Abstract
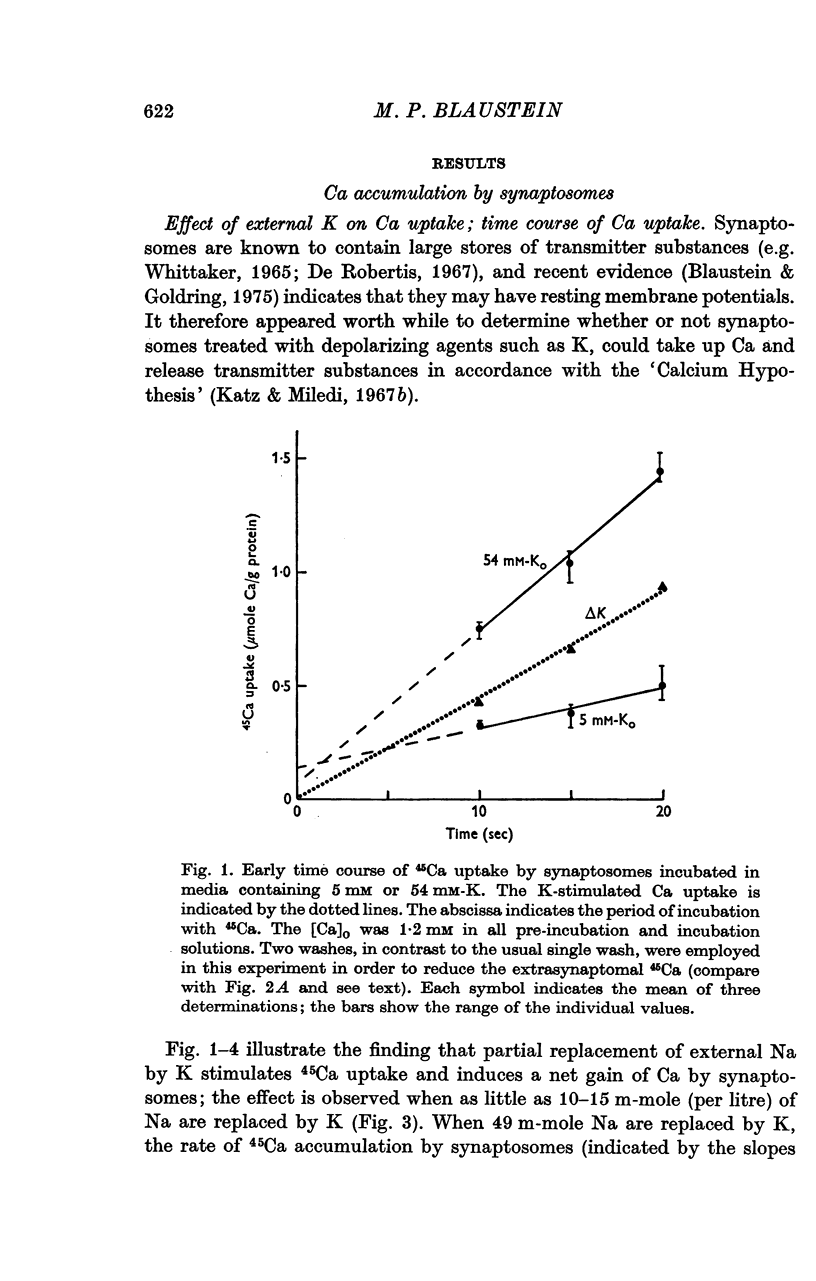
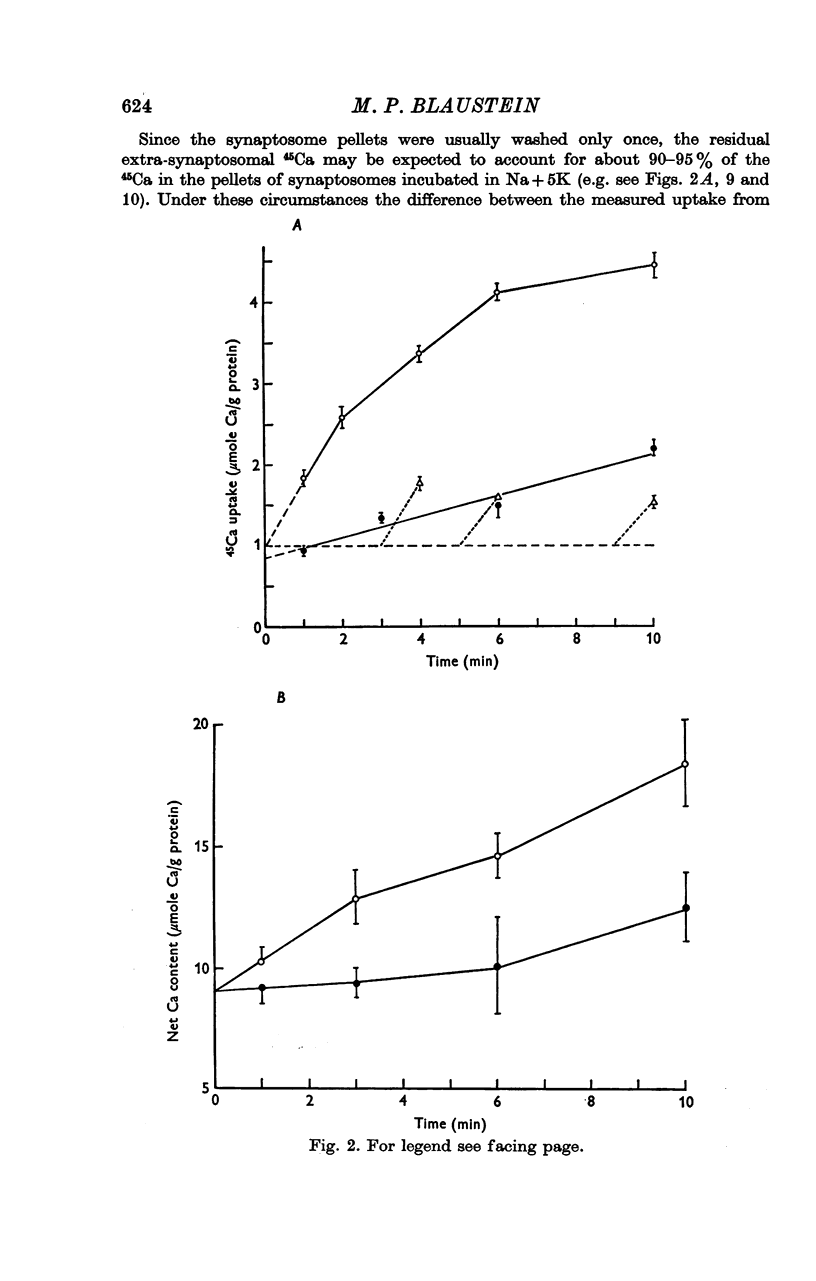
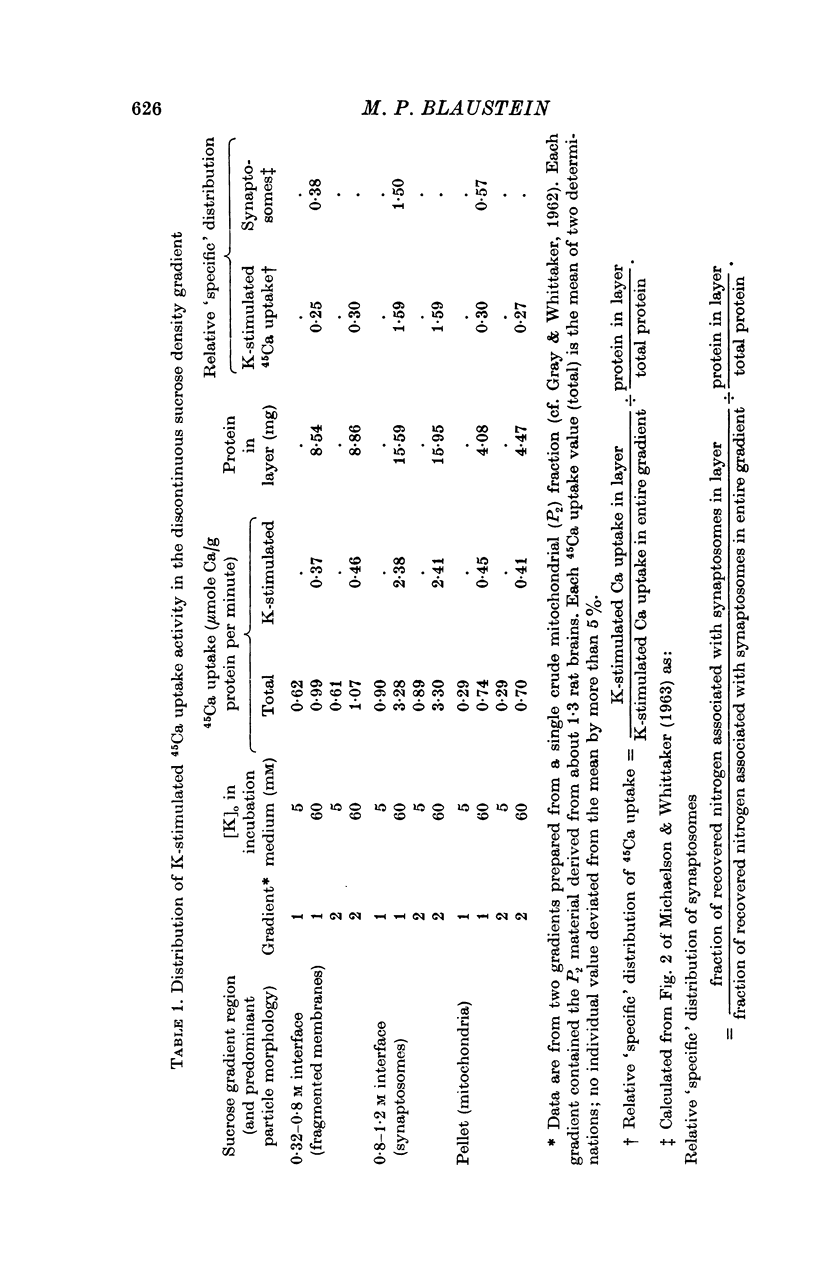
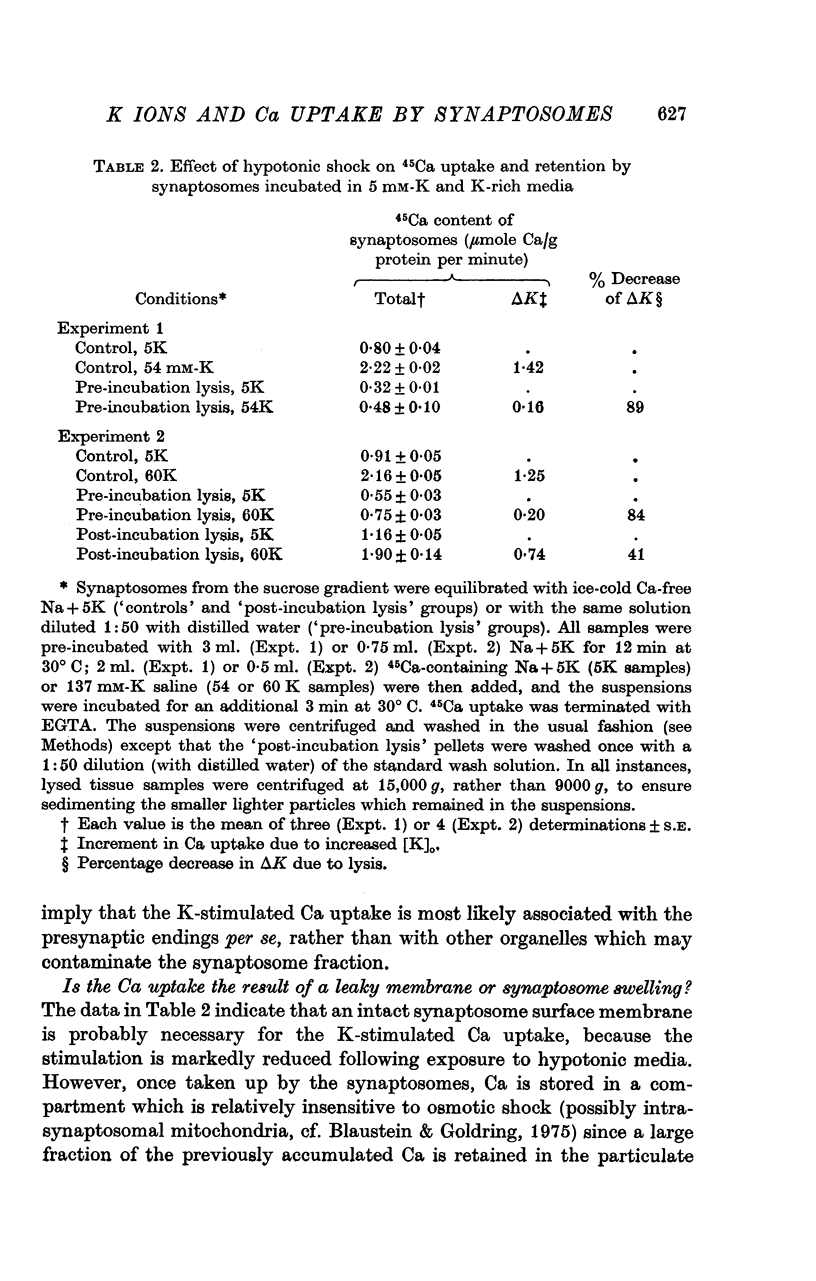
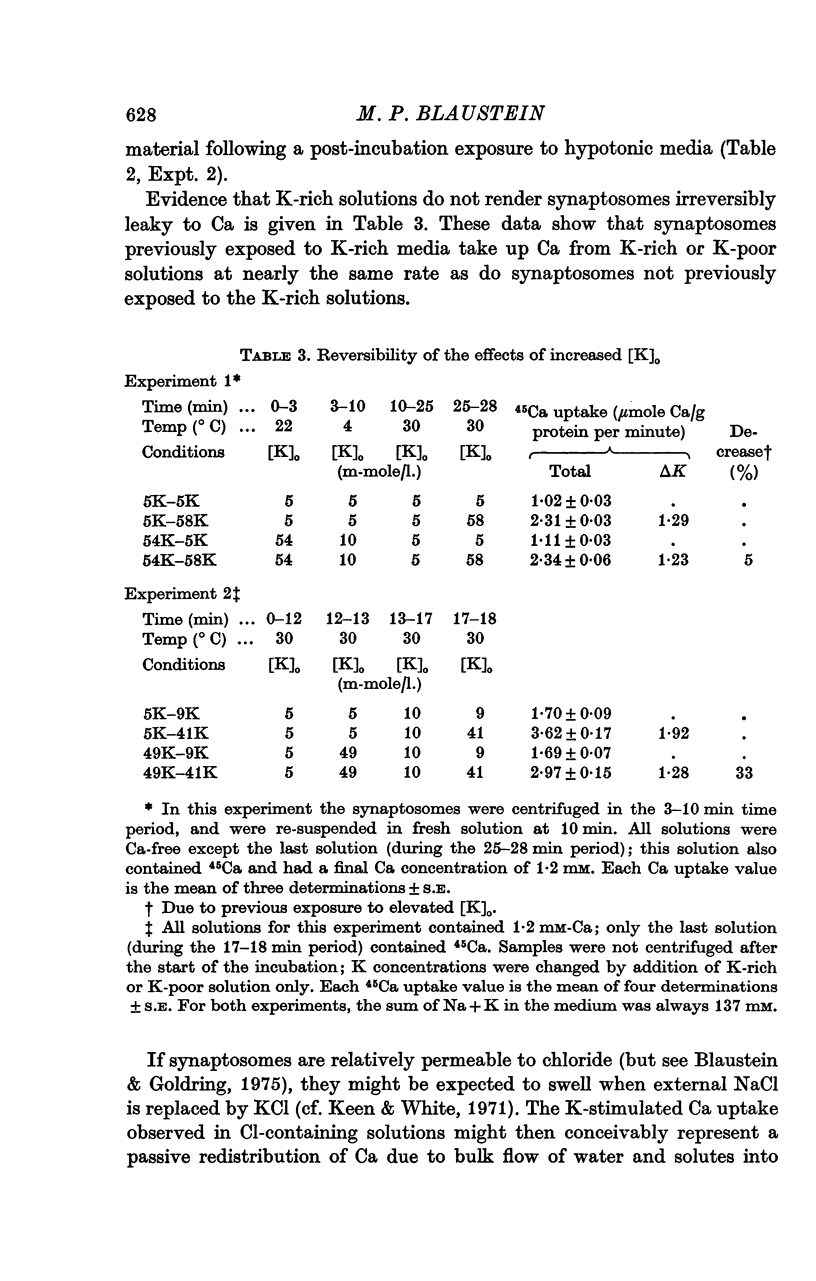
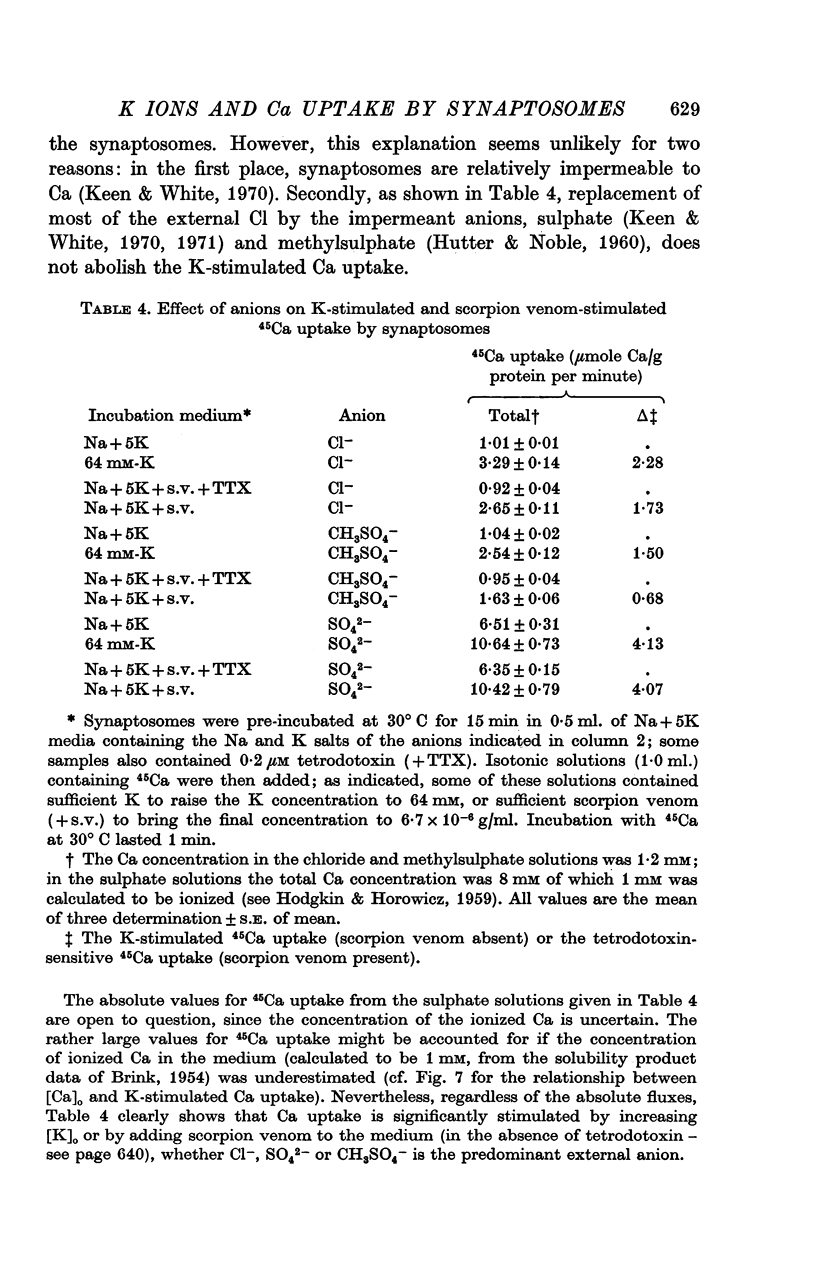
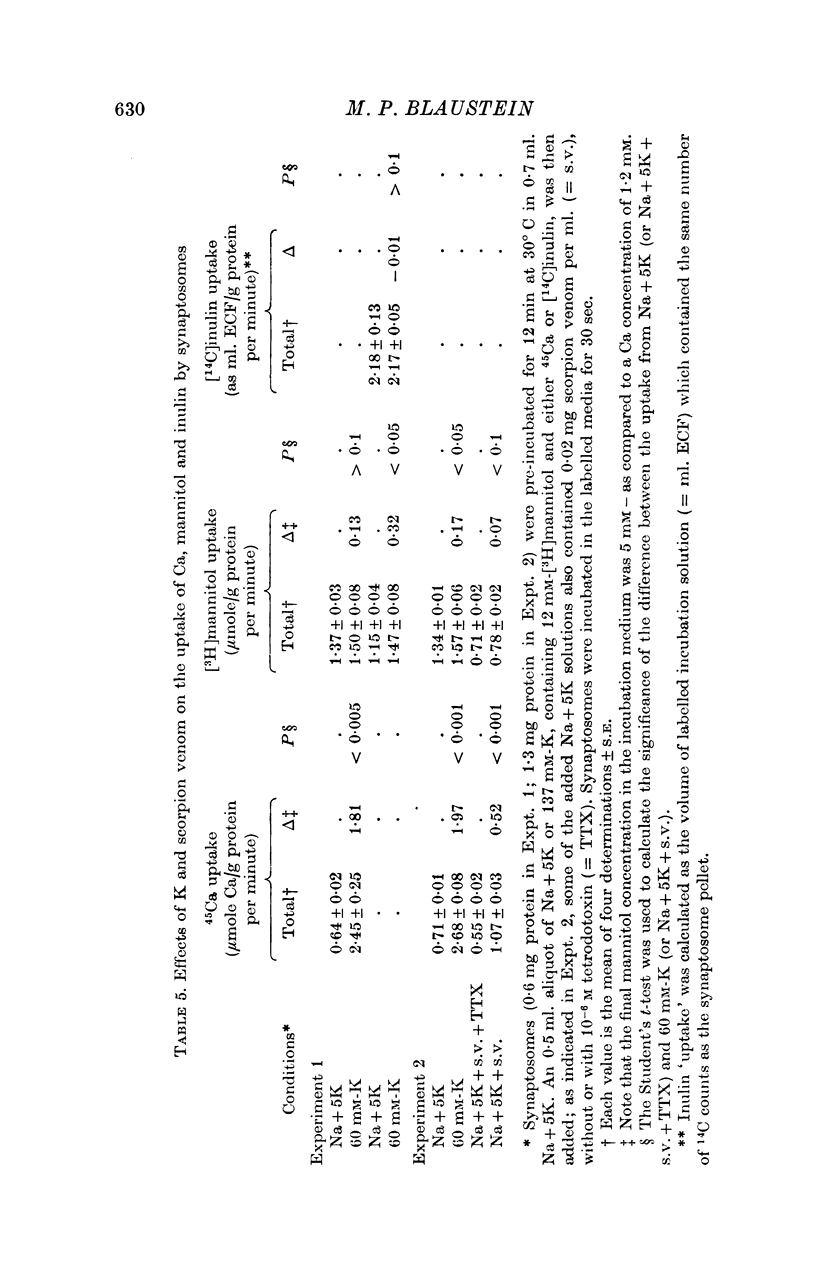
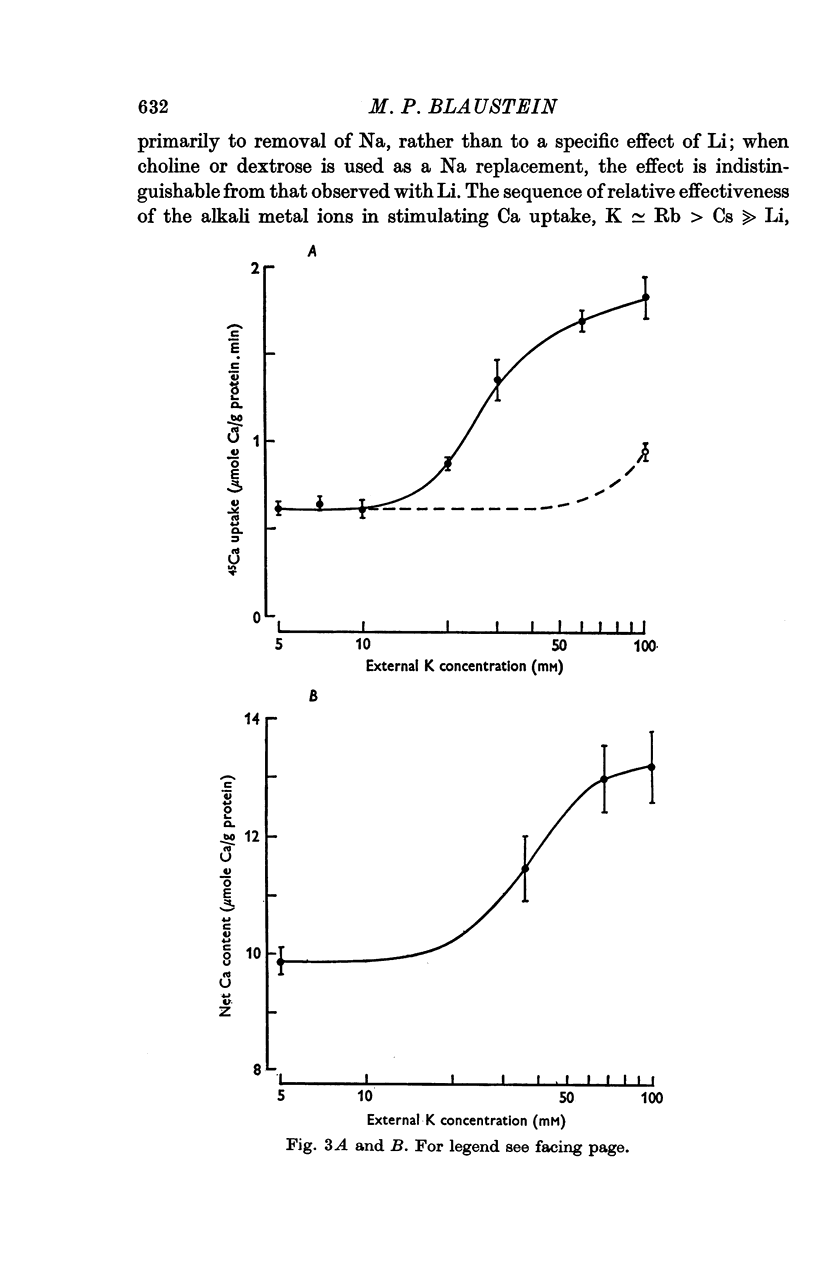
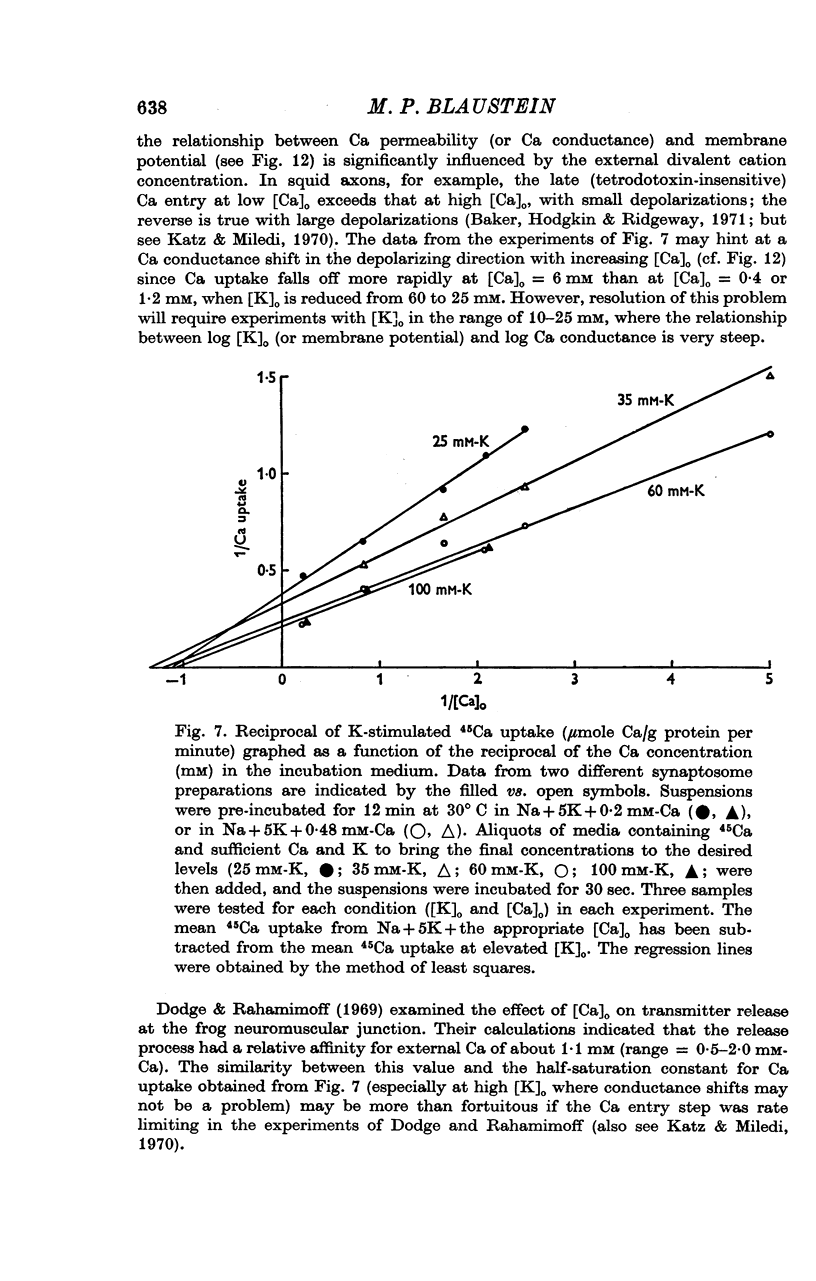
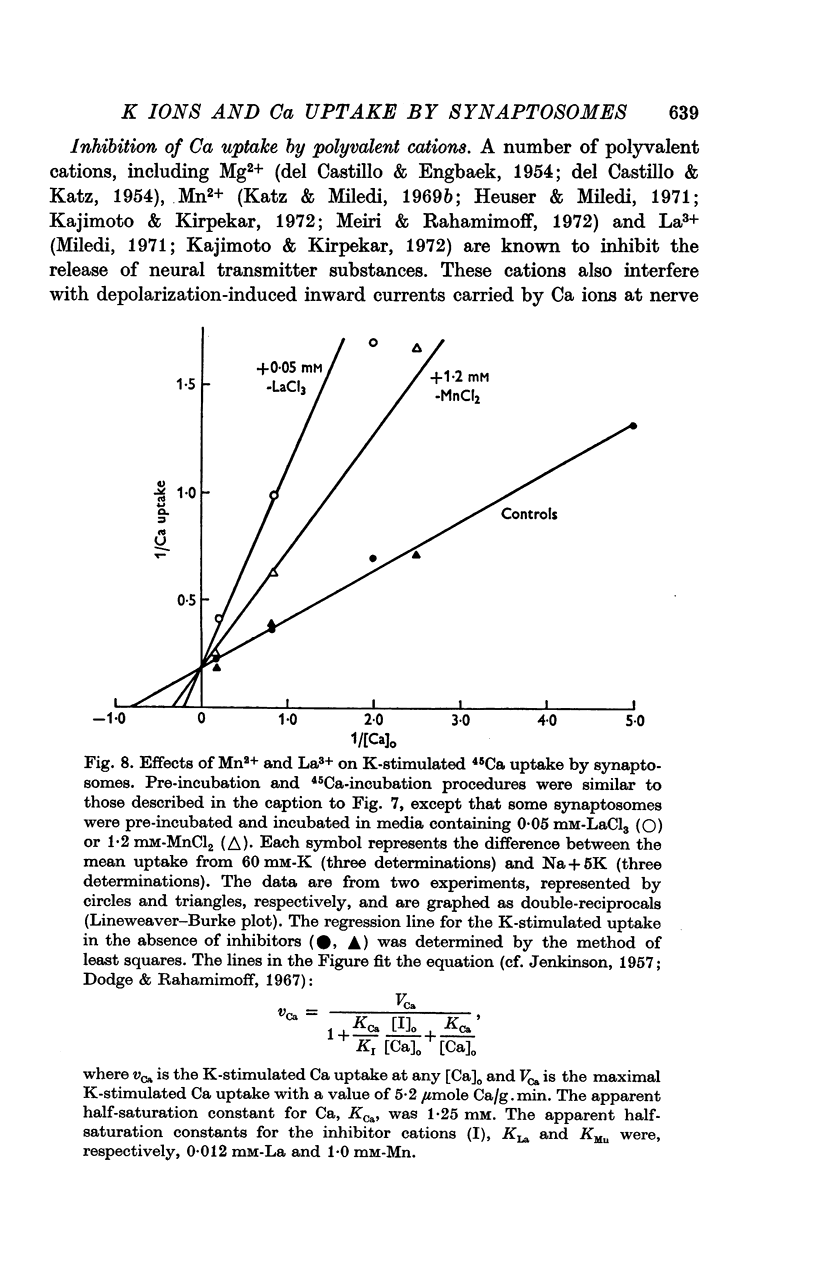
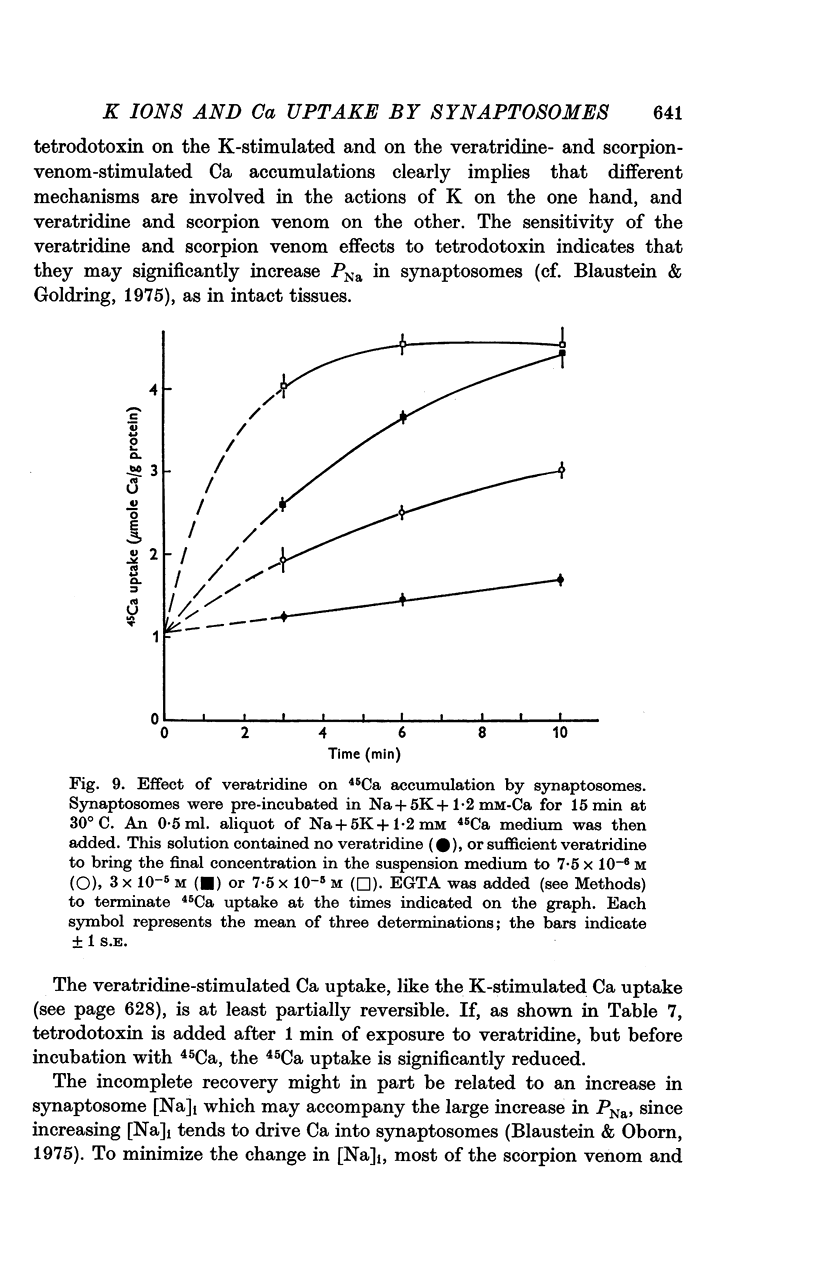
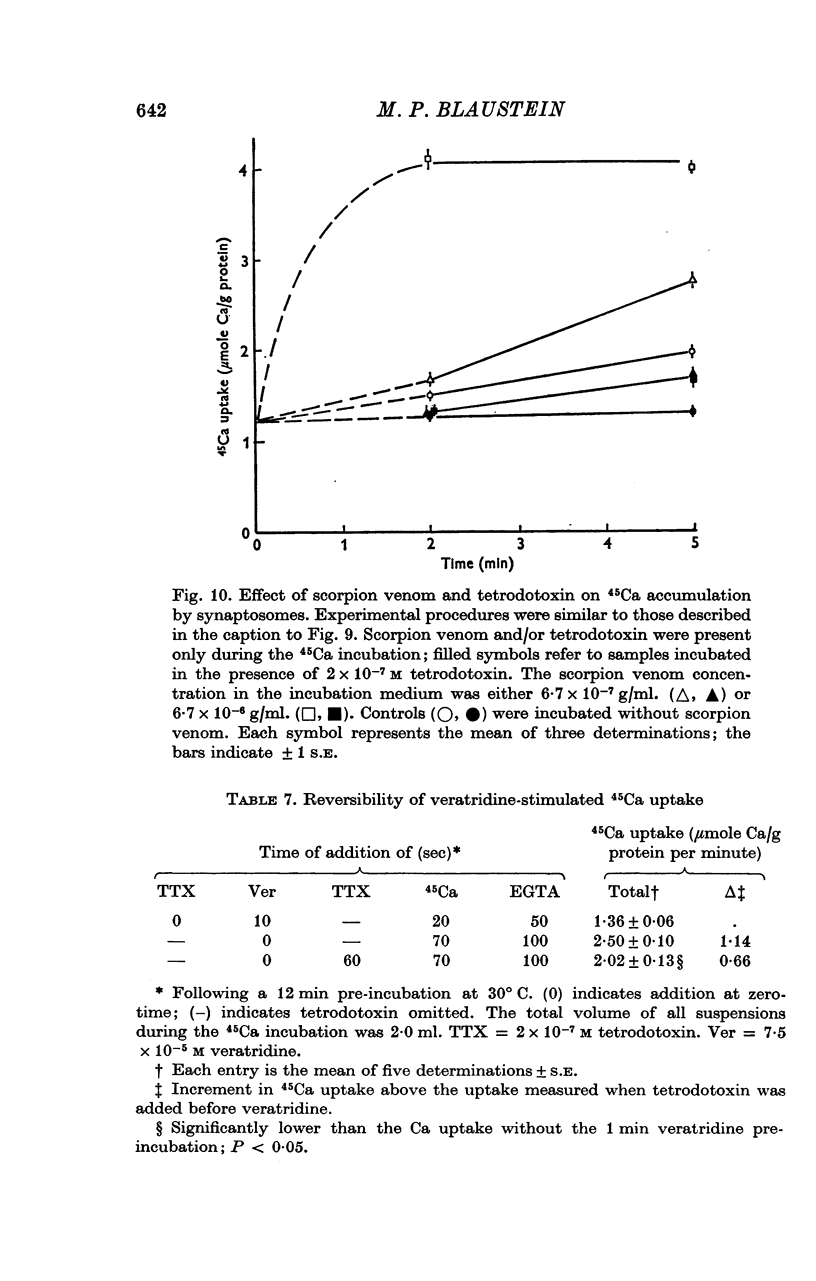
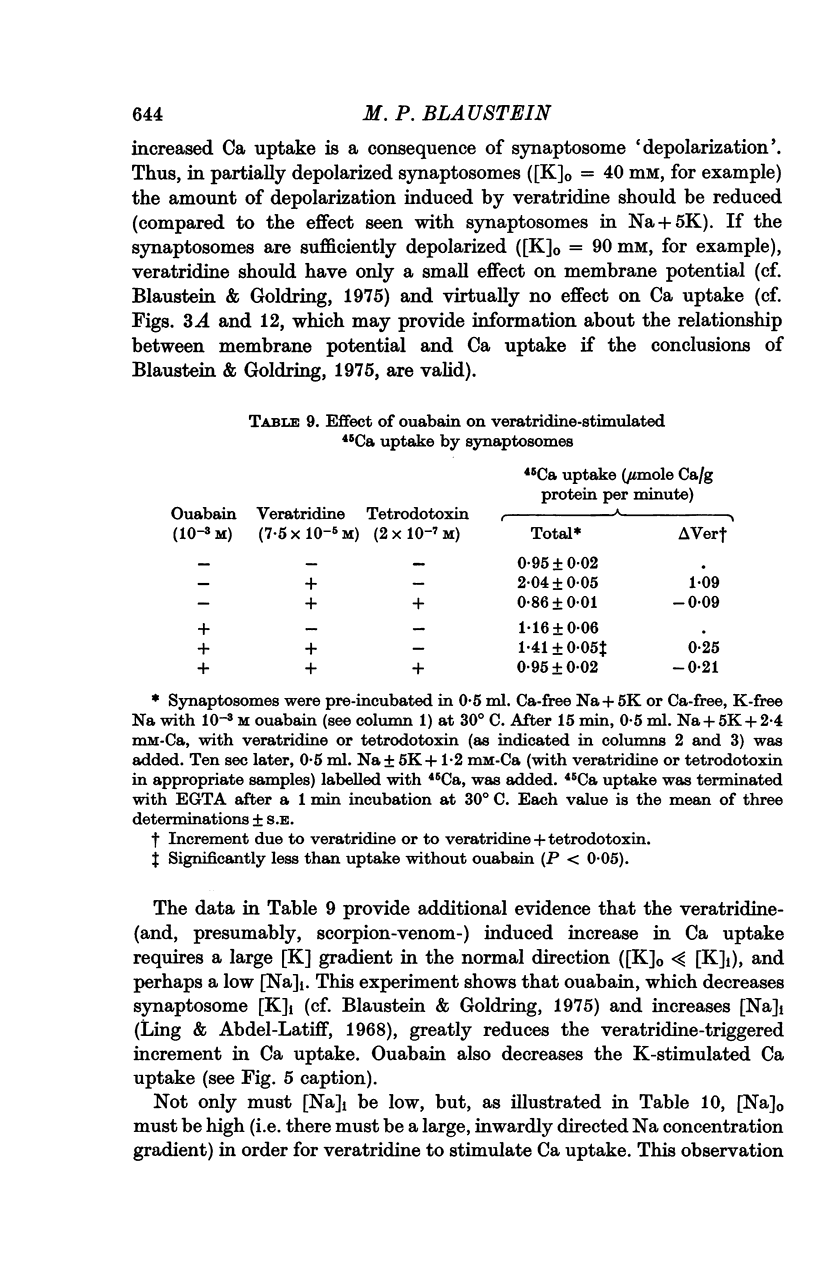
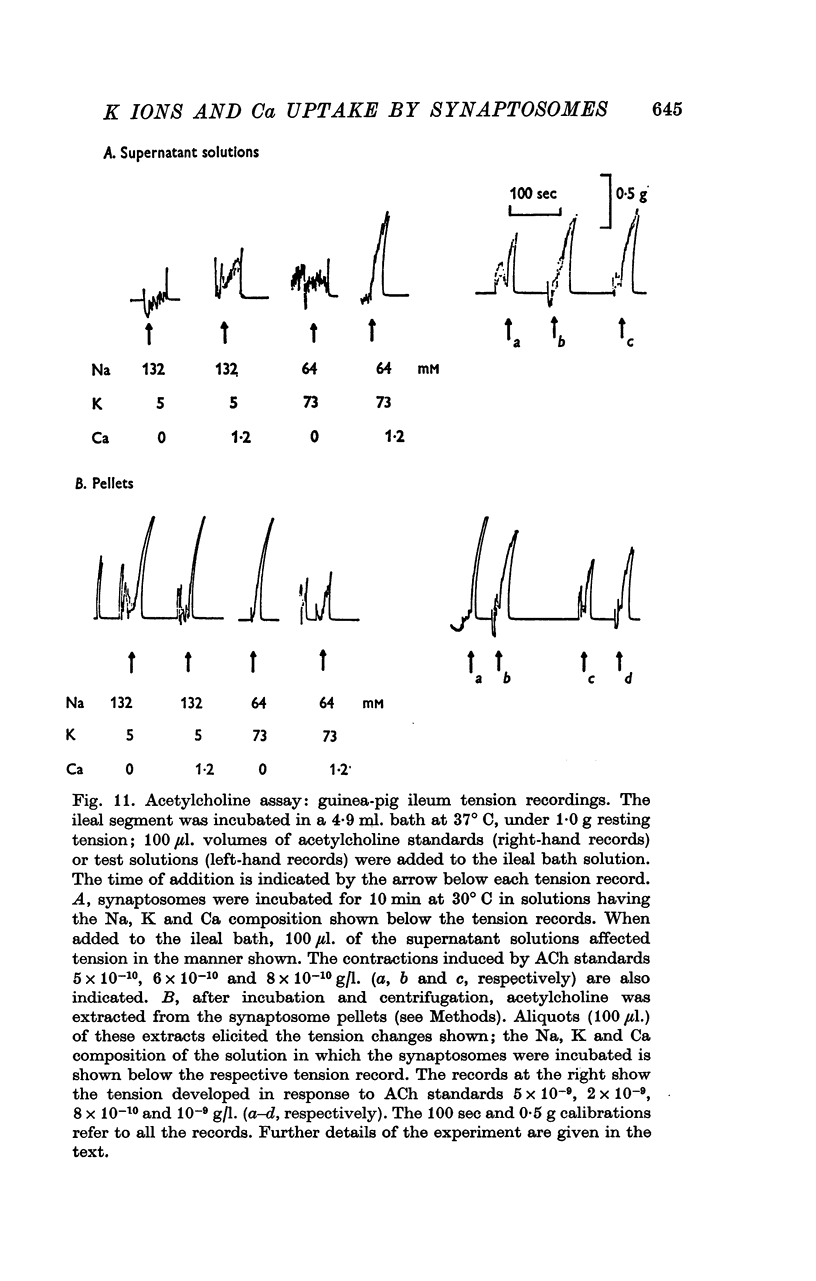
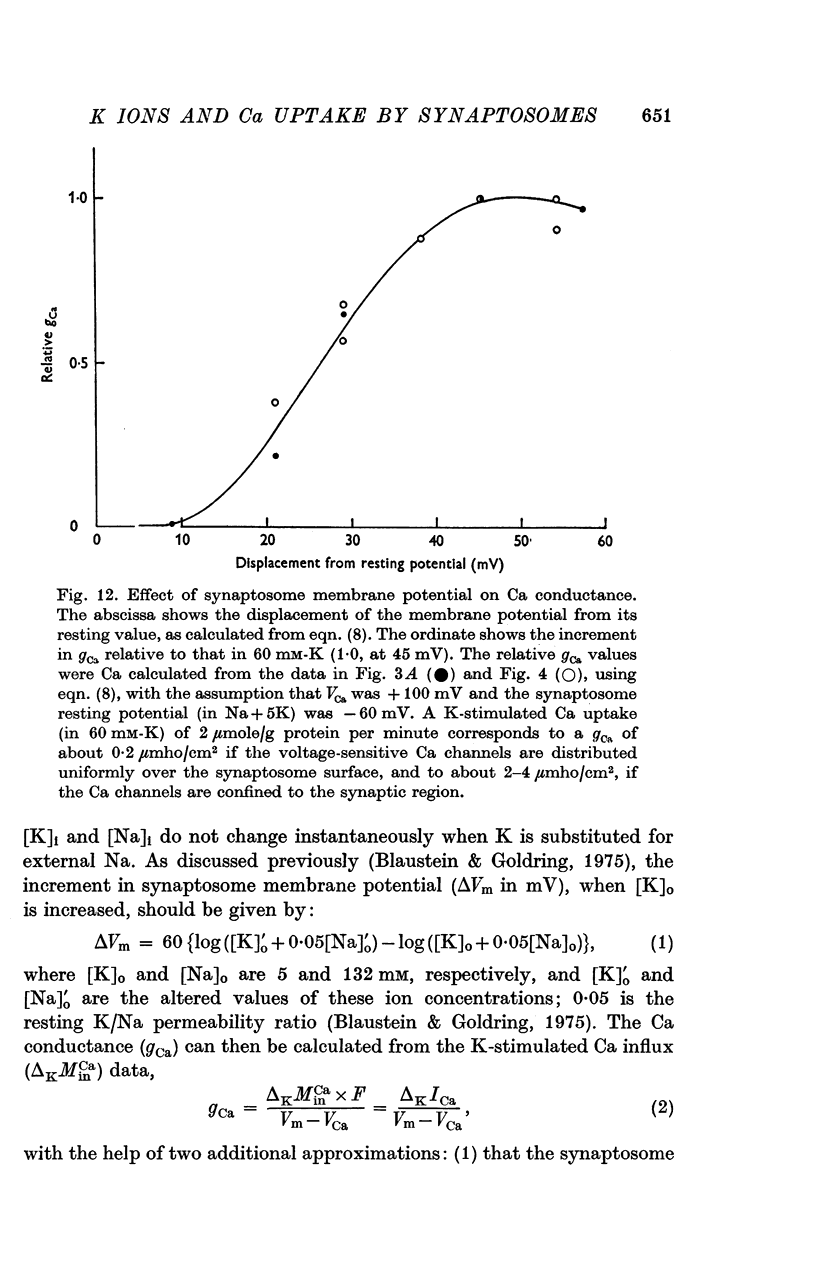
1. 45-Ca uptake by pinched-off nerve terminals (synaptosomes) of rat brain incubated in standard physiological saline (including 132 mM-Na + 5mM-K + 1-2 mM-Ca) at 30 degrees C averages about 0-5 mumole Ca per g protein per minute. This may be equivalent to a Ca influx of about 0-03 p-mole/cm-2 sec. 2. The rate of 45-Ca uptake is increased when the concentration of K in the medium is increased above 15-20 mM, K replacing Na isosmotically. Maximum stimulation, a three- to six-fold increase in the rate of Ca uptake, occurs when [K]o is about 60 mM. The effect of increased [K]o is reversible. 3. The K-stimulated Ca uptake is associated primarily with the nerve terminal fraction of brain homogenates. The entering Ca is not accompanied by extracellular markers such as mannitol or inulin. Replacement of external chloride by methylsulphate or sulphate does not prevent the stimulation by K. 4. The effects of external K are quantitatively mimicked by Rb. Caesium also stimulates Ca uptake, but is only about one fifth as effective as K or Rb; Li is ineffective. 5. Two other depolarizing agents also stimulate Ca uptake by synaptosomes: veratridine (7-5 times 10- minus 6 to 7-5 times 10- minus 5 M) and scorpion (Leirus quinquestriatus) venom (6-7 times 10- minus 7 to 6-7 times 10- minus g/ml.). The stimulatory effects of veratridine and scorpion venom, but not of increased [K] are blocked by 2 times 10- minus 7 M tetrodotoxin. 6. Internal K also influences the rate of 45-Ca uptake by synaptosomes: lowering [K]i reduces the stimulatory effect of external K and veratridine. 7. Replacement of external Na by choline markedly inhibits the response to veratridine, but has a much smaller effect on the response to increased [K]o. 8. The Ca uptake mechanism has an apparent dissociation constant for Ca (KCa) of about 0-8 mM. Increasing [K]o increases the maximal rate of Ca uptake, but has no effect on KCa. The K-induced 45-Ca uptake is competitively inhibited by Mg-2+, Mn-2+ and La-3+. 9. The release of acetylcholine and noradrenaline was also studied. Increasing [K]o stimulates external Ca-dependent acetylcholine release. Scorpion venom stimulates noradrenaline release from synaptosomes; this effect could be prevented by adding tetrodotoxin or removing external Ca. 10. These results indicate that synaptosomes may increase their permeability to Ca, accumulate Ca and release neural transmitter substances, when stimulated by depolarizing agents under appropriate physiological conditions.
Full text
PDF







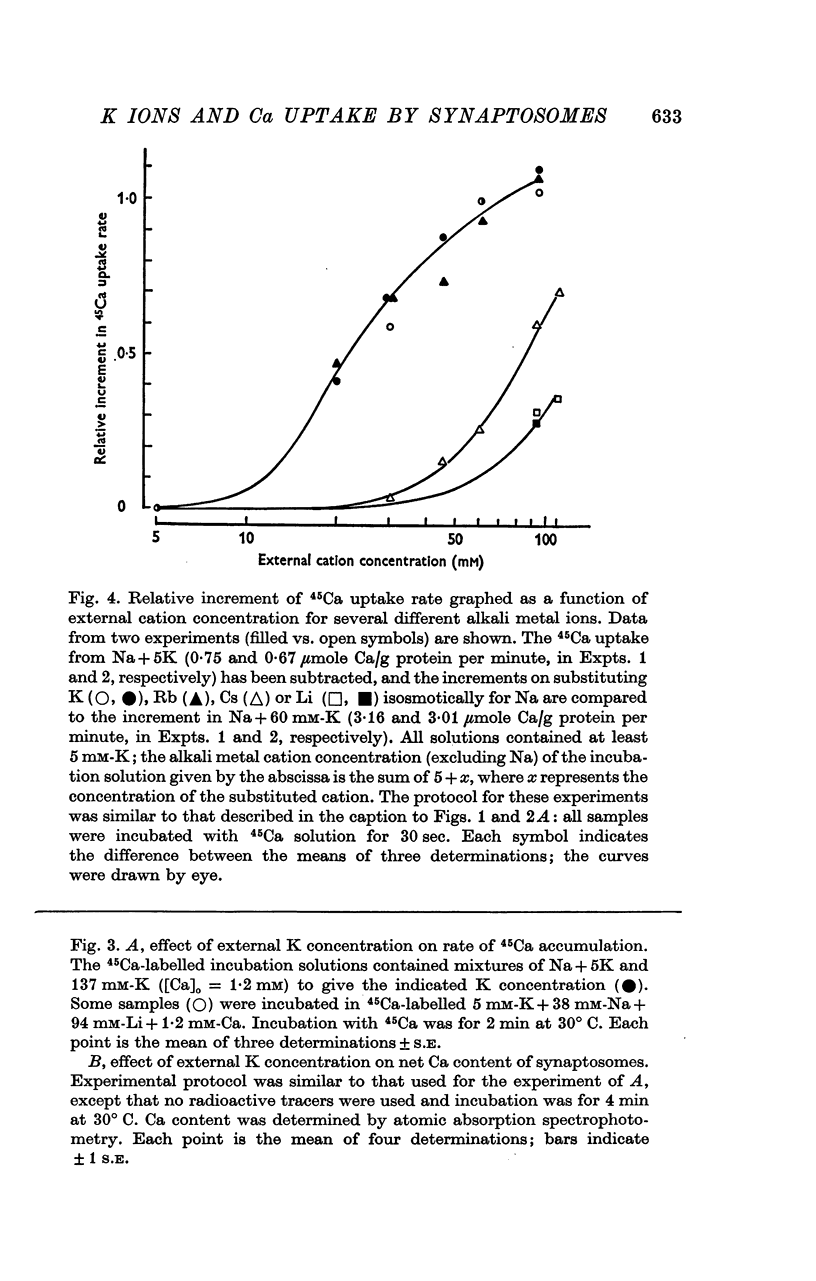
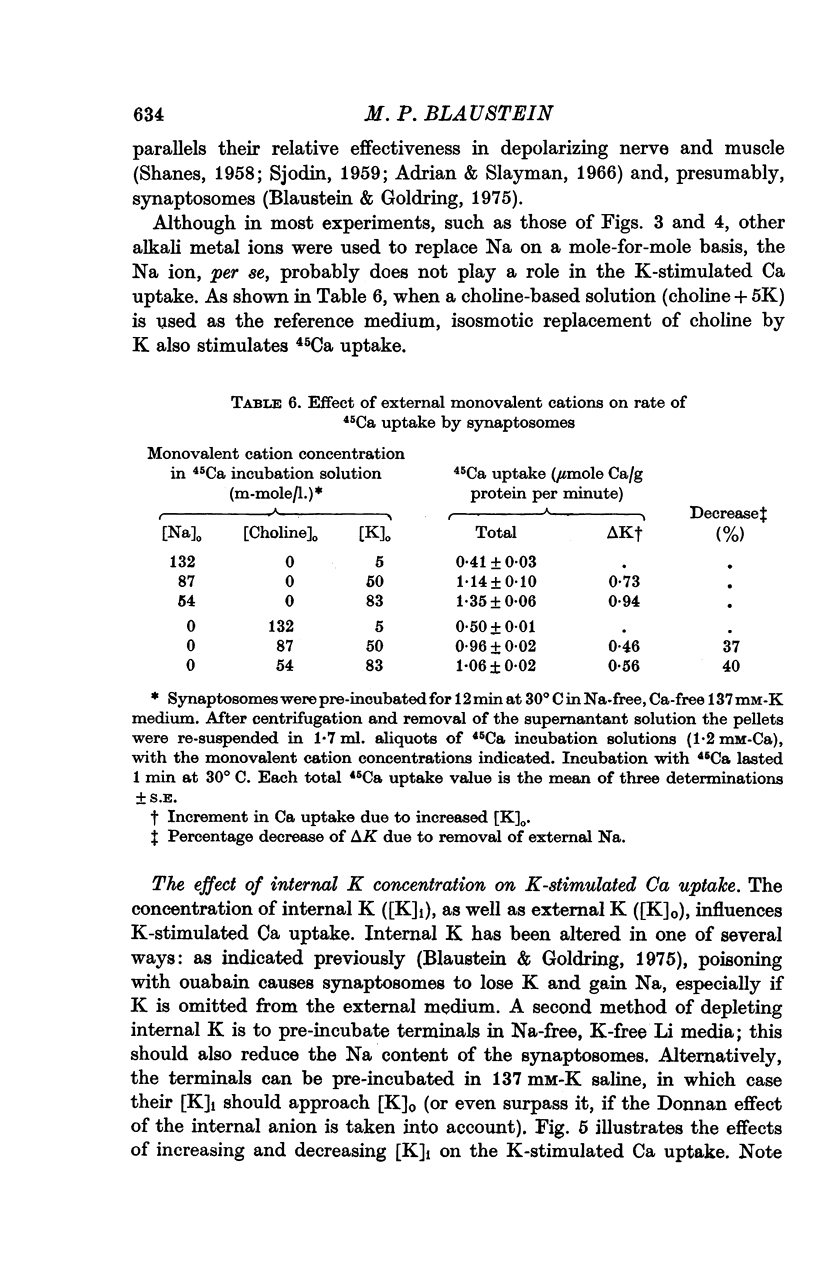
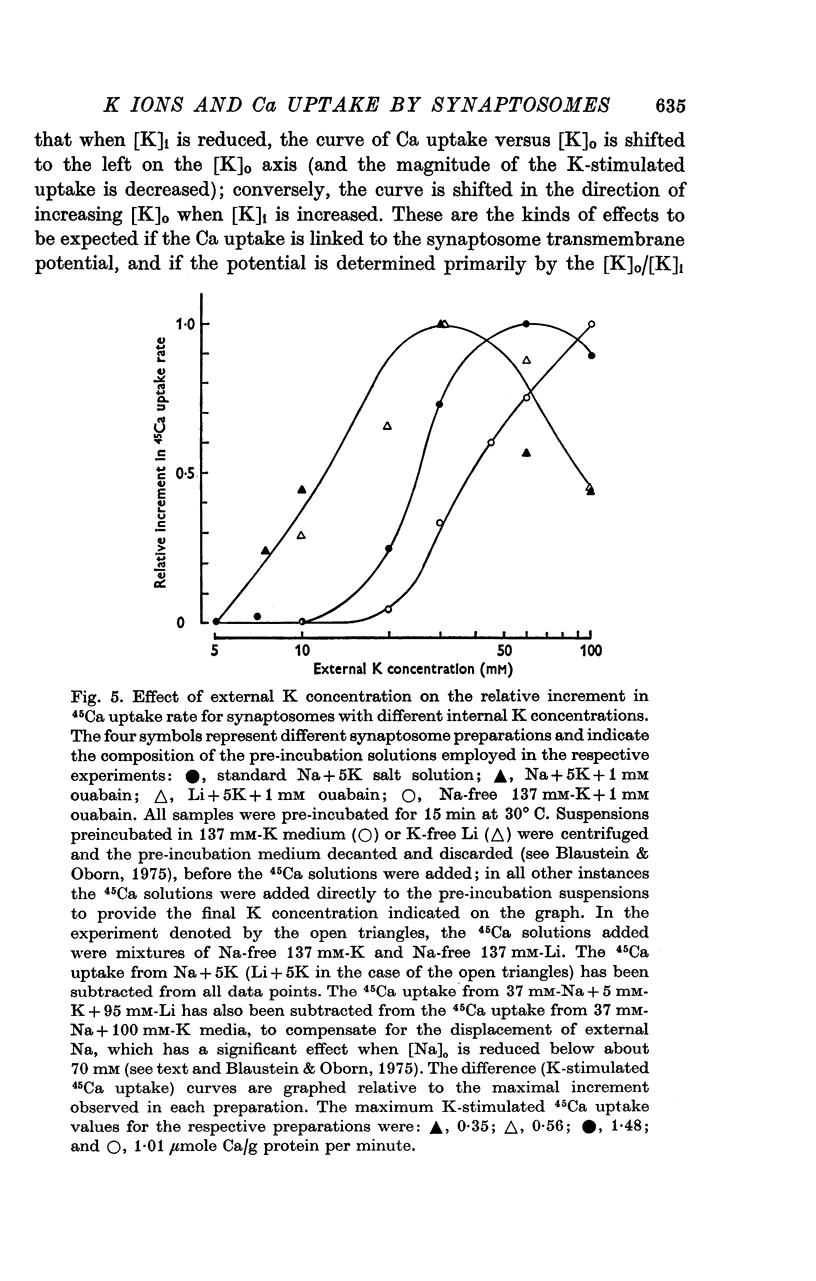

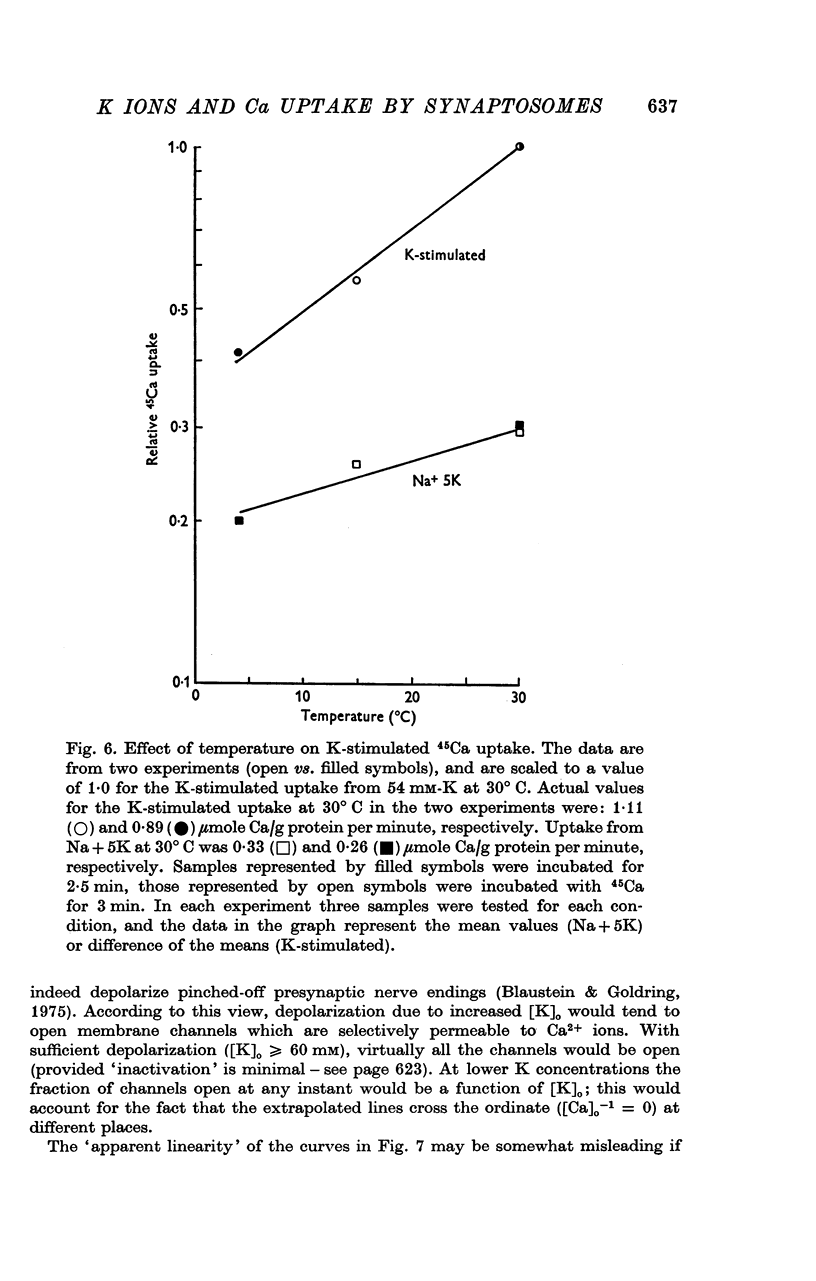











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAM K. R., WEISS C. Actions of scorpion venom on skeletal muscle. Br J Pharmacol Chemother. 1959 Sep;14:334–339. doi: 10.1111/j.1476-5381.1959.tb00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINK F. The role of calcium ions in neural processes. Pharmacol Rev. 1954 Sep;6(3):243–298. [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Ravazzola M., Malaisse-Lagae F. Secretion-dependent uptake of extracellular fluid by the rat neurohypophysis. Nature. 1974 Jul 12;250(462):155–157. doi: 10.1038/250155a0. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Goldring J. M. Membrane potentials in pinched-off presynaptic nerve ternimals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J Physiol. 1975 Jun;247(3):589–615. doi: 10.1113/jphysiol.1975.sp010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Johnson E. M., Jr, Needleman P. Calcium-dependent norepinephrine release from presynaptic nerve endings in vitro. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2237–2240. doi: 10.1073/pnas.69.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Oborn C. J. The influence of sodium on calcium fluxes in pinched-off nerve terminals in vitro. J Physiol. 1975 Jun;247(3):657–686. doi: 10.1113/jphysiol.1975.sp010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. Preganglionic stimulation increases calcium uptake by sympathetic ganglia. Science. 1971 Apr 23;172(3981):391–393. doi: 10.1126/science.172.3981.391. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Wiesmann W. P. Effect of sodium ions on calcium movements in isolated synaptic terminals. Proc Natl Acad Sci U S A. 1970 Jul;66(3):664–671. doi: 10.1073/pnas.66.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B., Briggs F. N. Microdetermination of calcium in biological material by automatic fluorometric titration. Anal Chem. 1968 Feb;40(2):339–344. doi: 10.1021/ac60258a056. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in HeLa cell cultures. I. Calcium influx. J Gen Physiol. 1969 Jan;53(1):43–56. doi: 10.1085/jgp.53.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford H. F. Metabolic response of synaptosomes to electrical stimulation: release of amino acids. Brain Res. 1970 Apr 14;19(2):239–247. doi: 10.1016/0006-8993(70)90437-3. [DOI] [PubMed] [Google Scholar]
- Bradford H. F. Respiration in vitro of synaptosomes from mammalian cerebral cortex. J Neurochem. 1969 May;16(5):675–684. doi: 10.1111/j.1471-4159.1969.tb06444.x. [DOI] [PubMed] [Google Scholar]
- Bradford H. F., Thomas A. J. Metabolism of glucose and glutamate by synaptosomes from mammalian cerebral cortex. J Neurochem. 1969 Nov;16(11):1495–1504. doi: 10.1111/j.1471-4159.1969.tb09904.x. [DOI] [PubMed] [Google Scholar]
- CHANG C. C. A SENSITIVE METHOD FOR SPECTROPHOTOFLUOROMETRIC ASSAY OF CATECHOLAMINES. Int J Neuropharmacol. 1964 Dec;3:643–649. doi: 10.1016/0028-3908(64)90089-9. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Gamble R. L., Rossi C. S., Lehninger A. L. Super-stoichiometric ratios between ion movements and electron transport in rat liver mitochondria. J Biol Chem. 1967 Mar 25;242(6):1199–1204. [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P., Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi F., Whittaker V. P., Sheridan M. N. The yield of synaptosomes from the cerebral cortex of guinea pigs estimated by a polystyrene bead "tagging" procedure. Z Zellforsch Mikrosk Anat. 1966;72(1):126–138. doi: 10.1007/BF00336902. [DOI] [PubMed] [Google Scholar]
- Curtis B. A. Ca fluxes in single twitch muscle fibers. J Gen Physiol. 1966 Nov;50(2):255–267. doi: 10.1085/jgp.50.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAHOTA Z., LEHNINGER A. L. MOVEMENTS OF H+, K+, AND NA+ DURING ENERGY-DEPENDENT UPTAKE AND RETENTION OF CA++ IN RAT LIVE MITOCHONDRIA. Biochem Biophys Res Commun. 1965 Apr 23;19:351–356. doi: 10.1016/0006-291x(65)90467-5. [DOI] [PubMed] [Google Scholar]
- De Belleroche J. S., Bradford H. F. The stimulus-induced release of acetylcholine from synaptosome beds and its calcium dependence. J Neurochem. 1972 Jul;19(7):1817–1819. doi: 10.1111/j.1471-4159.1972.tb06229.x. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escueta A. V., Appel S. H. Biochemical studies of synapses in vitro. II. Potassium transport. Biochemistry. 1969 Feb;8(2):725–733. doi: 10.1021/bi00830a038. [DOI] [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Gomez M. V., Dai M. E., Diniz C. R. Effect of scorpion venom, tityustoxin, on the release of acetylcholine from incubated slices of rat brain. J Neurochem. 1973 Apr;20(4):1051–1061. doi: 10.1111/j.1471-4159.1973.tb00076.x. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto N., Kirpekar S. M. Effect of manganese and lanthanum on spontaneous release of acetylcholine at frog motor nerve terminals. Nat New Biol. 1972 Jan 5;235(53):29–30. doi: 10.1038/newbio235029a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of prolonged depolarization on synaptic transfer in the stellate ganglion of the squid. J Physiol. 1971 Jul;216(2):503–512. doi: 10.1113/jphysiol.1971.sp009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Keen P., White T. D. A light-scattering technique for the study of the permeability of rat brain synaptosomes in vitro. J Neurochem. 1970 May;17(5):565–571. doi: 10.1111/j.1471-4159.1970.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Keen P., White T. D. The permeability of pinched-off nerve endings to sodium, potassium and chloride and the effects of gramicidin. J Neurochem. 1971 Jun;18(6):1097–1103. doi: 10.1111/j.1471-4159.1971.tb12038.x. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer E., Schmidt H. Incomplete sodium inactivation in nodes of Ranvier treated with scorpion venom. Experientia. 1968 Jan 15;24(1):41–42. doi: 10.1007/BF02136780. [DOI] [PubMed] [Google Scholar]
- Lazarewicz J. W., Haljamäe H., Hamberger A. Calcium metabolism in isolated brain cells and subcellular fractions. J Neurochem. 1974 Jan;22(1):33–45. doi: 10.1111/j.1471-4159.1974.tb12176.x. [DOI] [PubMed] [Google Scholar]
- Ling C. M., Abdel-Latif A. A. Studies on sodium transport in rat brain nerve-ending particles. J Neurochem. 1968 Aug;15(8):721–729. doi: 10.1111/j.1471-4159.1968.tb10316.x. [DOI] [PubMed] [Google Scholar]
- Llinás R., Blinks J. R., Nicholson C. Calcium transient in presynaptic terminal of squid giant synapse: detection with aequorin. Science. 1972 Jun 9;176(4039):1127–1129. doi: 10.1126/science.176.4039.1127. [DOI] [PubMed] [Google Scholar]
- MICHAELSON I. A., WHITTAKER V. P. The subcellular localization of 5-hydroxytryptamine in guinea pig brain. Biochem Pharmacol. 1963 Feb;12:203–211. doi: 10.1016/0006-2952(63)90185-0. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M. The osmotically sensitive potassium and sodium compartments of synaptosomes. Biochem J. 1967 Jul;104(1):148–157. doi: 10.1042/bj1040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Neuromuscular transmission: inhibition by manganese ions. Science. 1972 Apr 21;176(4032):308–309. doi: 10.1126/science.176.4032.308. [DOI] [PubMed] [Google Scholar]
- Miledi R. Lanthanum ions abolish the "calcium response" of nerve terminals. Nature. 1971 Feb 5;229(5284):410–411. doi: 10.1038/229410a0. [DOI] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Shapiro B. I., Deguchi T., Scuka M., Wang C. M. Effects of scorpion venom on squid axon membranes. Am J Physiol. 1972 Apr;222(4):850–857. doi: 10.1152/ajplegacy.1972.222.4.850. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. I. The resting cell and its alteration by extrinsic factors. Pharmacol Rev. 1958 Mar;10(1):59–164. [PubMed] [Google Scholar]
- SJODIN R. A. Rubidium and cesium fluxes in muscle as related to the membrane potential. J Gen Physiol. 1959 May 20;42(5):983–1003. doi: 10.1085/jgp.42.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P. The application of subcellular fractionation techniques to the study of brain function. Prog Biophys Mol Biol. 1965;15:39–96. doi: 10.1016/0079-6107(65)90004-0. [DOI] [PubMed] [Google Scholar]


