Abstract
Measurement of antibodies to human papillomavirus (HPV) is complicated by many factors. Although enzyme-linked immunosorbent assays (ELISAs) that use virus-like particles (VLPs) have proved useful, the assays have, in general, had moderate sensitivities and low signal-to-noise ratios. To enhance the performance of the assay, a systematic investigation was undertaken to examine key variables used in ELISAs for the detection of antibodies to VLPs of HPV. Incorporation of two vinyl polymers, polyvinyl alcohol (molecular weight, 50,000) (PVA-50) and polyvinylpyrrolidone (molecular weight, 360,000) (PVP-360), was found to increase the sensitivity as well as the specificity of the assay for the detection of antibodies to VLPs of HPV. In particular, the addition of PVA-50 to the blocking solution reduced the amount of nonspecific binding of antibodies to VLPs and the microplate surface, whereas the addition of PVP-360 increased the sensitivity of antibody detection. The new ELISA demonstrated increased sensitivity and specificity for the detection of cervical HPV type 16 infection compared to those of a prototype assay with coded clinical serum samples from women with known cervicovaginal HPV infection status. It is anticipated that the enhanced ELISA conditions will have wide application to a large number of clinical diagnostic assays.
Human papillomavirus (HPV) infection of the cervicovaginal region is the most common sexually transmitted disease in young adults and adolescents (6, 10). The association of HPV with preinvasive and invasive cervical cancer (26) makes genital HPV infection of particular medical importance. Initial attempts to characterize the humoral response to HPV virions were made as early as 1965 (1). However, research in this area has been impeded by an inability to grow large amounts of HPV virions in the laboratory and the poor sensitivities and specificities of antibody assays based on denatured recombinant HPV proteins reported to date.
A major innovation in HPV serology was the use of recombinant DNA technology to produce HPV capsid proteins that assembled into virus-like particles (VLPs) (18, 29, 37, 42). These methods produce conformationally intact HPV capsids in adequate amounts for use in enzyme-linked immunosorbent assays (ELISAs) (12, 28). Of major significance was the observation that antibodies to conformational epitopes on these synthetically produced HPV VLPs develop in response to type-specific infections and that such antibodies were neutralizing in cell culture systems and in an animal model (13-15, 34, 35, 40, 41).
Clinical applications of assays for HPV antibody detection, however, have been limited due to the lack of highly sensitive and reproducible assay systems. The majority of serum specimens have low titers of antibodies to VLPs of HPV, perhaps due to the immune isolation of the cervix and/or the fact that infection with HPV has no viremic phase. In addition, low titers of antibodies to conformational epitopes of HPV make background reactivity a major barrier to the development of a clinically useful assay for HPV antibody screening. The specific stereometry of HPV epitopes further complicates matters, in that no laboratory has yet succeeded in the development of a confirmatory antibody test (e.g., Western blotting) to verify the results of ELISA or to resolve the results for samples with borderline results.
In this report, we present data on a novel ELISA that uses a new combination of reagents to improve detection of antibodies to VLPs of HPV. This second-generation assay for HPV VLPs has improved sensitivity, specificity, and reproducibility for the detection of HPV antibodies, on the basis of analyses with samples from women known to have cervicovaginal HPV infections.
MATERIALS AND METHODS
HPV16 VLP preparation.
HPV type 16 (HPV16) L1 and L2 proteins were heterologously expressed in Trichoplusia ni cells (High Five cells; Invitrogen, Inc., Carlsbad, Calif.) infected with recombinant baculovirus encoding the complete open reading frame for L1 and L2 downstream of the polyhedrin promoter, as described previously (28). A high-titer virus stock (>108 PFU/ml) was created by amplification of a single recombinant plaque. High Five cells were grown to a density of 2 × 106 to 3 × 106 cells/ml (viability, >98%) and infected at a multiplicity of infection of 10 PFU/cell. VLPs were purified by a modification of the protocol described by McCarthy et al. (31). Briefly, cells were harvested at approximately 72 h postinfection and were pelleted by gentle centrifugation (1,500 to 2,000 rpm) in an IEC Centra-7R centrifuge (Damon Co., Needham, Mass.). The cell pellets were resuspended in homogenization buffer (20 mM NaH2PO4 and 150 mM NaCl [pH 7.4] containing 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin per ml, and 1 μg of pepstatin A per ml) and disrupted with 20 strokes of a Dounce homogenizer. The nuclear fraction was pelleted by differential centrifugation at 32,000 rpm for 90 min in a 50.2Ti fixed-angle rotor, and the supernatant was removed. The nuclear pellet was resuspended in 27% CsCl (wt/wt) in a 1× phosphate-buffered saline (PBS) solution (CsCl-PBS), sheared by multiple sequential passes through 18- and 19-gauge needles, and sonicated on ice for 30 s. The lysate was diluted in CsCl-PBS and centrifuged at 22,000 rpm for 90 min in an SW28 rotor at 4°C. The contaminating buoyant chromatin fraction layer was discarded, and the clarified lysate was then centrifuged to equilibrium for at least 16 h at 32,000 rpm in a VTi 50 vertical rotor at 4°C.
The fraction containing VLPs was visualized as an opalescent band and was collected from the top of the tube with a 15-cm blunt-end pipetting needle. The density of the VLP fraction was determined (i.e., density = 1.27 to 1.28 g/cm3), and the solution was dialyzed (Slide-A-Lyzer 10K dialysis cassette; Pierce, Inc., Rockford, Ill.) against 1× PBS for 30 min and layered on top of a two-component step gradient composed of 30 and 63% (wt/wt) sucrose in 1× PBS-0.5 M NaCl. Rate-zonal centrifugation was performed at 42,000 rpm in a VTi 50 rotor for 3 h at 4°C, and the VLP band was collected from the interface. The collected fractions were combined, CsCl-PBS solution was added to make the final CsCl concentration 27%, and the VLPs were rebanded by isopycnic centrifugation as described above. VLPs were stored in 27% CsCl-PBS at 4°C.
Analysis and quantitation of VLPs.
VLP structures were confirmed by transmission electron microscopy. The purities and the concentrations of the L1 and L2 proteins comprising the VLPs were determined by Coomassie brilliant blue (ICN Biomedicals, Inc., Irvine, Calif.) staining of the gel after sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 10% Tris-glycine precast gels (Novex, Inc., San Diego, Calif.). The only visible band was the 55-kDa L1 protein that reacted with the Camvir-1 monoclonal antibody (Pharmingen, Inc., San Diego, Calif.) by Western blotting analysis. The protein concentrations of the final dialyzed VLPs were determined by the Bio-Rad automated microplate assay with bovine serum albumin (BSA) as a standard (7).
ELISA material and reagents.
Polyvinyl alcohol (molecular weight, 50,000) (PVA-50), polyvinylpyrrolidone (molecular weight, 360,000) (PVP-360), 10× PBS concentrate, and Tween 20 were obtained from Sigma Chemical Co. (St. Louis, Mo.); nonfat milk was obtained from Bio-Rad Laboratories (Hercules, Calif.); BSA and goat anti-human immunoglobulin G (IgG) Fcγ fragment-specific horseradish peroxidase were obtained from Jackson Immunoresearch Laboratories, Inc. (West Grove, Pa.); PolySorp C96 microtiter plates and 0.45-μm-pore-size filter units were obtained from Nalgene Nunc International (Naperville, Ill.); and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) peroxidase solution and peroxidase stop solution from were obtained Kirkegaard & Perry Laboratories, Inc. (Gaithersburg, Md.). A Nunc-Immuno Wash12 appliance (Naglene Nunc International) was used to wash the wells of the microtiter plates. Optical densities (ODs) were determined with an MRX microtiter plate reader (Dynex Technologies, Chantilly, Va.).
Serum specimens.
Serum samples from studies that assessed cervical HPV infection were used (10, 23). For assay development, a seropositive control panel was constructed by making 10 pools of sera, with each pool consisting of serum samples from five different subjects; 8 pools consisted of sera from women in whom HPV16 DNA was detected in cervicovaginal cells and who had histologically confirmed cervical intraepithelial neoplasia or cancer (24), and 2 pools consisted of sera from college women in whom HPV16 DNA was detected in their cervicovaginal cells but who were cytologically negative (10).
Polymer ELISA conditions.
Stock solutions of PVA-50 and PVP-360 were prepared by dissolution of the polymers in boiling water and filtration through a 0.45-μm-pore-size filter unit. Blocking, serum, and conjugate buffers were prepared on the day of the assay. HPV VLPs were diluted to a concentration of 500 ng/ml in 10 ml of ice-cold 1× PBS buffer (pH 6.0) and were treated by gentle sonication for 15 s. A total of 100 μl of the purified HPV16 VLPs was added to precooled wells (i.e., 50 ng/well) (PolySorp C96 microtiter plates; Nalgene Nunc International), and the plates were incubated overnight at 4°C. Following incubation, the plates were rinsed six times with washing buffer (1× PBS, 0.05% Tween 20), and thereafter, 300 μl of blocking solution (1× PBS, 0.5% PVA-50) was added to each well. The plates were incubated for 3 h at room temperature. The plates were then rinsed six times with washing buffer and vigorously cleared by tapping them on paper towels. The samples were diluted 1:100 in blocking solution, and 100 μl was added to each of duplicate wells. The plates were incubated for 1 h at 37°C. The plates were then rinsed 10 times with washing buffer and vigorously cleared. A total of 100 μl of goat anti-human IgG Fcγ fragment conjugated with horseradish peroxidase diluted 1:10,000 in conjugate buffer (1× PBS, 0.025% Tween 20, 0.5% PVA-50, 0.8% PVP-360) was added to each well, and the plates were incubated for 30 min at 37°C. The plates were rinsed 10 times with washing buffer and vigorously cleared. A total of 100 μl of prewarmed (37°C) ABTS peroxidase solution was added to each well, and the plates were incubated for up to 40 min. The time interval between the additions of substrate to the different plates was about 3 to 5 min. After 30 min, the first plate was placed in an MRX ELISA reader programmed to read the OD at 405 nm until the weakly reactive control reached an OD of 0.3. The plate was then taken from the reader, 100 μl of peroxidase stop solution was added to each well, and the plate was shaken vigorously. The procedure was repeated with all of the plates. The final reading was made at 405 nm, with a 490-nm filter used as a reference.
The day-to-day reproducibility of the ELISA was monitored by inclusion of five specimens that, because of their characteristics, were particularly useful as quality control reagents for use on every plate. Specifically, it was noted that for a set of samples obtained from the National Cancer Institute and previously tested in multiple laboratories for antibodies to VLPs of HPV16 (38), some positive and negative samples gave very reproducible results, whereas other samples gave variable results. From this observation, we identified five controls that were run in each assay. These controls included (i) a stable, highly reactive positive control (i.e., OD > 1.3); (ii) a stable, weakly reactive positive control (i.e., OD ≈ 0.3to 0.38); (iii) a sensitive negative control (i.e., a sample sensitive to ELISA conditions); (iv) a stable negative control (i.e., OD < 0.1); and (5) a blank control (no serum). The mean ODs for four blank wells were subtracted from the OD for each antigen-containing well to create a background-adjusted OD.
The OD cutoff point for seropositivity for the HPV16 ELISA was determined by receiver operating characteristic (ROC) analyses (43). The log-transformed ODs for 51 subjects who were positive for HPV16 DNA were compared with those for 70 virginal women who were HPV16 DNA negative (10, 25). Since women with HPV16 infection may not necessarily have detectable antibodies to VLPs of HPV16, the cutoff point for seropositivity was chosen to maximize the specificity among virginal women. The cutoff point of an OD of ≥0.16 gave a sensitivity of 70.4% and a specificity of 100%.
Statistical methods.
The ODs of all samples were downloaded into the Revelation software package (Dynex Technologies) for initial analyses. On the basis of the results obtained with cervical HPV DNA and the mean ODs obtained by the ELISAs, a ROC analysis was performed (43). For plausible thresholds of seropositivity, we cross-tabulated the percent sensitivity of the detection of women with cervicovaginal HPV16 DNA infection identified by PCR (y axis) with 1 − specificity (x axis). The value of 1 − specificity represents the percentage of virginal women who would have been seropositive at a given OD cutoff point. A curve of (x, y) points indicating how the ELISA performed using different cutoff points for seropositivity is shown (see Fig. 1). The theoretical optimal cutoff point for each assay (x = 100% sensitivity, y = 0% nonspecificity) would find all women with cervical HPV16 infections but none of the virginal women to be positive. The statistical significance of the ELISA conditions was analyzed by an analysis of variance by the procedure provided with SAS software (version 8.0; SAS, Inc., Cary, N.C.).
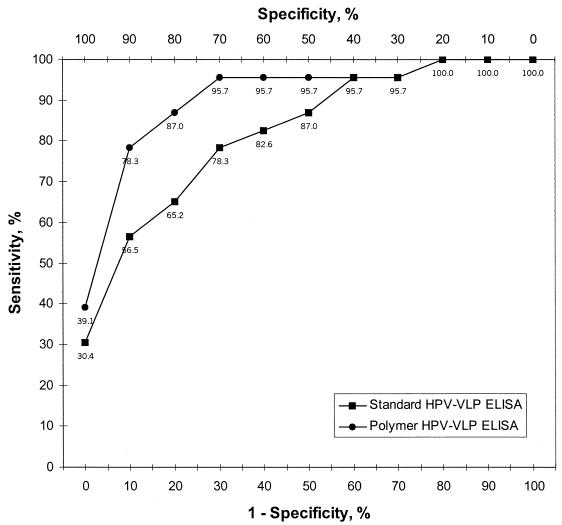
FIG. 1.
Performance of polymer and standard HPV VLP ELISAs. A ROC curve was plotted by using the mean ODs for 200 women with known cervical HPV16 DNA infection status (23). The y axis represents percent seropositivity among HPV16 DNA-positive women. The x axis shows 1 − specificity as percent seropositivity among virginal women. The results obtained by the polymer HPV VLP ELISA and the standard HPV VLP ELISA (28) are indicated. The numbers under the symbols indicate the sensitivities at different levels of specificity. Each laboratory prepared its own VLPs. The polymer ELISA is described in the Material and Methods section. For the standard ELISA, 200 ng of VLPs adsorbed at 37°C for 2 h was used for each well. After three washes with PBS, the wells were blocked with 0.5% milk and 0.1% fetal bovine serum in PBS. The sera were diluted 1:20 in 0.5% milk-1× PBS, and 50 μl was added to each well. After incubation for 2.5 h at room temperature, the wells were washed five times with PBS and an anti-human IgG F(ab′)2 horseradish peroxidase-conjugated secondary antibody diluted 1:10,000 in 0.5% milk-1× PBS was added prior to reaction with ABTS substrate.
RESULTS
To optimize the ELISA for VLPs of HPV (the HPV VLP ELISA), 20 samples presumed to be negative (i.e., from virginal women) and 10 samples from HPV16 DNA-positive women (10, 25) were repeatedly tested with HPV16 VLPs by using the conditions used for the first-generation HPV VLP ELISA (38). It was noted that approximately 30% of samples reacted with the wells when no antigen was present (data not shown). To improve the specificity, the concentrations of the VLP antigen and the primary antibody were sequentially reduced. After extensive testing, the optimal concentration of antigen was found to be 50 ng/well with a 1:100 dilution of test sample (data not shown). The effect of adsorption of VLPs at different temperatures is shown in Table 1. The use of a temperature of 4°C led to a significant improvement in the specificity (i.e., lower rates of positivity for the serum samples from HPV-negative women), lower ODs for HPV-negative women, and greater intra-assay reproducibility (i.e., decreased coefficient of variance [CV] values). Thus, these conditions were used in subsequent tests.
TABLE 1.
Influence of VLP adsorption temperature on ELISA resultsa
| HPV16 DNA status of the sample | No. of samples | 25°C
|
4°C
|
||||
|---|---|---|---|---|---|---|---|
| No. (%) of samples with OD of 0.16 | Mean OD | CV (%) | No. (%) of samples with OD of 0.16 | Mean OD | CV (%) | ||
| Positiveb | 11 | 9 (82) | 0.581 | 25.6 | 9 (82) | 0.703 | 2.8 |
| Negativec | 29 | 11 (38) | 0.188 | 45.1 | 2 (7) | 0.065 | 8.7 |
Serum samples were from women infected with known cervical HPV DNA types and with known disease status (24). OD and CV values between VLP adsorption at 25 and 4°C were compared by the paired t test. The difference in the proportion of seropositive samples between the two conditions among the HPV DNA-negative samples was evaluated by the McNemar test.\
Among the HPV16 DNA-positive samples, comparison of VLP adsorption at 25 and 4°C for differences in ODs, P = 0.206; comparison for differences in CVs, P = 0.014.\
Among the HPV16 DNA-negative samples, comparison of VLP adsorption at 25 and 4°C for differences in ODs, P ≤ 0.001; comparison for differences in CVs, P < 0.001; comparison for differences in the proportions of samples with false-positive results, P = 0.004.
Panels of serum samples were used to compare the sensitivities, specificities, and reproducibilities of different ELISA blocking reagents. Since seroreactivity to the plastic well was noted for a large number of samples initially tested, we reasoned that use of a plastic polymer might block this undesired immunoreactivity. After comparison of PVAs with different molecular weights, PVA-50 at a concentration of 0.5% in 1× PBS showed maximum and reproducible blocking activities and an enhanced level of sensitivity for the detection of positive samples (data not shown). The blocking effectiveness of 0.5% PVA-50 was then compared with those of other commonly used blocking agents (Tables 2 and 3). A test panel consisting of 10 pools of samples from HPV16 DNA-positive women (see Materials and Methods) and 10 samples from HPV-negative virginal women (10, 25) was evaluated. The maximum ODs for the samples from women with HPV16 infection and cervical neoplasia (pools 1 to 8) were observed when 0.5% PVA-50 was combined with Tween 20 (Table 2). In contrast, Tween 20 added to milk and BSA caused decreases in the ODs. Among the samples in pools 9 and 10, from college women with HPV16 DNA-positive, Pap smear-negative results (10, 25), only those in pool 9 showed a weak reaction (i.e., ODs of 0.16 to 0.20) with each blocking agent except milk and milk with Tween 20. To evaluate the specificities of the blocking agents, the 10 samples from virginal women were tested (Table 3). In contrast to the results for sensitivity, milk alone demonstrated optimal blocking for the 10 control samples. However, PVA-50 and PVA-50 with Tween 20 also resulted in very low ODs.
TABLE 2.
Influence of ELISA blocking solutions on samples from women positive for HPV16 DNAa
| Reagent | Mean OD
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pool 1 | Pool 2 | Pool 3 | Pool 4 | Pool 5 | Pool 6 | Pool 7 | Pool 8 | Pool 9 | Pool 10 | |
| Milk | 0.133 | 1.272 | 0.261 | 1.968 | 1.143 | 1.565 | 1.270 | 0.715 | 0.049 | 0.000 |
| Milk + Tween 20 | 0.140 | 1.122 | 0.239 | 1.547 | 0.900 | 1.263 | 1.006 | 0.533 | 0.050 | 0.010 |
| BSA | 0.604 | 1.645 | 0.581 | 2.038 | 1.488 | 1.861 | 1.589 | 1.110 | 0.198 | 0.091 |
| BSA + Tween 20 | 0.468 | 1.271 | 0.469 | 1.616 | 1.244 | 1.569 | 1.274 | 0.804 | 0.143 | 0.067 |
| Tween | 0.505 | 1.334 | 0.464 | 1.868 | 1.334 | 1.689 | 1.495 | 0.930 | 0.157 | 0.096 |
| PVA-50 | 0.607 | 1.586 | 0.600 | 1.946 | 1.205 | 2.010 | 1.115 | 0.668 | 0.072 | 0.006 |
| PVA-50 + Tween | 0.653 | 1.745 | 0.633 | 2.237 | 1.605 | 2.010 | 1.703 | 1.156 | 0.160 | 0.036 |
The compositions of the serum pools are described in Materials and Methods. A two-way analysis of variance was used to assess the statistical significance between the average ODs obtained with different blocking solutions (P < 0.0001). Milk and BSA were added to the blocking solutions at final concentrations of 5% each, Tween 20 was added at a final concentration of 0.05%, and PVA-50 was added at a final concentration of 0.5%. Values represent the mean OD for each pooled sample run twice.
TABLE 3.
Influence of ELISA blocking solutions on control samplesa
| Reagent | Mean OD
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control 1 | Control 2 | Control 3 | Control 4 | Control 5 | Control 6 | Control 7 | Control 8 | Control 9 | Control 10 | |
| Milk | 0.000 | 0.000 | 0.006 | 0.000 | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Milk + Tween 20 | 0.024 | 0.006 | 0.018 | 0.003 | 0.008 | 0.001 | 0.004 | 0.004 | 0.012 | 0.070 |
| BSA | 0.055 | 0.012 | 0.026 | 0.003 | 0.032 | 0.010 | 0.012 | 0.006 | 0.061 | 0.018 |
| BSA + Tween 20 | 0.088 | 0.019 | 0.088 | 0.124 | 0.064 | 0.166 | 0.054 | 0.038 | 0.12 | 0.021 |
| Tween | 0.095 | 0.070 | 0.061 | 0.030 | 0.068 | 0.028 | 0.061 | 0.069 | 0.065 | 0.057 |
| PVA-50 | 0.032 | 0.018 | 0.026 | 0.012 | 0.032 | 0.000 | 0.000 | 0.000 | 0.011 | 0.028 |
| PVA-50 + Tween 20 | 0.042 | 0.007 | 0.014 | 0.003 | 0.012 | 0.007 | 0.008 | 0.008 | 0.027 | 0.020 |
Control samples were obtained from HPV DNA-negative virginal women (25). A two-way analysis of variance was used to assess the statistical significance between the average ODs obtained with different blocking solutions (P < 0.0001). Milk and BSA were added to the blocking solutions at final concentrations of 5% each, Tween 20 was added at a final concentration of 0.05%, and PVA-50 was added at a final concentration of 0.5%. Values represent the mean OD for each sample run twice.
Previous studies had demonstrated that another hydrophilic polymer, PVP, improved adsorption of secondary antibody in Western blotting assays (5, 20). To improve the signal of the HPV VLP ELISA, we evaluated different concentrations of high-molecular-weight PVP, PVP-360, added to the conjugate buffer. An optimal concentration of 0.8% PVP-360 was shown to enhance the OD signal (data not shown). We then evaluated the effect of PVP-360 in combination with the other blocking agents (Table 4). The addition of PVP-360 resulted in improved ODs when it was combined with milk and PVA-50 (Table 4). Compared with milk, the combination of PVA-50, PVP-360, and Tween 20 maximized the ODs of the positive samples. More importantly, previously negative pools 9 and 10 consisting of samples from HPV16 DNA-positive, Pap smear-negative college women gave ODs in the positive range (i.e., ODs ≥ 0.16). There was no significant difference in the ODs of the 10 control samples with the addition of PVP-360 (Table 5).
TABLE 4.
Influence of PVP-360 on assays with samples from women positive for cervical HPV16 DNAa
| Reagent | Mean OD
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pool 1 | Pool 2 | Pool 3 | Pool 4 | Pool 5 | Pool 6 | Pool 7 | Pool 8 | Pool 9 | Pool 10 | |
| Milk | 0.134 | 0.974 | 0.224 | 1.562 | 0.938 | 1.17 | 0.953 | 0.522 | 0.042 | 0.006 |
| Milk + PVP-360 | 0.232 | 1.090 | 0.376 | 1.538 | 1.131 | 1.315 | 1.153 | 0.764 | 0.087 | 0.019 |
| PVA-50 + Tween 20 | 0.576 | 1.188 | 0.449 | 1.688 | 1.222 | 1.489 | 1.202 | 0.827 | 0.144 | 0.07 |
| PVA-50 + Tween 20 + PVP-360 | 0.785 | 1.281 | 0.676 | 1.564 | 1.264 | 1.444 | 1.267 | 1.010 | 0.275 | 0.165 |
The compositions of the serum pools are described in Materials and Methods. A two-way analysis of variance was used to assess the statistical significance between the average ODs obtained with different blocking solutions (P < 0.0001). Milk, PVP-360, Tween 20, and PVA-50 were added to the blocking solution at final concentrations of 5, 0.8, 0.05, and 0.5%, respectively. Values represent the mean OD for each serum pool run twice.
TABLE 5.
Influence of PVP-360 on assays with control samplesa
| Reagent | Mean OD
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control 1 | Control 2 | Control 3 | Control 4 | Control 5 | Control 6 | Control 7 | Control 8 | Control 9 | Control 10 | |
| Milk | 0.008 | 0.000 | 0.018 | 0.004 | 0.002 | 0.004 | 0.001 | 0.002 | 0.002 | 0.000 |
| Milk + PVP-360 | 0.017 | 0.010 | 0.045 | 0.003 | 0.015 | 0.001 | 0.007 | 0.003 | 0.014 | 0.011 |
| PVA-50 + Tween 20 | 0.054 | 0.030 | 0.034 | 0.016 | 0.037 | 0.017 | 0.027 | 0.043 | 0.046 | 0.028 |
| PVA-50 + Tween 20 + PVP-360 | 0.074 | 0.037 | 0.043 | 0.023 | 0.053 | 0.032 | 0.043 | 0.057 | 0.059 | 0.063 |
Control samples were obtained from HPV16 DNA-negative virginal women (serum set 1). PVP-360 was added to the conjugate buffers at a final concentration of 0.8%, Tween 20 was added at a final concentration of 0.05%, milk was added at a final concentration of 5%, and PVA-50 was added at a final concentration of 0.5%. Values represent the mean OD of each sample run twice.
On the basis of the results of these experiments, an improved polymer ELISA protocol was established with PVA-50 and PVP-360. To directly compare this assay to a first-generation (standard) HPV VLP ELISA, a set of 200 coded serum samples from women with known cervical HPV16 DNA infection status (23) was tested. Each laboratory prepared its own VLPs for use in the ELISA. Both laboratories were blinded to the clinical and HPV results of the samples. The performance of the two assays was compared by a ROC analysis. As shown in Fig. 1, detection of antibodies to VLPs of HPV was strongly correlated with cervicovaginal HPV DNA status in both assays. The sensitivity of the polymer HPV VLP ELISA was higher than that of the standard HPV VLP ELISA from 0 to 50% nonspecificity. For instance, with an OD cutoff point that resulted in 10% of virginal women scoring positive, 78.3 and 56.5% of HPV16 DNA-positive women scored seropositive by the polymer ELISA and the standard ELISA, respectively. Thus, improvements in both sensitivity and specificity were observed with the polymer ELISA.
DISCUSSION
Serological assays for the detection of exposure to and infection with viral pathogens is a classic mode of documentation of viral infection. We performed a study to optimize the conditions of a type-specific serological test for infection with HPV. Based on the hypothesis that nonspecific reactivity with the microplate well involves a reaction with a plastic polymer, we systematically evaluated polymer-blocking agents and identified a novel combination of blocking and enhancing agents, PVA-50 and PVP-360, that increased the reactivities of weakly responding human sera in a VLP-based ELISA. Together with the use of more highly purified VLPs and adsorption to the plates at a low temperature, the use of these agents improved the sensitivity and specificity of the VLP ELISA for the detection of concurrent cervical HPV16 infection.
To date, there is no standardized test for the detection of antibodies to HPV VLPs, in part because of the inherent difficulties with VLP production, low antibody titers, and nonspecific reactivity. Three classes of tests for the detection of type-specific genital HPV antibodies have been described previously. The most commonly reported assay is an ELISA based on the direct adsorption of VLPs to the plastic well (21, 22, 28, 36). An alternative approach is the use of a VLP-specific antibody to first capture VLPs and then to detect subject antibodies (11, 30). Recently, a competitive radioimmunoassay has been applied to the detection of HPV VLP-specific antibodies (9). In addition to different types of assays, the conditions between laboratories using similar assay formats also vary considerably. For instance, the quantities of VLPs used for each test vary between 50 and 1,000 ng, serum dilutions vary between 1:4 and 1:100, blocking solutions vary, and many laboratories subtract the reactivity of serum to wells lacking intact VLPs. Nevertheless, interlaboratory agreement was reasonable when ODs near the cutoff values for seropositivity were omitted, suggesting that detection of strongly seropositive samples is adequate (38). Development of an improved standardized test would improve interstudy comparisons.
In the case of HPV infection, the majority of serum specimens display ODs in the low to middle range. This makes selection of a blocking agent of critical importance for HPV antibody screening. Blocking agents that have been successfully used in ELISAs can be classified into three categories: proteins, polymers, and surfactants (19). The polymer group has a number of advantages over protein and surfactant blocking agents: (i) polymers represent a homogeneous solution and do not contain potentially interfering proteins (e.g., the presence of immunoglobulins in BSA preparations, cross-reacting proteins in milk, or endogenous peroxidase activity in a variety of different protein blockers) (16, 17, 19, 39); (ii) polymer solutions are stable over long periods of time and do not require preservatives, which can influence the immunoreaction; (iii) polymers are inexpensive and readily available; and (iv) polymers present a surface similar to that found on the plastic microtiter plate wells and can thus block nonspecific reactivity with plastic.
In recent years, polymers have been used as a new class of blocking and enhancing agents in immunoassays. Polymers such as polyethylene glycol (27), PVA (33; K. May, M. E. Prion, and I. Richards, 9 November 1988, U.K. Patent Application G.B. 2204398), polyvinyl alcohol-glutaraldehyde (2-4), and PVP (5, 20) have been shown to have significant blocking abilities and do not interfere with specific binding. In addition, PVA has been shown to stabilize the immunoreactive activities of proteins (8, 32) and improve antibody adsorption. Rodda and Yamazaki (33) demonstrated a significant improvement in the specificity of the secondary antibody reaction using PVA with rabbit IgG compared with the specificity obtained with traditional blocking agents (e.g., BSA and milk). Previous studies have demonstrated that another hydrophilic polymer, PVP, could be used as a quenching agent to improve the adsorption of the secondary antibody in a Western blotting assay (5, 20).
In conclusion, this is the first report of an improved HPV VLP ELISA based on the use of synthetic polymers. These reagents should have wide use in other immunological assays. The ELISA protocol described in this report can be used in conjunction with HPV DNA detection techniques for accurate clinical diagnosis, epidemiological studies, and evaluations of responsiveness to VLP-based vaccines. However, the eventual utility of this assay will require confirmation in additional studies with samples from diverse populations.
Acknowledgments
This work was supported in part by grants from NIH to R.D.B.
REFERENCES
- 1.Almeida, J. D., and A. P. Goffe. 1965. Antibody to wart virus in human sera demonstrated by electron microscopy and precipitin tests. Lancet ii:1205-1207. [DOI] [PubMed]
- 2.Araujo, A. M., G. H. Barbosa, J. R. Diniz, E. Malagueno, W. M. Azevedo, and L. B. Corvalho, Jr. 1997. Polyvinyl alcohol-glutaraldehyde as solid-phase in ELISA for shistosomiasis. Rev. Inst. Med. Trop. Sao Paulo 39:155-158. [DOI] [PubMed] [Google Scholar]
- 3.Araujo, A. M., A. T. Petribu, G. H. Barbosa, J. R. Diniz, A. M. Almeida, W. M. Azevedo, E. Malagueno, and L. B. Corvalho, Jr. 1996. The use of polyvinyl alcohol glutaraldehyde as solid-phase in ELISA for plague. Mem. Inst. Oswaldo Cruz 91:195-198. [DOI] [PubMed] [Google Scholar]
- 4.Araujo, A. M., A. T. Petribu, G. H. Barbosa, J. R. Diniz, A. M. Almeida, and L. B. Corvalho, Jr. 1998. Rapid ELISA for plague. Mem. Inst. Oswaldo Cruz 93:111-112. [DOI] [PubMed] [Google Scholar]
- 5.Bartles, J. R., and A. L. Hubbard. 1984. 125I-wheat germ agglutinin blotting: increased sensitivity with polyvinylpyrrolidone quenching and periodate oxidation/reductive phenylamination. Anal. Biochem. 140:284-292. [DOI] [PubMed] [Google Scholar]
- 6.Bauer, H. M., A. Hildesheim, M. H. Schiffman, A. G. Glass, B. B. Rush, D. R. Scott, D. M. Cadell, R. J. Kurman, and M. M. Manos. 1993. Determinants of genital human papillomavirus infection in low-risk women in Portland, Oregon. Sex. Transm. Dis. 20:274-278. [DOI] [PubMed] [Google Scholar]
- 7.Baumgarten, H. 1985. A simple microplate assay for the determination of cellular protein. J. Immunol. Methods 82:25-37. [DOI] [PubMed] [Google Scholar]
- 8.Boyd, S., and H. Yamazaki. 1995. Stability of polypeptide immunoreactants and polyvinyl alcohol as a blocking agent on polyester cloth during dry storage. Immunol. Investig. 24:795-803. [DOI] [PubMed] [Google Scholar]
- 9.Brown, D. R., J. T. Bryan, J. M. Schroeder, T. S. Robinson, K. H. Fife, C. M. Wheeler, E. Barr, P. R. Smith, L. Chiacchierini, A. DiCello, and K. U. Jansen. 2001. Neutralization of human papillomavirus type 11 (HPV-11) by serum from women vaccinated with yeast-derived HPV-11 L1 virus-like particles: correlation with competitive radioimmunoassay titer. J. Infect. Dis. 184:1183-1186. [DOI] [PubMed] [Google Scholar]
- 10.Burk, R. D., G. Y. F. Ho, L. Beardsley, M. Lempa, M. Peters, and R. Bierman. 1996. Sexual behavior and partner selection are the predominant risk factors for genital HPV infection in young women. J. Infect. Dis. 174:679-689. [DOI] [PubMed] [Google Scholar]
- 11.Carter, J. J., L. A. Koutsky, G. C. Wipf, N. D. Christensen, S.-K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 1996. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J. Infect. Dis. 174:927-936. [DOI] [PubMed] [Google Scholar]
- 12.Carter, J. J., G. C. Wipf, M. E. Hagensee, B. McKnight, L. A. Habel, S. K. Lee, J. Kuypers, N. Kiviat, J. R. Daling, L. A. Koutsky, et al. 1995. Use of human papillomavirus type 6 capsids to detect antibodies in people with genital warts. J. Infect. Dis. 172:11-18. [DOI] [PubMed] [Google Scholar]
- 13.Christensen, N. D., R. Hopfl, S. L. DiAngelo, N. M. Cladel, S. D. Patrick, P. A. Welsh, L. R. Budgeon, C. A. Reed, and J. W. Kreider. 1994. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J. Gen. Virol. 75:2271-2276. [DOI] [PubMed] [Google Scholar]
- 14.Christensen, N. D., J. W. Kreider, K. V. Shah, and R. F. Rando. 1992. Detection of human serum antibodies that neutralize infectious human papillomavirus type 11 virions. J. Gen Virol. 73:1261-1267. [DOI] [PubMed] [Google Scholar]
- 15.Giroglou, T., M. Sapp, C. Lane, C. Fligge, N. D. Christensen, R. E. Streeck, and R. C. Rose. 2001. Immunological analyses of human papillomavirus capsids. Vaccine 19:1783-1793. [DOI] [PubMed] [Google Scholar]
- 16.Graig, W. Y., S. E. Poulin, M. F. Collins, T. B. Ledue, and R. F. Ritchie. 1993. Background staining in immunoblot assay. Reduction of signal caused by cross-reactivity with blocking agent. J. Immunol. Methods 158:67-76. [DOI] [PubMed] [Google Scholar]
- 17.Graig, W. Y., S. E. Poulin, C. P. Nelson, and R. F. Ritchie. 1994. ELISA of IgG antibody to oxidized low-density lipoprotein: effects of blocking buffer and method of data expression. Clin. Chem. 40:882-888. [PubMed] [Google Scholar]
- 18.Hagensee, M. E., N. Yaegashi, and D. A. Galloway. 1993. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 67:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey, M., R. Kremer, and L. Vickers. 2000. Guide to diagnostic rapid test device components, 2nd ed. Shleicher & Schuell, Keene, N.H.
- 20.Haycock, J. W. 1993. Polyvinylpirrolidone as a blocking agent in immunochemical studies. Anal. Biochem. 208:397-399. [DOI] [PubMed] [Google Scholar]
- 21.Heim, K., N. D. Christensen, R. Hoepfl, B. Wartusch, G. Pinzger, A. Zeimet, P. Baumgartner, J. W. Kreider, and O. Dapunt. 1995. Serum IgG, IgM, and IgA reactivity to human papillomavirus types 11 and 6 virus-like particles in different gynecologic patient groups. J. Infect. Dis. 172:395-402. [DOI] [PubMed] [Google Scholar]
- 22.Heino, P., C. Eklund, V. Fredriksson-Shanazarian, S. Goldman, J. T. Schiller, and J. Dillner. 1995. Association of serum immunoglobulin G antibodies against human papillomavirus type 16 capsids with anal epidermoid carcinoma. J. Natl. Cancer Inst. 87:437-440. [DOI] [PubMed] [Google Scholar]
- 23.Herrero, R., A. Hildesheim, B. Concepcion, M. E. Sherman, M. Hutchinson, J. Morales, I. Balmaceda, M. D. Greenberg, M. Alfaro, R. D. Burk, S. Wacholder, and M. Schiffman. 1999. Population-based study of human papillomavirus infection and all grades of cervical neoplasia in rural Costa Rica. J. Natl. Cancer Inst. 921:464-474. [DOI] [PubMed] [Google Scholar]
- 24.Ho, G. Y., P. R. Palan, J. Basu, S. L. Romney, A. S. Kadish, M. Mikhail, S. Wassertheil-Smoller, C. Runowicz, and R. D. Burk. 1998. Viral characteristics of human papillomavirus infection and antioxidant levels as risk factors for cervical dysplasia. Int. J. Cancer 78:594-599. [DOI] [PubMed] [Google Scholar]
- 25.Ho, G. Y. F., R. Bierman, L. Beardsley, C. J. Chang, and R. D. Burk. 1998. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 338:423-428. [DOI] [PubMed] [Google Scholar]
- 26.International Agency for Research on Cancer. 1995. IARC monographs on the evaluation of carcinogenic risks to humans, vol. 64. International Agency for Research on Cancer, Lyon, France.
- 27.Kilpatrick, D. C. 1998. Factors affecting cardiolipin antibody assays: modification with polyethylene glycol compound. Br. J. Hematol. 137:27-35. [DOI] [PubMed] [Google Scholar]
- 28.Kirnbauer, R., N. L. Hubbert, C. M. Wheeler, T. M. Becker, D. R. Lowy, and J. T. Schiller. 1994. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J. Natl. Cancer Inst. 86:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Durst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Cann, P., A. Touze, N. Enogat, D. Leboulleux, C. Mougin, M. C. Legrand, C. Calvet, J. M. Afoutou, and P. Coursaget. 1995. Detection of antibodies against human papillomavirus (HPV) type 16 virions by enzyme-linked immunosorbent assay using recombinant HPV 16 L1 capsids produced by recombinant baculovirus. J. Clin. Microbiol. 33:1380-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy, M. P., W. I. White, F. Palmer-Hill, S. Koenig, and J. A. Suzich. 1998. Quantitative disassembly and reassembly of human papillomavirus type 11 viruslike particles in vitro. J. Virol. 72:32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghuvanshi, R. S., S. Goyal, O. Singh, and A. K. Panda. 1998. Stabilization of dichloromethane-induced protein denaturation during microencapsulation. Pharm. Dev. Technol. 3:269-276. [DOI] [PubMed] [Google Scholar]
- 33.Rodda, D. J., and H. Yamazaki. 1994. Poly(vinyl alcohol) as a blocking agent in enzyme immunoassays. Immunol. Investig. 23:421-428. [DOI] [PubMed] [Google Scholar]
- 34.Roden, R. B., H. L. Greenstone, R. Kirnbauer, F. P. Booy, J. Jessie, D. R. Lowy, and J. T. Schiller. 1996. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J. Virol. 70:5875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roden, R. B., N. L. Hubbert, R. Kirnbauer, N. D. Christensen, D. R. Lowy, and J. T. Schiller. 1996. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J. Virol. 70:3298-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose, R. C., W. Bonnez, C. Da Rin, D. J. McCance, and R. C. Reichman. 1994. Serological differentiation of human papillomavirus types 11, 16 and 18 using recombinant virus-like particles. J. Gen. Virol. 75:2445-2449. [DOI] [PubMed] [Google Scholar]
- 37.Rose, R. C., W. Bonnez, R. C. Reichman, and R. L. Garcea. 1993. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J. Virol. 67:1936-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strickler, H. D., A. Hildesheim, R. P. Viscidi, K. V. Shah, B. Goebel, J. Drummond, D. Waters, Y. Sun, N. L. Hubbert, S. Wacholder, L. A. Brinton, C. L. Han, P. C. Nasca, R. McClimens, K. Turk, V. Devairakkam, S. Leitman, C. Martin, and J. T. Schiller. 1997. Interlaboratory agreement among results of human papillomavirus type 16 enzyme-linked immunosorbent assays. J. Clin. Microbiol. 35:1751-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogt, R. F., D. L. Phillips, L. O. Henderson, W. Whitfield, and F. W. Spierto. 1987. Quantitative differences among various proteins as blocking agents for ELISA microtiter plates. J. Immunol. Methods 101:43-50. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Z. H., L. Kjellberg, H. Abdalla, F. Wiklund, C. Eklund, P. Knekt, M. Lehtinen, I. Kallings, P. Lenner, G. Hallmans, C. G. Mahlck, G. Wadell, J. Schiller, and J. Dillner. 2000. Type specificity and significance of different isotypes of serum antibodies to human papillomavirus capsids. J. Infect. Dis. 181:456-462. [DOI] [PubMed] [Google Scholar]
- 41.Wideroff, L., M. Schiffman, P. Haderer, A. Armstrong, C. E. Greer, M. M. Manos, R. D. Burk, D. R. Scott, M. E. Sherman, J. T. Schiller, R. N. Hoover, R. E. Tarone, and R. Kirnbauer. 1999. Seroreactivity to human papillomavirus types 16, 18, 31, and 45 virus- like particles in a case-control study of cervical squamous intraepithelial lesions. J. Infect. Dis. 180:1424-1428. [DOI] [PubMed] [Google Scholar]
- 42.Zhou, J., X. Y. Sun, D. J. Stenzel, and I. H. Frazer. 1991. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 185:251-257. [DOI] [PubMed] [Google Scholar]
- 43.Zweig, M. H., and G. Campbell. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561-577. [PubMed] [Google Scholar]