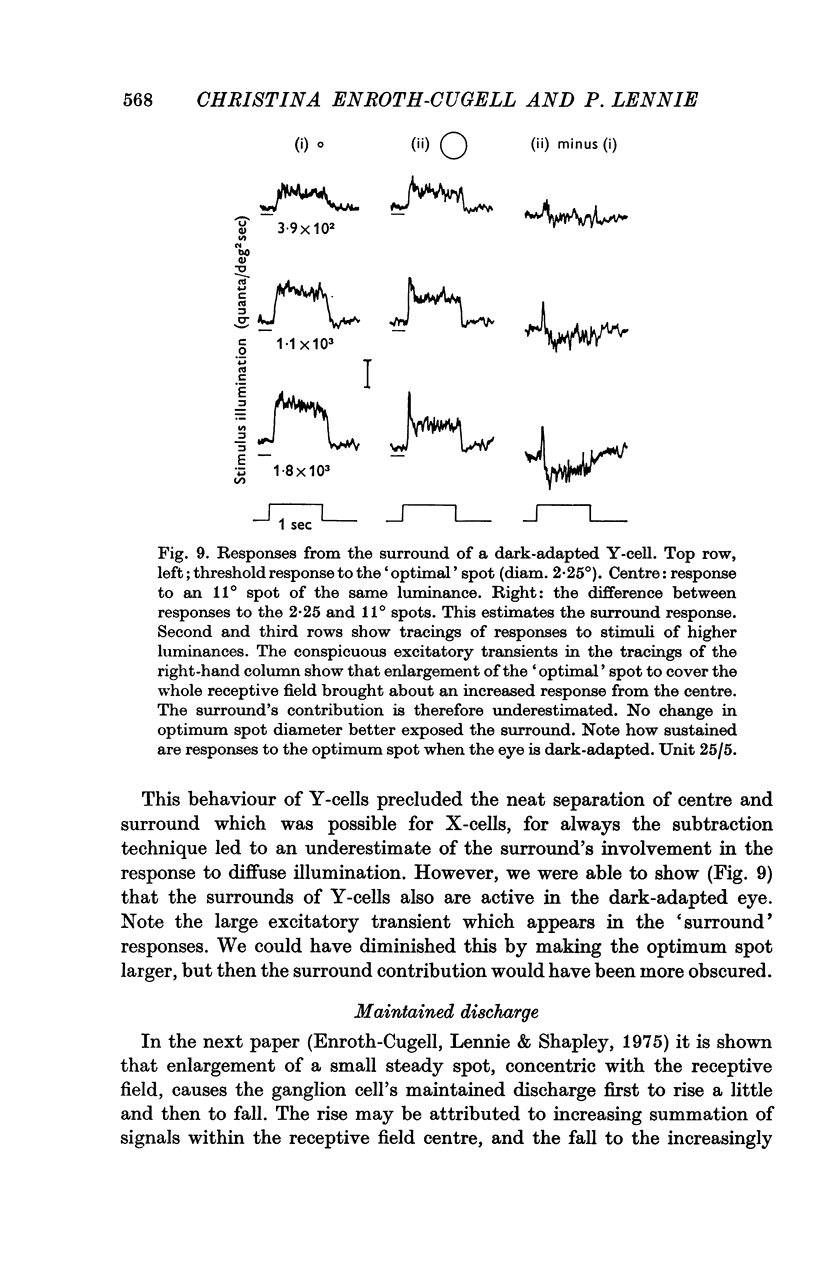

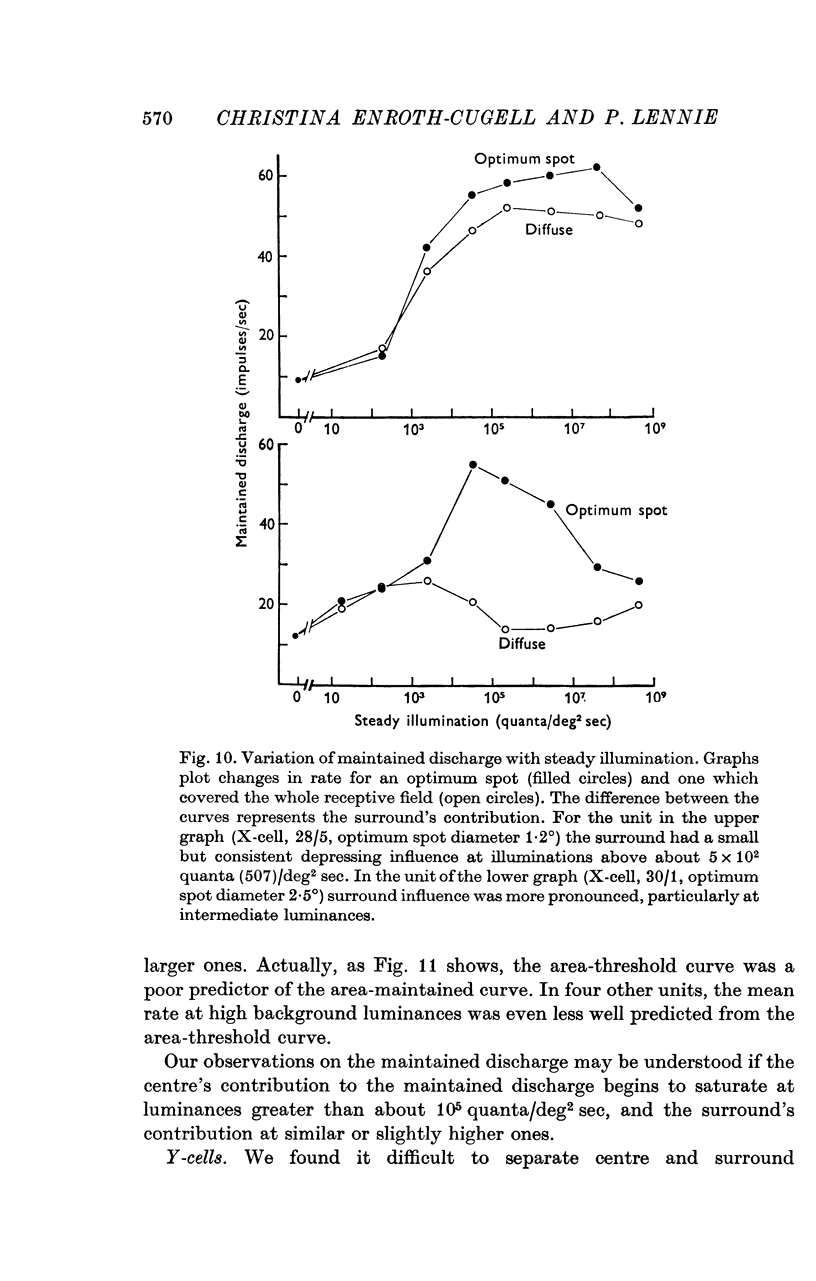
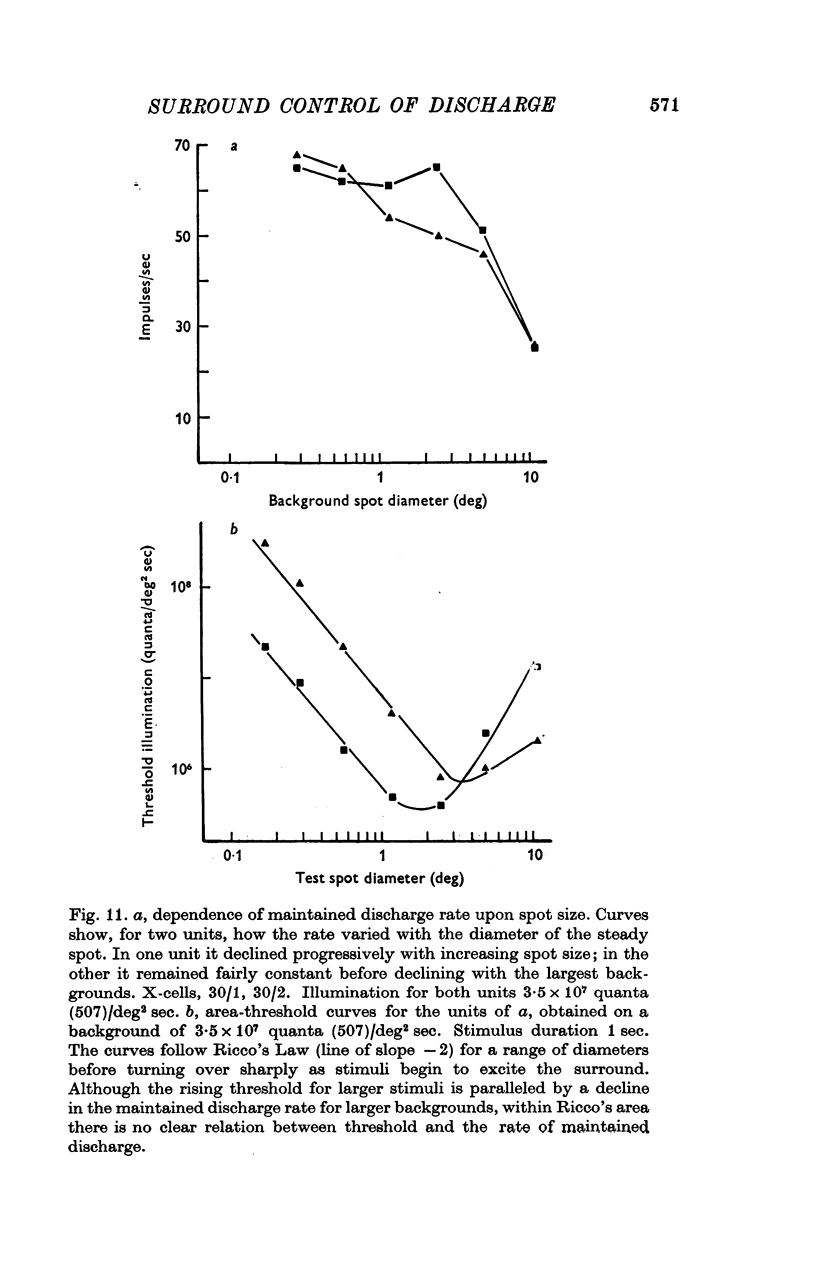
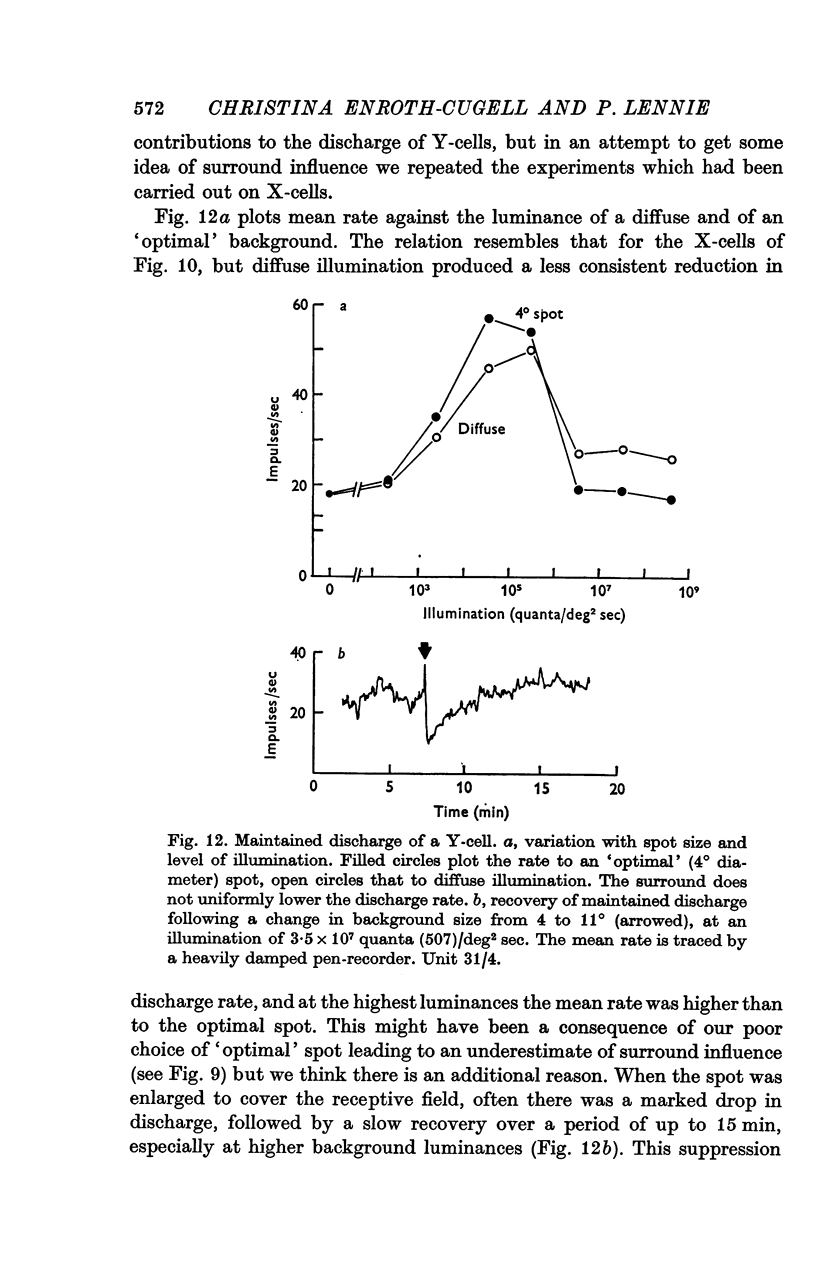






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R., Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res. 1971;Suppl 3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Lennie P., Shapley R. M. Surround contribution to light adaptation in cat retinal ganglion cells. J Physiol. 1975 Jun;247(3):579–588. doi: 10.1113/jphysiol.1975.sp010948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Properties of the surround response mechanism of cat retinal ganglion cells and centre-surround interaction. J Physiol. 1972 Jan;220(2):403–439. doi: 10.1113/jphysiol.1972.sp009714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Adaptation and dynamics of cat retinal ganglion cells. J Physiol. 1973 Sep;233(2):271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Krüger J., Fischer B. Dependence of surround effects on receptive field center illumination in cat retinal ganglion cells. Exp Brain Res. 1973 Oct 26;18(3):304–315. doi: 10.1007/BF00234600. [DOI] [PubMed] [Google Scholar]
- Maffei L., Cervetto L. Dynamic interactions in retinal receptive fields. Vision Res. 1968 Oct;8(10):1299–1303. doi: 10.1016/0042-6989(68)90051-5. [DOI] [PubMed] [Google Scholar]
- Maffei L., Cervetto L., Fiorentini A. Transfer characteristics of excitation and inhibition in cat retinal ganglion cells. J Neurophysiol. 1970 Mar;33(2):276–284. doi: 10.1152/jn.1970.33.2.276. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A., Cervetto L. Homeostasis in retinal receptive fields. J Neurophysiol. 1971 Jul;34(4):579–587. doi: 10.1152/jn.1971.34.4.579. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Maintained activity of cat retinal ganglion cells. J Neurophysiol. 1967 Sep;30(5):1043–1071. doi: 10.1152/jn.1967.30.5.1043. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Response of cat retinal ganglion cells to moving visual patterns. J Neurophysiol. 1965 Sep;28(5):819–832. doi: 10.1152/jn.1965.28.5.819. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Creutzfeldt O. D. Scotopic and mesopic light adaptation in the cat's retina. Pflugers Arch. 1969;313(2):168–185. doi: 10.1007/BF00586245. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in the human retina during scotopic vision. J Physiol. 1965 Dec;181(4):881–894. doi: 10.1113/jphysiol.1965.sp007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters R. W., Hickey T. L., Pollack J. G. Effect of variations of target location upon the peripheral responses of on-center retinal ganglion cells in the cat. Vision Res. 1973 Aug;13(8):1487–1498. doi: 10.1016/0042-6989(73)90008-4. [DOI] [PubMed] [Google Scholar]
- Yoon M. Influence of adaptation level on response pattern and sensitivity of ganglion cells in the cat's retina. J Physiol. 1972 Feb;221(1):93–104. doi: 10.1113/jphysiol.1972.sp009741. [DOI] [PMC free article] [PubMed] [Google Scholar]



