Abstract
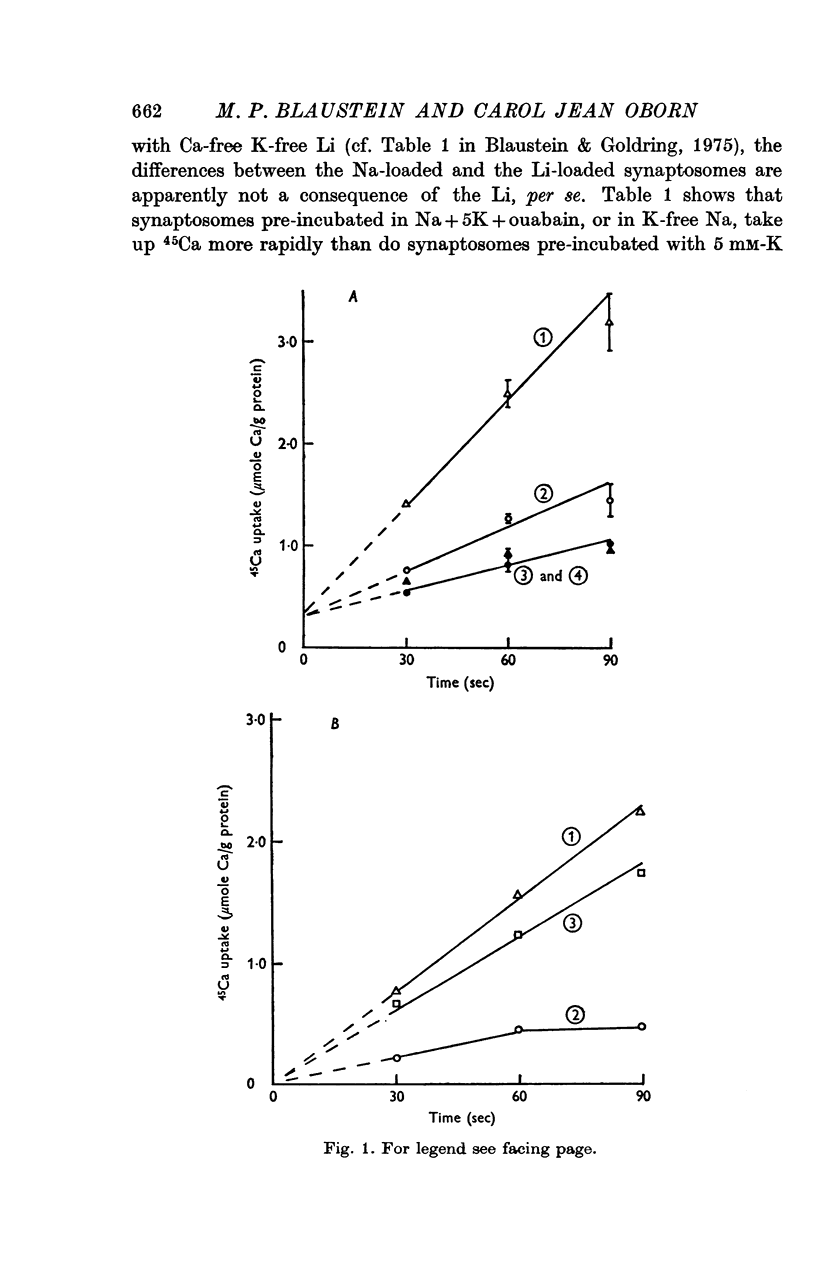
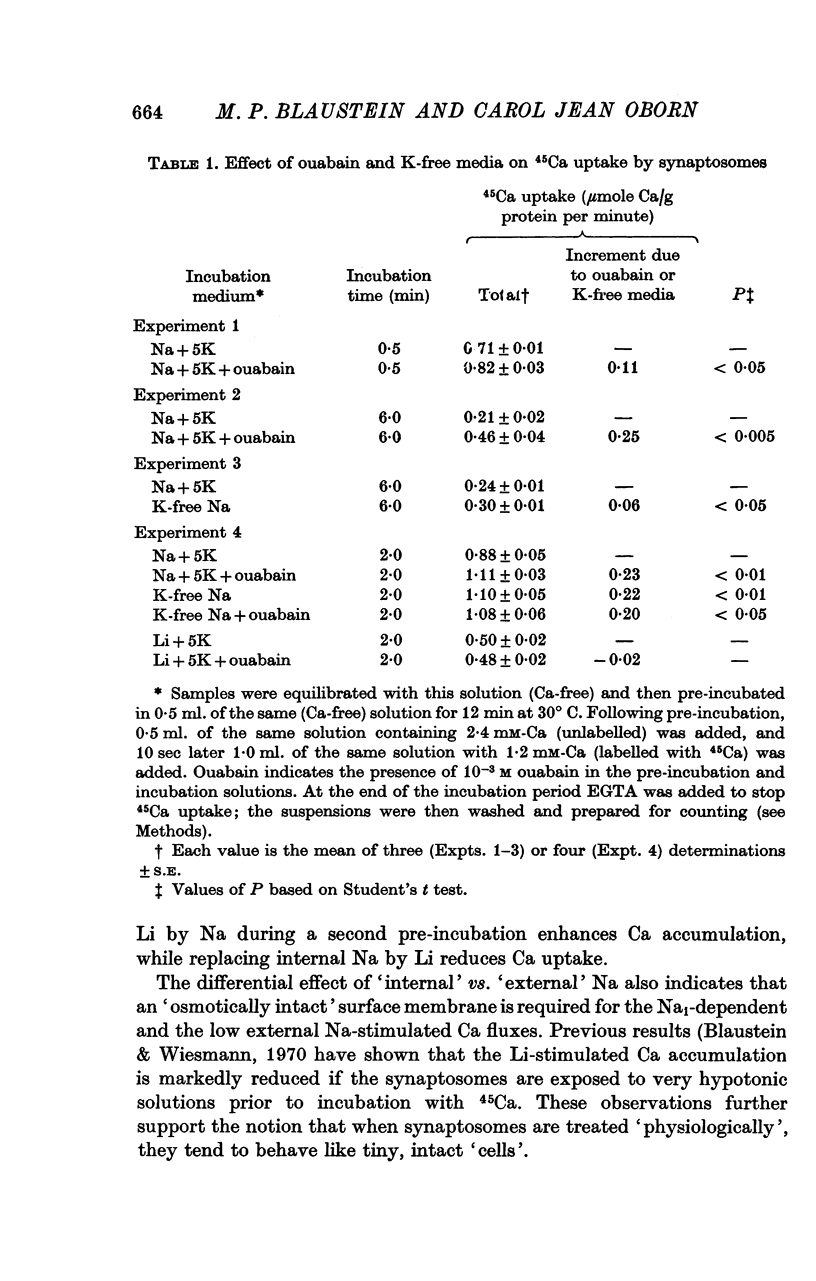
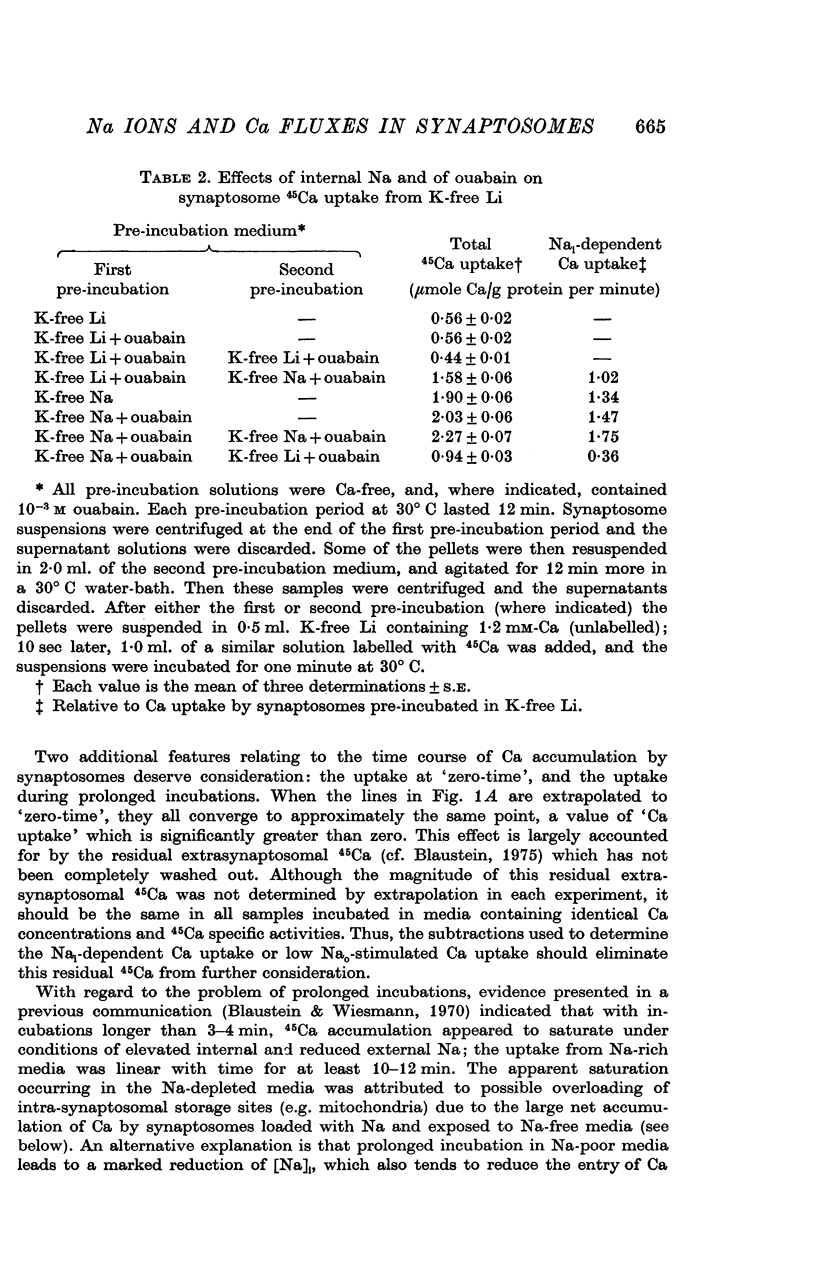
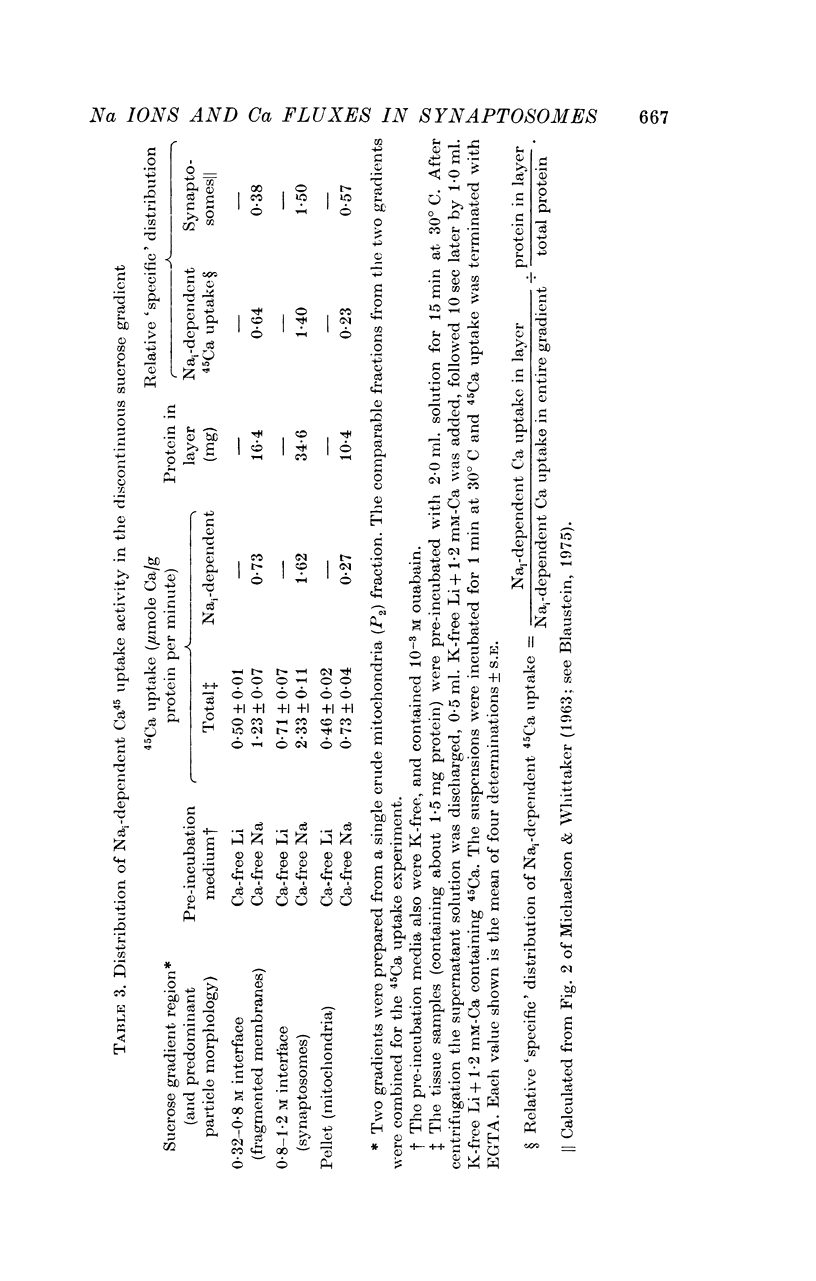
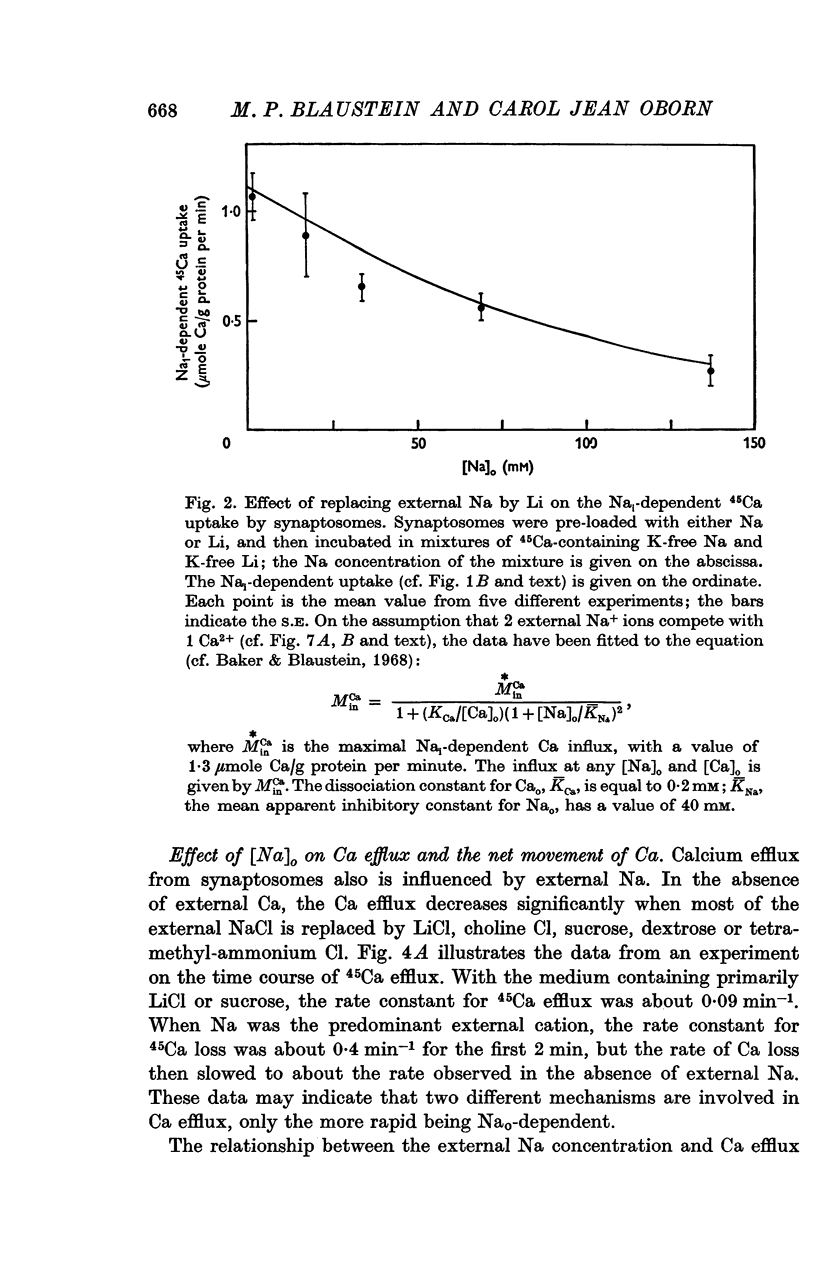
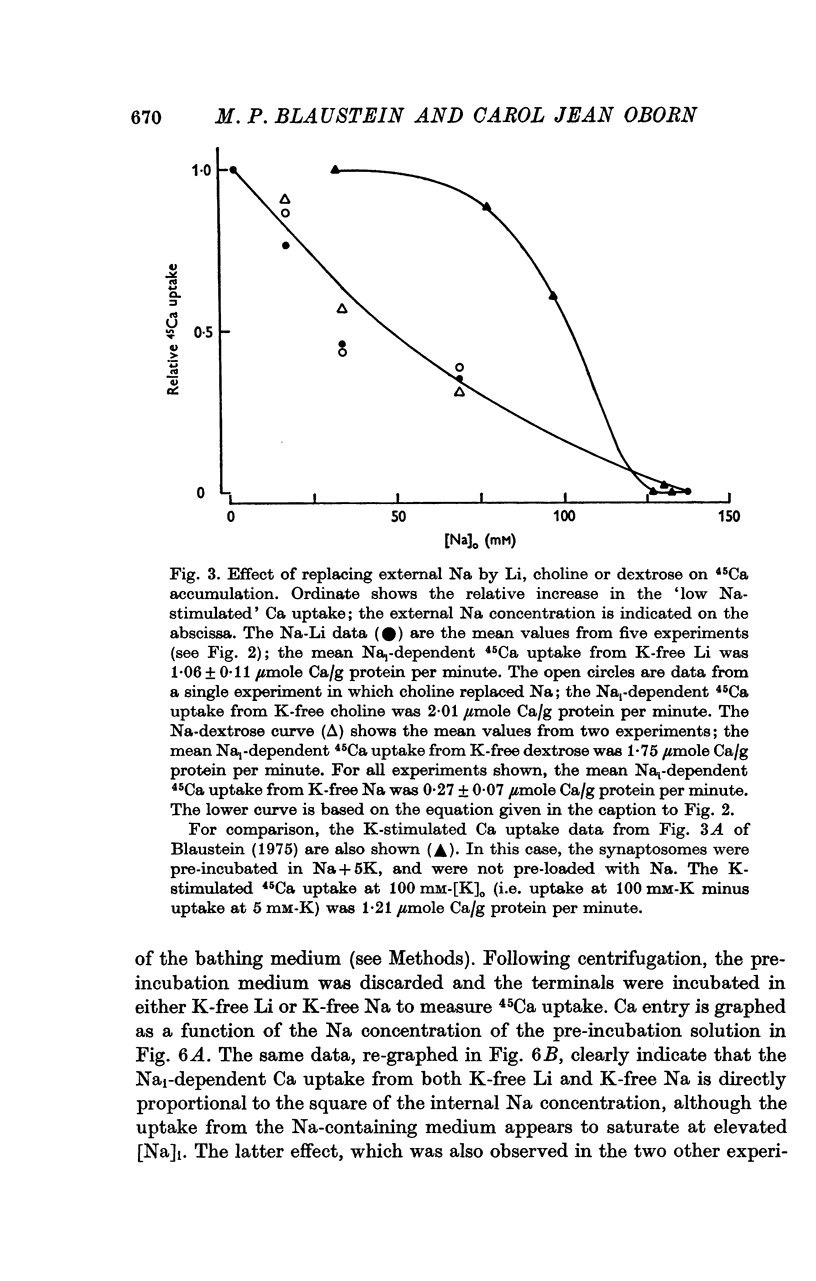
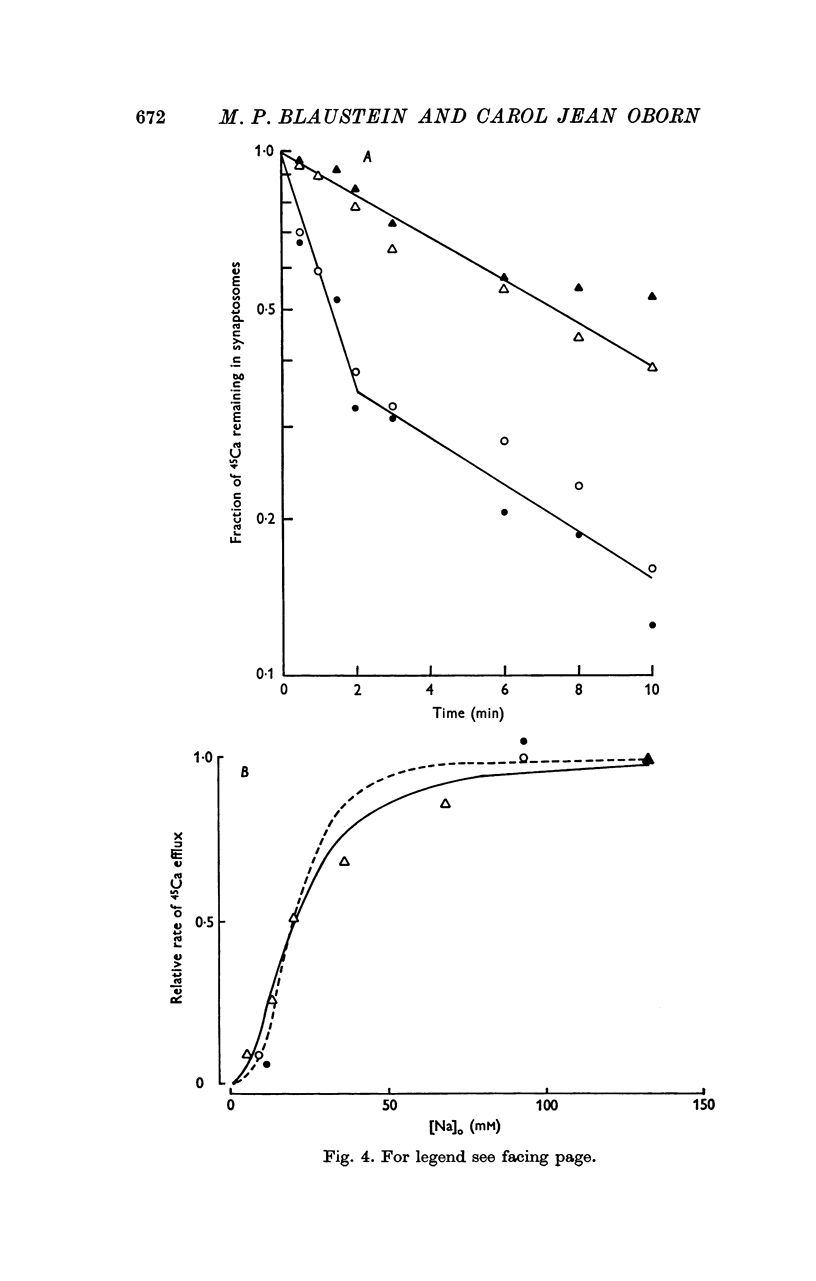
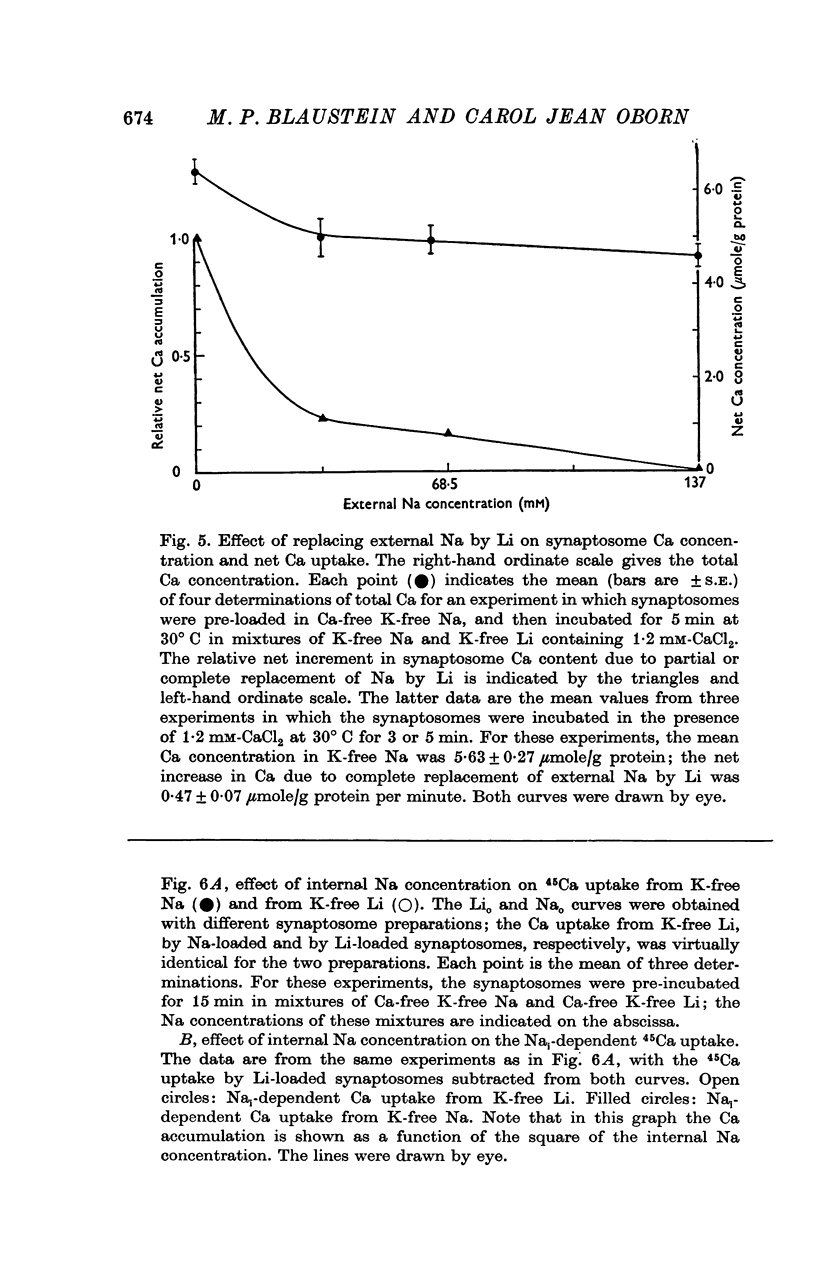
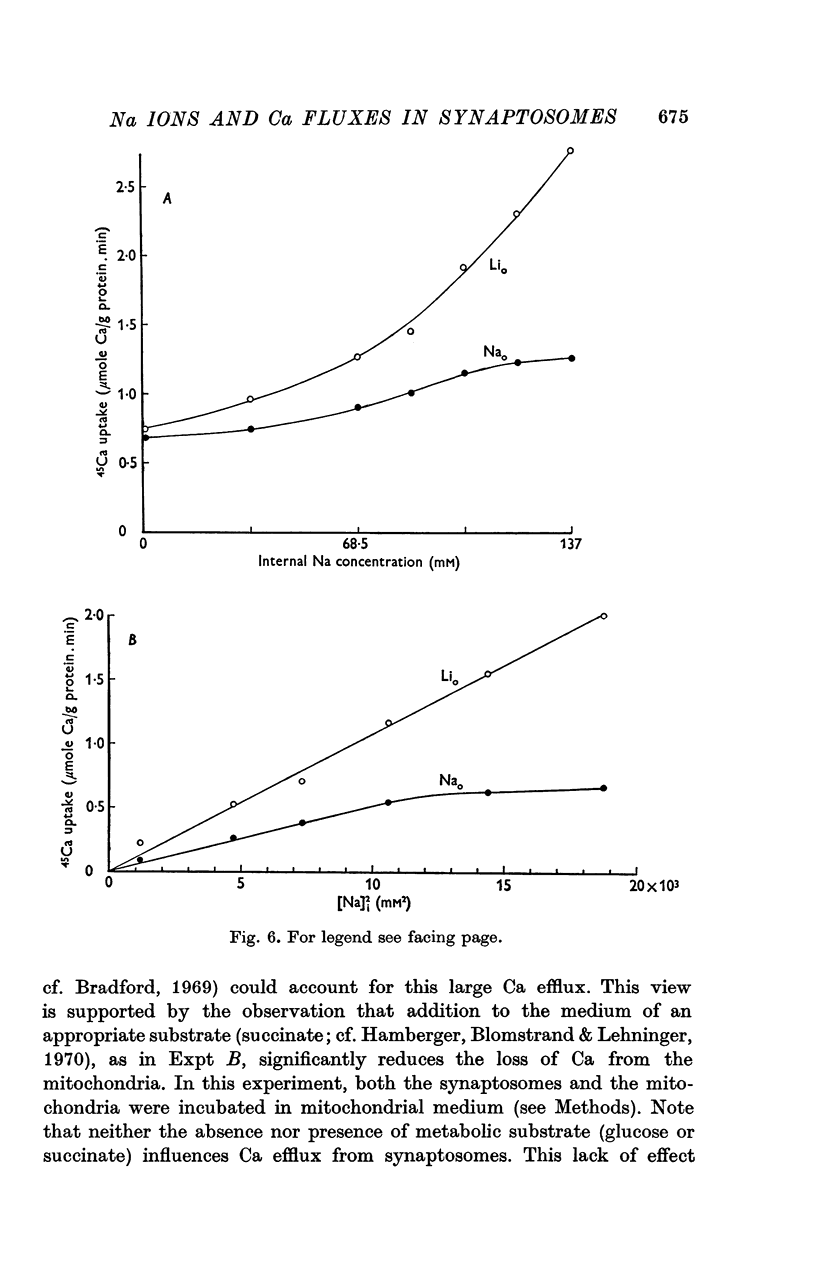
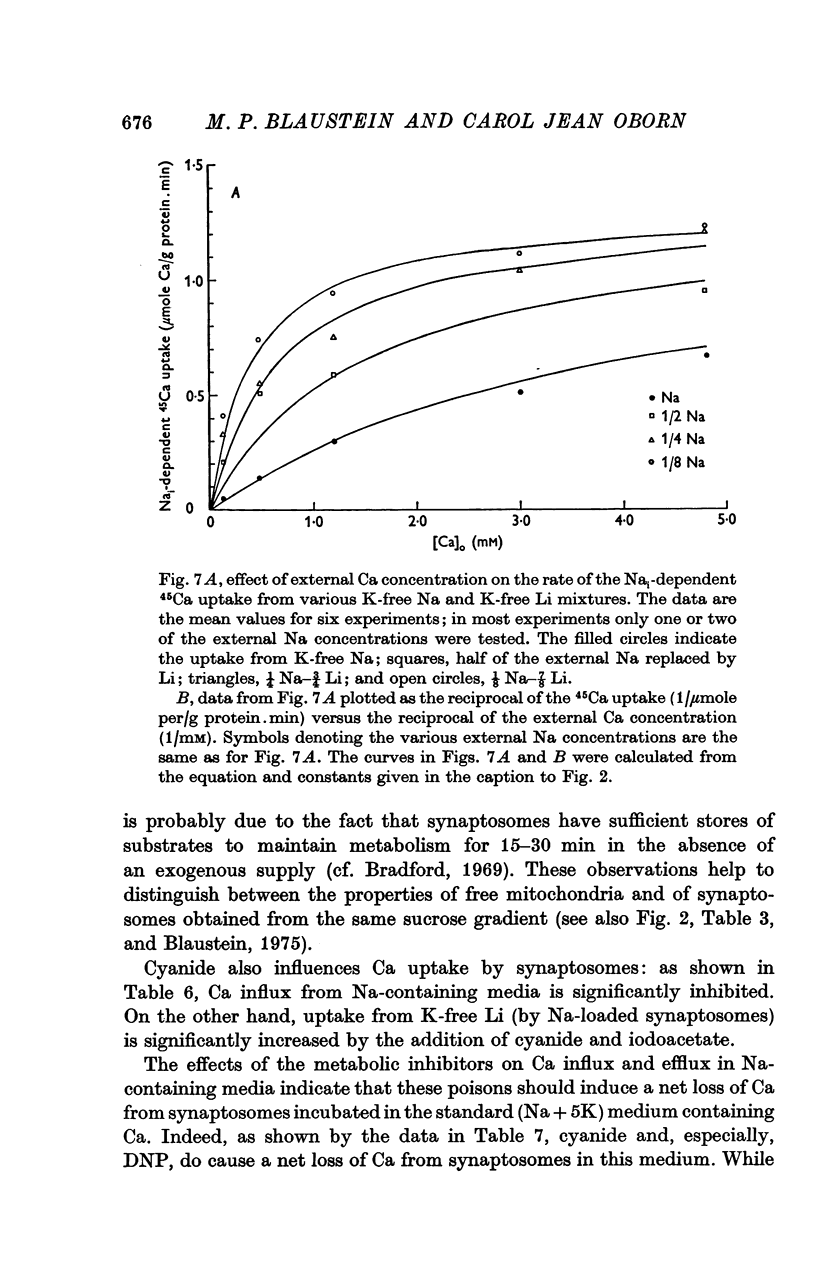
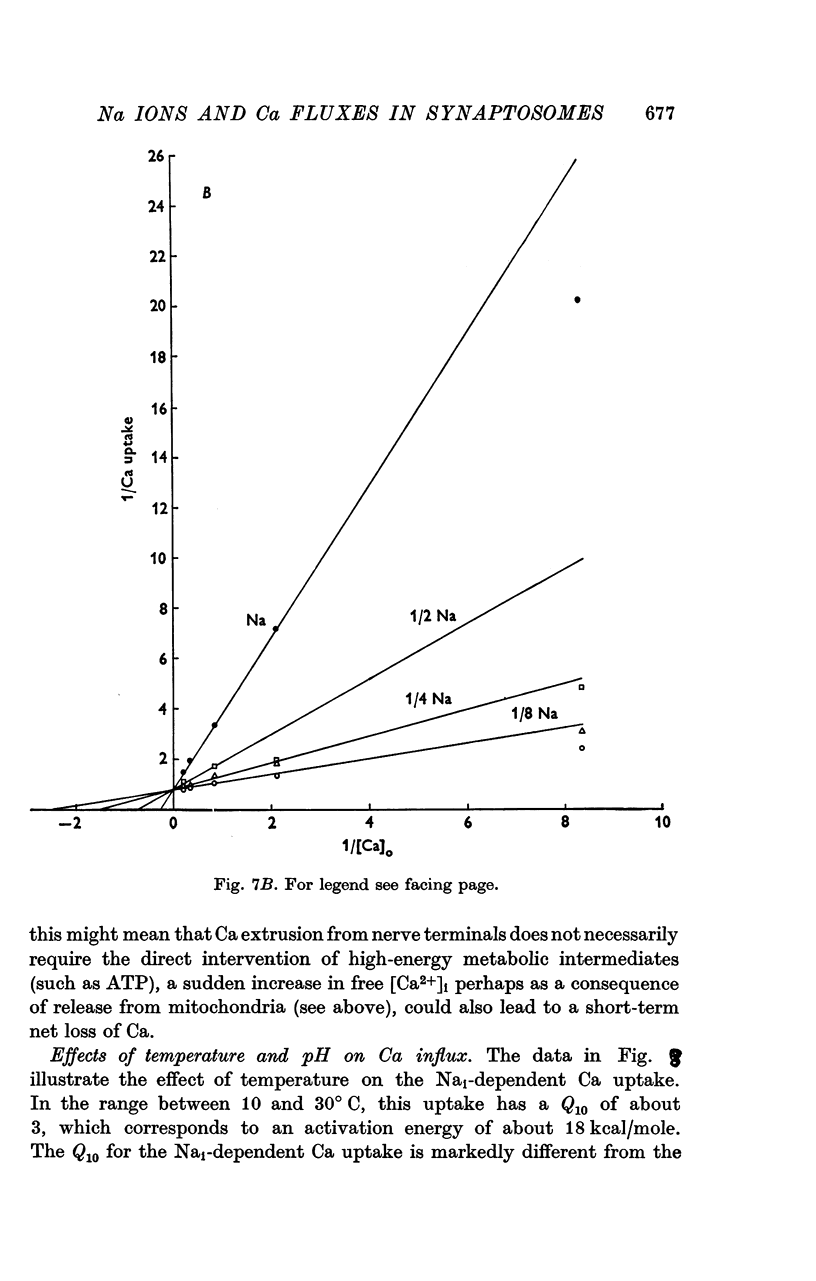
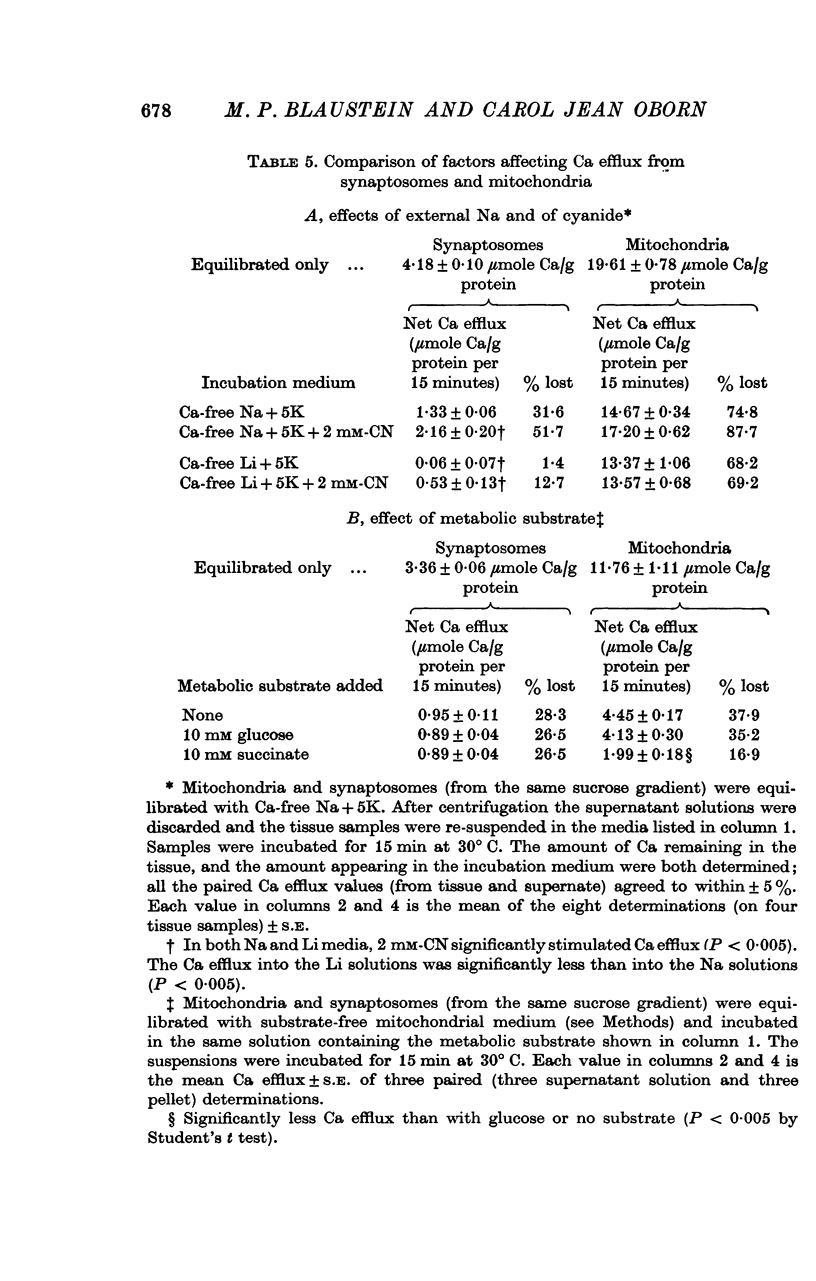
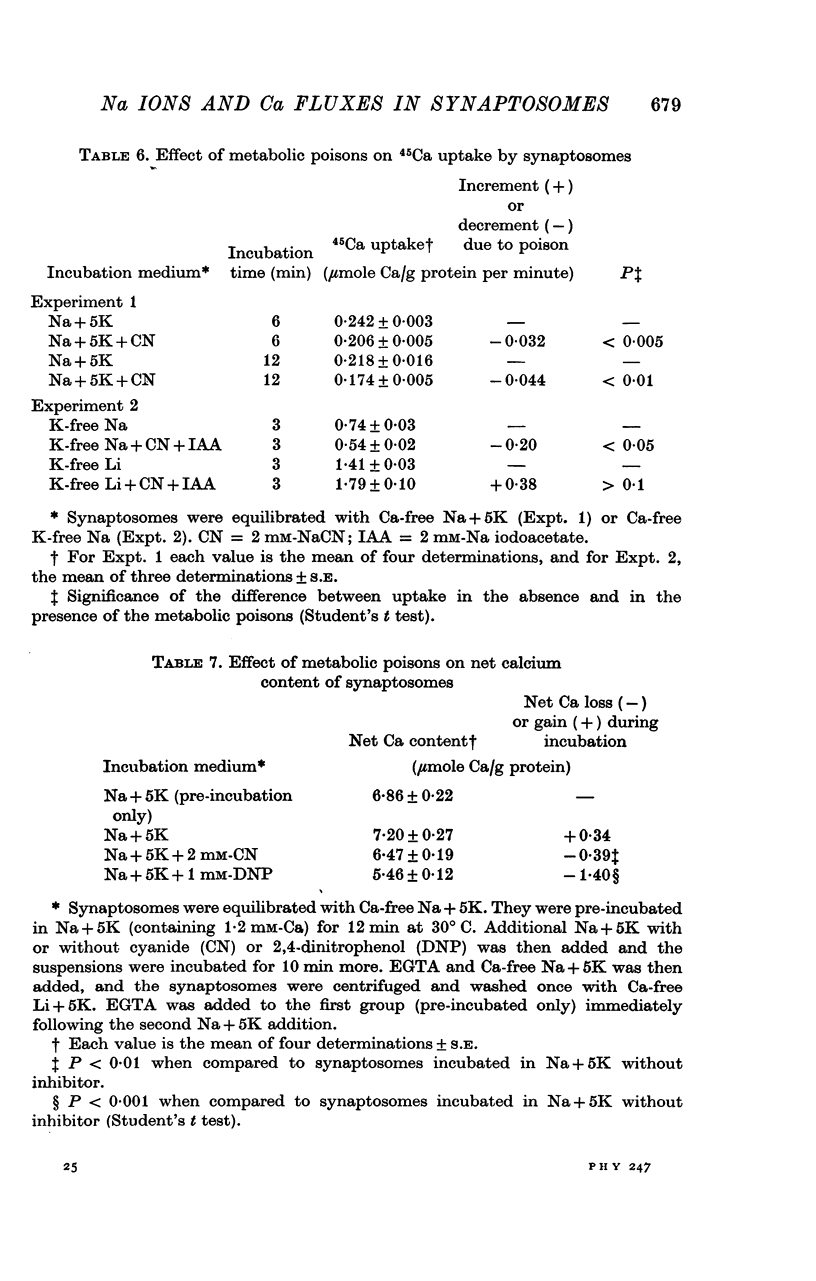
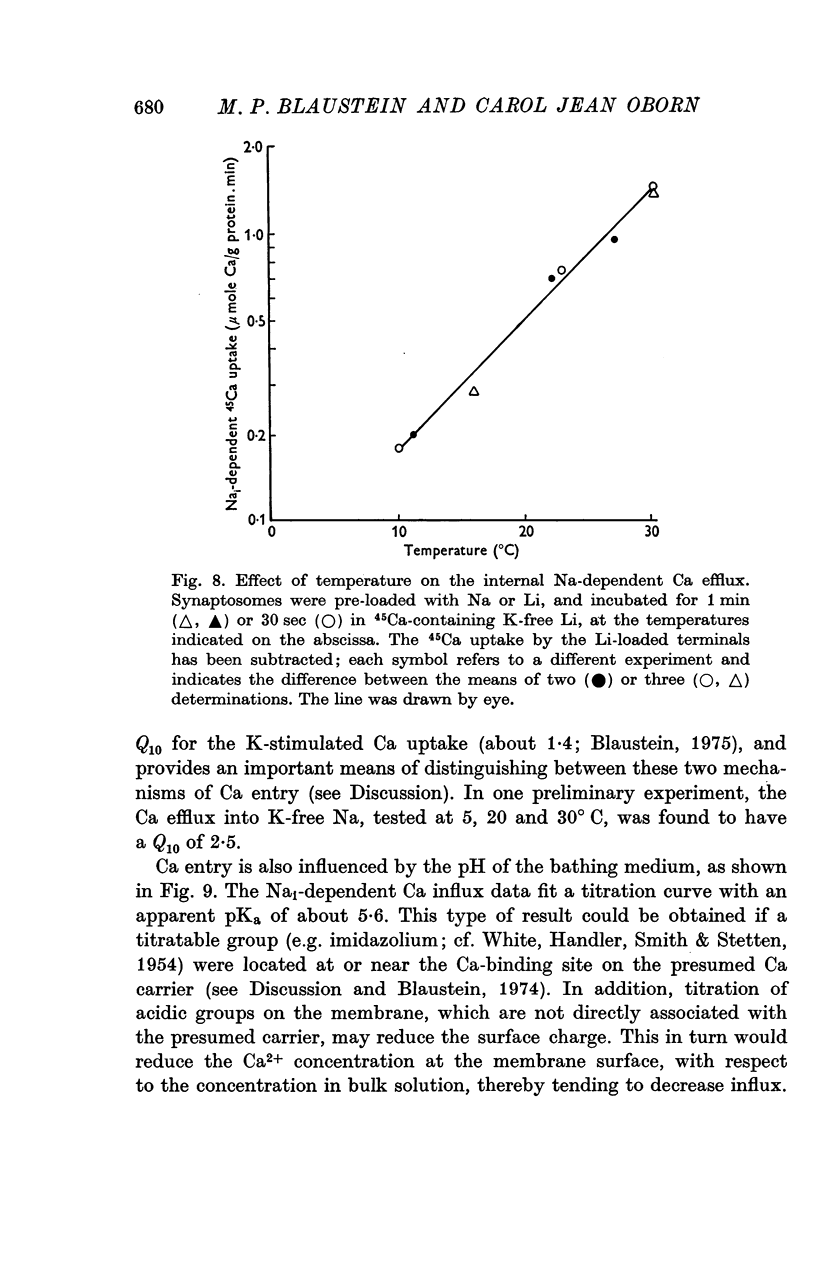
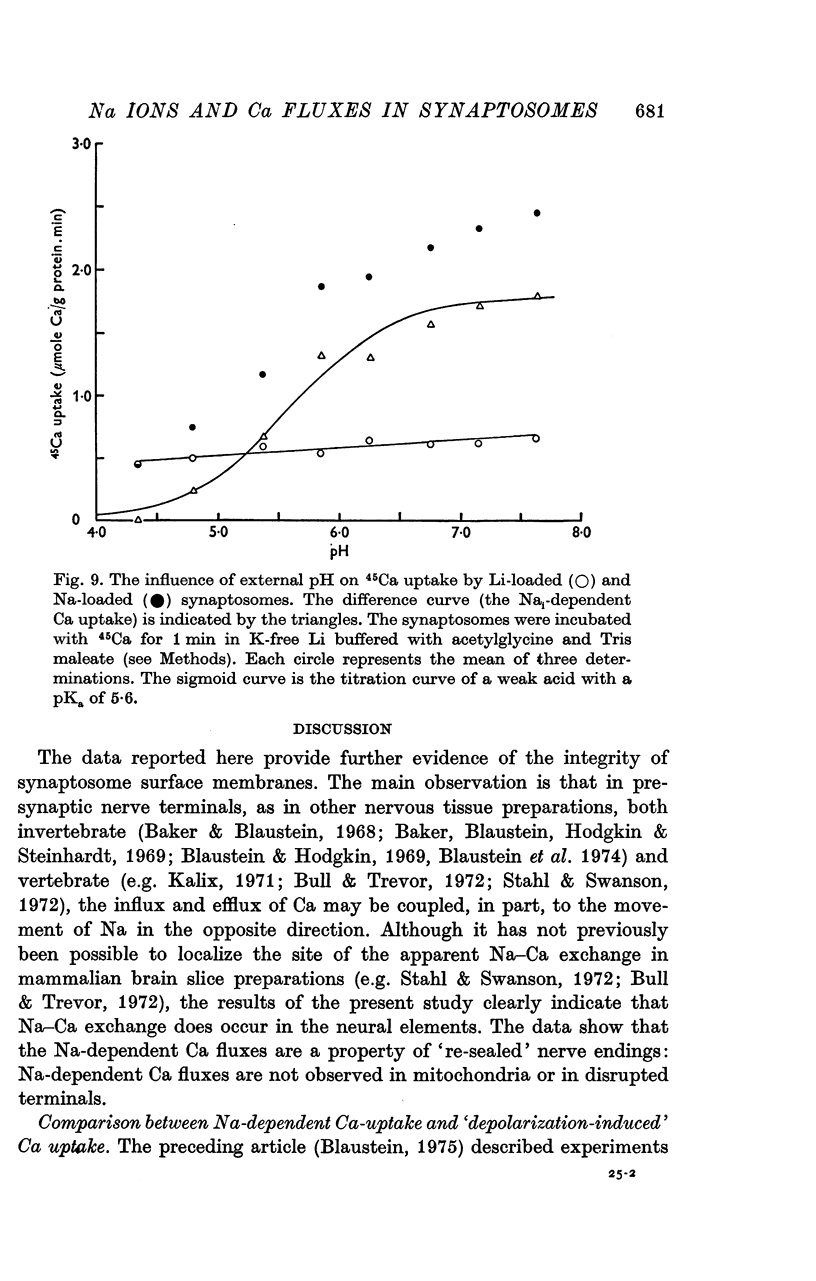
1. The influence of internal and external Na concentrations on Ca movements have been measured in pinch-off presynaptic nerve terminals (synaptosomes). Ca uptake is enhanced when external Na (Nao) is replaced by Li, choline or dextrose, in Na-loaded synaptosomes. Depletion of internal Na (Nai) abolishes the stimulatory effect of external Na removal. 2. Ca uptake from Na-depleted media is proportional to [Na]i -2, and averages about 1-5 mumole Ca/g synaptosome protein per minute when [Na]i is approximately 137 mM. This may correspond to a Ca influx of about 0-1 p-mole/cm-2 sec. 3. External Na is a competitive inhibitor of the Nai-dependent Ca uptake. The interrelationship between [Na]o, [Ca]o and Ca uptake indicate that two external Na ions may compete with one Ca at each uptake site. 4. The distribution of particles with Nai-dependent Ca uptake activity parallels the distribution of synaptosomes in the preparative sucrose gradient. Thus, this Ca uptake activity is probably a property of the pinched-off nerve terminals per se, and not of the mitochondria which may contaminate the synaptosome fraction. 5. The Nai-dependent Ca uptake mechanism requires an intact surface membrane, since synaptosomes subjected to osmotic lysis lose the ability to accumulate Ca by this route. 6. Ca efflux into Ca-free media is largely dependent upon the presence of external Na. The curve relating Ca efflux to [Na]o is sigmoid, and suggests that more than one external Na ion (perhaps 2 or 3) is needed to activate the efflux of each Ca ion. 7. The net Ca gain exhibited by Na-loaded synaptosomes incubated in Na-depleted media can be accounted for by the increased Ca uptake and decreased Ca loss observed under these conditions. 8. Treatment of synaptosomes with cyanide or 2,4-dinitrophenol decreases Ca uptake and enhances Ca efflux into Na-containing media. This results in a net loss of Ca from the terminals, even in the presence of external Ca. 9. In contrast to the Ca efflux from synaptosomes, the Ca efflux from brain mitochondria is not dependent upon external Na, and is reduced by succinate, a substrate which is known to fuel mitochondrial respiration. 10. The temperature coefficient (Q10) of the Nai-dependent Ca uptake is about 3. 11. The Nai-dependent Ca uptake is reduced at low pH. The relationship between this Ca uptake and pH approximates a titration curve with a pKa of about 5-6. 12. The data indicate that Ca transport in rat brain presynaptic terminals may involve a carrier-mediated Na-Ca exchange mechanism, and that some of the energy required for Ca extrusion may come from the Na electrochemical gradient across the surface membranes.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Effects of potassium, veratridine, and scorpion venom on calcium accumulation and transmitter release by nerve terminals in vitro. J Physiol. 1975 Jun;247(3):617–655. doi: 10.1113/jphysiol.1975.sp010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldring J. M. Membrane potentials in pinched-off presynaptic nerve ternimals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J Physiol. 1975 Jun;247(3):589–615. doi: 10.1113/jphysiol.1975.sp010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Wiesmann W. P. Effect of sodium ions on calcium movements in isolated synaptic terminals. Proc Natl Acad Sci U S A. 1970 Jul;66(3):664–671. doi: 10.1073/pnas.66.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford H. F. Respiration in vitro of synaptosomes from mammalian cerebral cortex. J Neurochem. 1969 May;16(5):675–684. doi: 10.1111/j.1471-4159.1969.tb06444.x. [DOI] [PubMed] [Google Scholar]
- Bull R. J., Trevor A. J. Sodium and the flux of calcium ions in electrically-stimulated cerebral tissue. J Neurochem. 1972 Apr;19(4):1011–1022. doi: 10.1111/j.1471-4159.1972.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Gamble R. L., Rossi C. S., Lehninger A. L. Super-stoichiometric ratios between ion movements and electron transport in rat liver mitochondria. J Biol Chem. 1967 Mar 25;242(6):1199–1204. [PubMed] [Google Scholar]
- Carafoli E., Rossi C. S. Calcium transport in mitochondria. Adv Cytopharmacol. 1971 May;1:209–227. [PubMed] [Google Scholar]
- Cooke W. J., Robinson J. D. Factors influencing calcium movements in rat brain slices. Am J Physiol. 1971 Jul;221(1):218–225. doi: 10.1152/ajplegacy.1971.221.1.218. [DOI] [PubMed] [Google Scholar]
- DRAHOTA Z., LEHNINGER A. L. MOVEMENTS OF H+, K+, AND NA+ DURING ENERGY-DEPENDENT UPTAKE AND RETENTION OF CA++ IN RAT LIVE MITOCHONDRIA. Biochem Biophys Res Commun. 1965 Apr 23;19:351–356. doi: 10.1016/0006-291x(65)90467-5. [DOI] [PubMed] [Google Scholar]
- Dipolo R. Calcium efflux from internally dialyzed squid giant axons. J Gen Physiol. 1973 Nov;62(5):575–589. doi: 10.1085/jgp.62.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escueta A. V., Appel S. H. Biochemical studies of synapses in vitro. II. Potassium transport. Biochemistry. 1969 Feb;8(2):725–733. doi: 10.1021/bi00830a038. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger A., Blomstrand C., Lehninger A. L. Comparative studies on mitochondria isolated from neuron-enriched and glia-enriched fractions of rabbit and beef brain. J Cell Biol. 1970 May;45(2):221–234. doi: 10.1083/jcb.45.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalix P. Uptake and release of calcium in rabbit vagus nerve. Pflugers Arch. 1971;326(1):1–14. doi: 10.1007/BF00586791. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOLLEY R. N. THE CALCIUM CONTENT OF ISOLATED CEREBRAL TISSUES AND THEIR STEADY-STATE EXCHANGE OF CALCIUM. J Neurochem. 1963 Sep;10:665–676. doi: 10.1111/j.1471-4159.1963.tb08938.x. [DOI] [PubMed] [Google Scholar]
- Lazarewicz J. W., Haljamäe H., Hamberger A. Calcium metabolism in isolated brain cells and subcellular fractions. J Neurochem. 1974 Jan;22(1):33–45. doi: 10.1111/j.1471-4159.1974.tb12176.x. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C. M., Abdel-Latif A. A. Studies on sodium transport in rat brain nerve-ending particles. J Neurochem. 1968 Aug;15(8):721–729. doi: 10.1111/j.1471-4159.1968.tb10316.x. [DOI] [PubMed] [Google Scholar]
- MICHAELSON I. A., WHITTAKER V. P. The subcellular localization of 5-hydroxytryptamine in guinea pig brain. Biochem Pharmacol. 1963 Feb;12:203–211. doi: 10.1016/0006-2952(63)90185-0. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl W. L., Swanson P. D. Calcium movements in brain slices in low sodium or calcium media. J Neurochem. 1972 Oct;19(10):2395–2407. doi: 10.1111/j.1471-4159.1972.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Tower D. B. Ouabain and the distribution of calcium and magnesium in cerebral tissues in vitro. Exp Brain Res. 1968;6(4):273–283. doi: 10.1007/BF00233179. [DOI] [PubMed] [Google Scholar]
- WILBRANDT W., ROSENBERG T. The concept of carrier transport and its corollaries in pharmacology. Pharmacol Rev. 1961 Jun;13:109–183. [PubMed] [Google Scholar]


