Abstract
Mycobacterium tuberculosis complex isolates from cerebrospinal fluid of 67 meningitis patients were obtained from six fever hospitals in Egypt. One M. bovis and 66 M. tuberculosis isolates were identified by PCR-restriction fragment length polymorphism (RFLP) analysis of oxyR. Among the M. tuberculosis isolates, 53 unique strain types (with 3 to 16 copies of IS6110) were found by RFLP analyses. Nine clusters (eight with two isolates each and one with six isolates) were also found. Thirty-six spoligotypes, including at least 10 that have been previously reported from other countries, were also observed. Forty-one (62.1%) of the isolates were in spoligotype clusters, and 22 (33%) of the isolates were in RFLP clusters. Fifty-one of the isolates were susceptible in vitro to all of the antituberculosis drugs tested, 11 were monoresistant to capreomycin, rifampin, isoniazid (INH), pyrazinamide, or streptomycin (STR), 4 were resistant to STR and INH, and 1 was resistant to STR, INH, and ethambutol.
Tuberculosis continues to pose serious public health threats, especially in developing countries. Tuberculous meningitis (TBM) is life threatening and is the most common form of central nervous system tuberculosis (5). The disease is less common in technologically developed countries but is a serious cause of death and disease among persons, particularly children, in developing countries (11). Nearly one-half of the children admitted to a reference hospital in Bombay, India, in the 1970s with a diagnosis of tuberculosis also had central nervous system complications (20). Although implementation of appropriate treatment during the early phase of TBM can significantly improve the outcome, high mortality rates (which average 27%) are related to the variable clinical presentations and inefficient diagnosis (5). Acid-fast microscopy of cerebrospinal fluid (CSF) specimens is positive for 5 to 25% of TBM patients, but a concentration of ≥104 bacilli/ml is required for reliable detection of tubercle bacilli (5). Positive cultures of Mycobacterium tuberculosis are obtainable in 60 to 75% of patients, but the combined use of microscopy and culture of several CSF samples provides optimal laboratory diagnosis of TBM (5, 11). The sensitivity of PCR for detection of M. tuberculosis complex (MTC) DNA in CSF specimens is 33 to 85% based upon results from four studies recently cited in a review of nucleic amplification methods for mycobacterial diagnosis by Pfyffer (12).
M. tuberculosis was identified as the etiologic organism in 19.7% of 7,809 patients with infectious neurologic disease at the Abbassia Fever Hospital in Cairo, Egypt, from 1966 to 1989. This prevalence was second only to that of meningococci, and TBM was the most frequent cause of death at this hospital (6). In a subsequent study of 1,430 patients with a presumptive diagnosis of TBM at the same hospital from 1976 to 1996, MTC organisms were cultured from CSF samples of 857 (59.9%) of the patients. Drug susceptibility testing of 150 of these isolates (all M. tuberculosis) showed that 3% were resistant to rifampin (RIF), 7% were resistant to ethambutol (EMB), and 10% were resistant to isoniazid (INH); none were resistant to pyrazinamide (PZA) or multidrug resistant (7). In 1998, a joint effort was undertaken by U.S. Naval Medical Research Unit No. 3, the Centers for Disease Control and Prevention (CDC), and the Field Epidemiology Training Program within the Egyptian Ministry of Health and Population (MOHP) to develop a more effective surveillance network for patients with meningitis and encephalitis in six Egyptian fever hospitals and to strengthen the capacity of MOHP to conduct infectious disease outbreak investigations and surveillance. Laboratory studies aimed at identifying specific strains of M. tuberculosis are an important component of this project, since they allow outbreaks to be tracked with greater precision. Previous typing data have shown, overall, that there are greater degrees of strain diversity in regions such as western Europe, where the incidence of tuberculosis is low and the majority of cases result from reactivation of existing infections (18). Less strain diversity is typically observed in countries with high tuberculosis case rates due to active transmission (8, 19).
The standard method for typing of M. tuberculosis strains is restriction fragment length polymorphism (RFLP) analysis based on the insertion element IS6110 (15, 16). A more recently described alternative to the IS6110 RFLP method is spoligotyping (spacer oligonucleotide typing). This method is based on polymorphisms in the MTC direct repeat (DR) chromosomal region consisting of identical 36-bp DRs alternating with 35- to 41-bp unique spacer sequences. Although spoligotyping is PCR based, faster, and easier to perform than IS6110 RFLP analysis (9), it has less ability to discriminate among M. tuberculosis strains (19). Spoligotyping used along with RFLP analysis has, however, been recently proposed as the definitive method by which to differentiate strains of M. tuberculosis that contain fewer than five copies of IS6110 (14).
In this study, we obtained isolates of mycobacteria from CSF that were available from the 1998 TBM joint surveillance project to establish a database of strain types and antimicrobial susceptibility patterns.
MATERIALS AND METHODS
Bacterial isolates.
Sixty-seven clinical isolates (one isolate per patient) were included in this study. All specimens were isolated from 1998 to 2000 from CSF samples from meningitis patients in six major fever hospitals in Egypt. Fifty (74.6%) of the isolates were from patients at Abbassia Hospital in Cairo, five each from were from hospitals in Alexandria and Assiut, four were from a hospital in Imbaba, two were from a hospital in Malawy, and one was from a hospital in Fayium. The hospitals were located in cities that are 100 to 375 km from Cairo, with the exception of those in Cairo and Imbaba (a suburb of Cairo). Primary isolation and identification of MTC were performed at the MOHP laboratory, and cultures were sent to CDC in a coded fashion with all patient identifiers removed and subsequent to patient discharge.
Nucleic acid procedures.
Crude lysates containing DNA for PCR amplification were prepared from cultures by agitation of cells in the presence of siliconized glass beads (13). Genomic DNA for insertion element IS6110 RFLP analyses was prepared as previously described (16, 17).
Identification of a 123-bp region of IS6110 was performed by PCR with primers described by Eisenach et al. (3). Reactions contained 12.5 μl of HotStarTaq polymerase Master Mix, which includes Taq DNA polymerase and nucleotides (Qiagen, Inc., Chatsworth, Calif.); 1 μl of template DNA (10 μl of CSF extracts); and 0.5 μmol of each primer in a total volume of 50 μl. Thermal cycling was performed with a Gene-Amp PCR System 2400 Thermocycler (Perkin-Elmer, Inc., Foster City, Calif.) set for 15 min at 95°C; 35 cycles of 30 s at 95°C, 30 s at 64°C, and 30 s at 72°C; and a final incubation for 10 min at 72°C. Isolates were further characterized by PCR-RFLP analysis of a region of oxyR as previously described (15). Isolates of M. tuberculosis were typed by the standard IS6110 RFLP method (16). Spoligotyping of M. tuberculosis isolates was performed as previously described (9). In brief, the DR region was amplified by PCR with primers derived from the DR sequence. The amplified DNA was hybridized to a set of 43 immobilized oligonucleotides derived from the spacer sequences of M. bovis BCG strain P3 and M. tuberculosis strain H37Rv by reverse line blotting. Hybridization membranes were charged nylon (Biodyne C) obtained from Pall Biosupport, Portsmouth, United Kingdom, and were activated as previously described (9). The 43-digit binary result was converted to a 15-digit octal designation as previously described (2).
Antimicrobial susceptibility testing.
Isolates of MTC were tested at CDC by the modified method of proportions (10) with Middlebrook and Cohn 7H10 agar plates containing the drugs RIF (1.0 μg/ml), INH (0.2, 1.0, and 5.0 μg/ml), streptomycin (STR) (2.0 and 10.0 μg/ml), capreomycin (CAP) (2.0 μg/ml), PZA (25.0 μg/ml), kanamycin (5.0 μg/ml), ethionamide (10.0 μg/ml), ciprofloxacin (2.0 μg/ml), cycloserine (30.0 μg/ml), and EMB (5.0 μg/ml). Statistical evaluation was performed as previously described (1).
RESULTS
MTC was cultured from CSF samples from 67 patients with a presumptive diagnosis of TBM from six reference fever hospitals in Egypt. All isolates contained IS6110 based upon PCR amplification of a 123-bp region of the insertion element. One isolate was determined to be M. bovis, and 66 were M. tuberculosis based upon PCR-RFLP analyses of oxyR.
Fifty-one isolates (76.1%) were susceptible to all 10 of the drugs tested, and 11 (16.4%) were monoresistant to STR, RIF, INH, PZA, or CAP (Table 1). The only isolate with PZA resistance was the M. bovis isolate. Four isolates were resistant to SM and INH (6%), and one isolate (1.5%) was resistant to INH, SM, and EMB (Table 1). Resistance to STR was low in level, i.e., MICs of >2 to 10 μg/ml. Resistance to INH was high in level (MIC, >5 μg/ml) in one isolate, intermediate (MIC, >1 to 5 μg/ml) in four isolates, and low in level (MIC, >0.2 to 1 μg/ml) in two isolates.
TABLE 1.
Antimicrobial susceptibility patterns of MTC isolates from CSF
| Resistance patterna | No. of isolates | RFLP pattern(s)b |
|---|---|---|
| RIF | 1 | 5 |
| INH | 2 | 24, 28 |
| CAP | 2 | 9, 41 |
| STR | 5 | 1, 7, 8, 35, 44 |
| PZA | 1c | M. bovis |
| INH + STR | 4 | 4, 22, 30, 37 |
| INH + STR + EMB | 1 | 48 |
| None | 51 | Multipled |
| Total | 67 |
The drugs (concentrations) tested were RIF (1.0 μg/ml), INH (0.2, 1.0, and 5.0 μg/ml), CAP (10.0 μg/ml), STR (2.0 and 10.0 μg/ml), PZA (25.0 μg/ml), kanamycin (5.0 μg/ml), ethionamide (10.0 μg/ml), ciprofloxacin (2.0 μg/ml), cycloserine (30.0 μg/ml), and EMB (5.0 μg/ml).\
See Fig. 1.
M. bovis.
38 RFLP patterns.
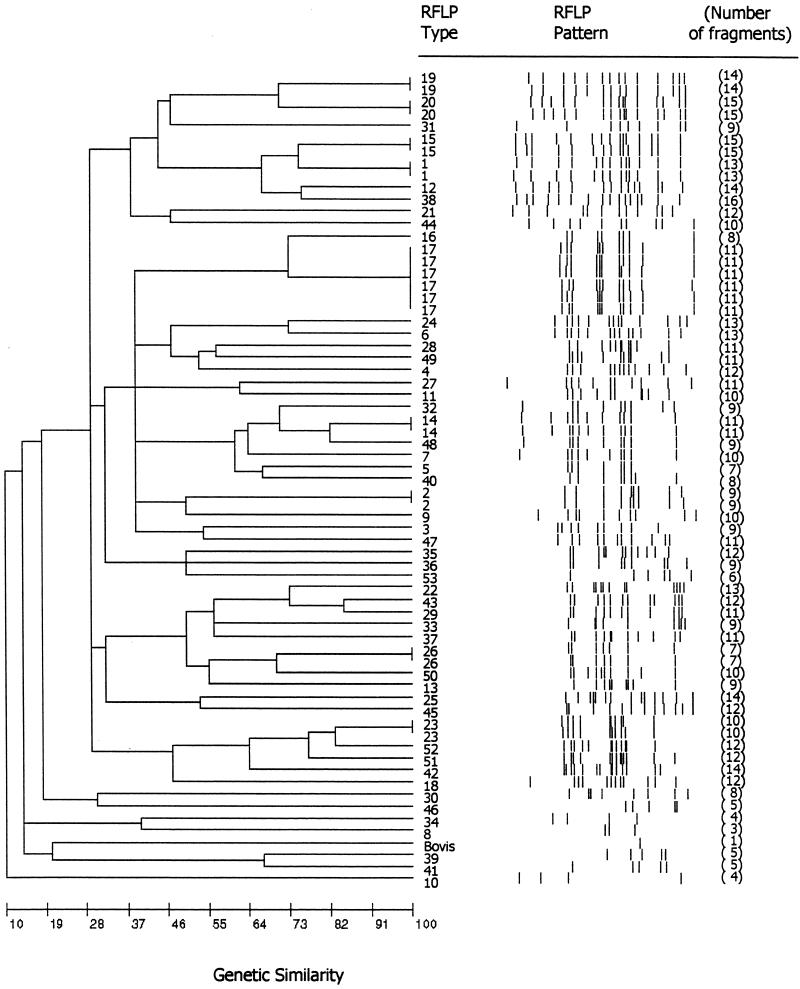
Fifty-three IS6110 RFLP patterns containing 3 to 16 bands were observed among the 66 M. tuberculosis isolates (Fig. 1). Forty-four unique strain types and nine clusters (eight with two isolates each and one with six isolates) were observed. Except for clusters, none of the isolates was more than 85% related to other isolates based upon the genetic distance revealed in the dendrogram (Fig. 1). Five of the six isolates with RFLP pattern 17 were from Abbassia Hospital, and the sixth isolate in this cluster was from a hospital in Alexandria. No additional information was available regarding any relationships among the six patients whose isolates had this pattern. All two-isolate clusters, except pattern 20, also contained isolates from Abbassia Hospital. One of the isolates with pattern 20 was from Abbassia Hospital, and the other was from the hospital in Imbaba. The 53 patterns were compared with those in a database containing more than 6,000 distinct patterns from U.S. isolates maintained at CDC, and only 3 (patterns 4, 5, and 26) showed similarities (i.e., pattern differences of no more than one band). Thirty-six spoligotypes were observed among the isolates (Table 2). The 2 most common patterns, no. 1 and 5, were found among 8 and 14 isolates, respectively, and the remaining 34 patterns were found in 1 to 3 isolates each. RFLP clusters were not further subdivided by spoligotyping. Isolates from four of the RFLP clusters that contained two isolates were spoligotype pattern 1. A different spoligotype was found for each of the remaining four RFLP clusters that contained two isolates, and the six isolates with RFLP pattern 17 were spoligotype 5. Nine RFLP patterns, including pattern 17, were found among isolates with the most common spoligotype (pattern 5), and four RFLP patterns were found among the eight isolates with spoligotype pattern 1. Spoligotype patterns 1, 5, 14, 17, 21, and 31 were also found among our collection of 25 isolates from pulmonary tuberculosis patients at Assiut University Hospital, which is 375 km south of Cairo (1). None of the RFLP patterns among the Assiut isolates, however, matched those found among the CSF isolates. Ten spoligotypes (no. 1, 5, 6, 15, 17, 22, 27, 31, 32, and 35) were present in the Rijksinstitut voor Volksgsondheit en Milieuhygiene international database (National Institute of Public Health and the Environment, Bilthoven, The Netherlands) (17). These patterns were reported from 1 to 16 countries (but not from Egypt), and spoligotypes 6 and 5 were reported from the most countries (13 and 16 countries, respectively). Spoligotypes 5, 6, 31, and 32 were reported in the Rijksinstitut voor Volksgsondheit en Milieuhygiene database from three African countries, and types 1, 5, and 15 were reported from Iran. The 22 isolates with the most common spoligotypes (no. 1 and 5) were susceptible to the 10 antimicrobial agents tested, and no isolates with matching susceptibility patterns shared any specific RFLP pattern. The five isolates with resistance to more than one drug were unrelated by RFLP analyses (Table 1).
FIG. 1.
Dendrogram, produced by BioImage whole-band analysis V.3.42 software (Genomic Solutions, Ann Arbor, Mich.), showing the relationship of 53 RFLP patterns identified in 67 MTC isolates from CSF cultures. RFLP patterns were compared by using the Jaccard matching method with an allowed band size deviation of 2.5%, and the dendrogram showing the relationships between patterns was generated with the unweighted pair group method using arithmetic averages.
TABLE 2.
Spoligotypes of 66 M. tuberculosis isolates from CSF cultures from meningitis patients at six Egyptian fever hospitals
| Spoligo- type | No. of isolates | Spoligotypea | RFLP type(s) (no. of isolates if >1) |
|---|---|---|---|
| 1b | 8 | 703777740003171 | 1 (2), 15 (2), 19 (2), 20 (2) |
| 2 | 2 | 577767777760771 | 2 (2) |
| 3 | 2 | 777767777620771 | 3, 47 |
| 4 | 2 | 777767607760771 | 4, 42 |
| 5b | 14 | 777777777760771 | 5, 7, 16, 17 (6), 25, 28, 29, 45, 53 |
| 6 | 3 | 777777607760771 | 6, 23 (2) |
| 7 | 1 | 777767404760771 | 8 |
| 8 | 1 | 777727637760771 | 9 |
| 9 | 1 | 777767637760771 | 10 |
| 10 | 1 | 777701007760771 | 11 |
| 11 | 1 | 577747404760671 | 12 |
| 12 | 1 | 000000007760770 | 13 |
| 13 | 2 | 773777777740171 | 14 (2) |
| 14b | 1 | 716377777760771 | 18 |
| 15 | 1 | 703777400001771 | 21 |
| 16 | 2 | 776367377760771 | 22, 33 |
| 17b | 1 | 377777607760771 | 24 |
| 18 | 2 | 516347777760771 | 26 (2) |
| 19 | 1 | 711762007760771 | 27 |
| 20 | 1 | 011203777760671 | 30 |
| 21 | 1 | 777777000360771 | 31 |
| 22 | 1 | 777777404760771 | 32 |
| 23 | 1 | 701773760003171 | 34 |
| 24 | 1 | 777727777760771 | 35 |
| 25 | 2 | 777767777760771 | 36, 51 |
| 26 | 1 | 514347776560761 | 37 |
| 27 | 1 | 777777757760771 | 38 |
| 28 | 1 | 777767770000000 | 39 |
| 29 | 2 | 437767777760771 | 40, 48 |
| 30 | 1 | 534307637760771 | 41 |
| 31b | 1 | 776377777760771 | 43 |
| 32b | 1 | 777737777760731 | 44 |
| 33 | 1 | 761767776000371 | 46 |
| 34 | 1 | 731047436540661 | 49 |
| 35 | 1 | 777767774020771 | 50 |
| 36 | 1 | 011340607740661 | 52 |
| Total | 66 | 53 |
15-digit octal code (2).\
Type found among Assiut isolates (1).
DISCUSSION
Among the 67 MTC isolates from CSF samples obtained from six national fever hospitals in Egypt, 66 were identified as M. tuberculosis and 1 was identified as M. bovis by PCR-RFLP analyses of oxyR. This finding suggests that M. bovis plays a minor role, compared to M. tuberculosis, in the etiology of TBM in Egypt. We found 53 IS6110 RFLP patterns among the 66 M. tuberculosis isolates. Three of these patterns may be considered low in copy number (fewer than five bands). This overall diversity of strains may not be surprising considering that these reference institutions accept patients with preexisting conditions from a variety of locations. Nonetheless, 22 isolates (33%) were in nine RFLP clusters, 6 isolates (9%) were in one cluster, and the remaining 16 isolates were in eight clusters consisting of 2 isolates each. This low level of strain relatedness is consistent with rates found in regions with low incidences of tuberculosis and contrasts rather sharply with the high degrees of clustering previously reported in two other regions of Africa. In Ethiopia and Tunisia, 52 and 62% of the M. tuberculosis isolates studied, respectively, were found to belong to three or four genotypic families (8). The relationships of the patients in our study from whom clustered isolates were obtained is unknown, other than the common diagnosis of meningitis, which most often is a sequela of pulmonary tuberculosis (19).
Strain identification by spoligotyping was less definitive than RFLP typing, since 41 (62.1%) of the 66 isolates were in spoligotype clusters: three clusters with 14, 8, and 3 isolates each and eight clusters with 2 isolates each. The spoligotyping procedure, however, may be more easily implemented than RFLP typing in laboratories without M. tuberculosis typing capabilities.
No clustering of resistant strains by RFLP analyses or spoligotyping was observed among the 67 isolates studied. The overall antimicrobial susceptibilities were similar to those reported among 857 MTC isolates at Abbassia Hospital from 1976 to 1996 (7). Less single-drug resistance to RIF and INH was found among our isolates, compared to 150 isolates tested in the previous study, and no PZA or EMB resistance was found (for RIF and INH, the P values were 0.48 and 0.17, respectively). Five of our isolates were resistant to more than one drug, but none was related by RFLP analyses and no multidrug-resistant isolates (as defined by resistance to at least INH and RIF) were found in the previous study (7). The overall prevalence of resistance among these isolates was lower than that previously reported in 1996 by the Egyptian National Tuberculosis Program (ENTP) for pulmonary isolates (4). Among 250 isolates tested in the ENTP study, 55.2% were resistant, compared to 23.9% of our isolates. Resistance to INH, however, was more common among our isolates (10.4%) than among the ENTP isolates (6.4%) (PINH = 0.10). A considerably higher prevalence of multidrug resistance was also found among the pulmonary M. tuberculosis isolates we examined previously that were from patients at the Assiut University Hospital. All of the patients in the Assiut study had been treated for tuberculosis for at least 1 year, and the prevalence of multidrug resistance was 44%, compared to 0% among the meningitis patient isolates. Continued surveillance for strains of M. tuberculosis involved in TBM in Egypt, especially for drug-resistant strains, is warranted.
REFERENCES
- 1.Abbadi, S., H. G. Rasheed, G. P. Morlock, C. L. Woodley, O. El Shanawy, and R. C. Cooksey. 2001. Characterization of IS6110 restriction fragment length polymorphism patterns and mechanisms of antimicrobial resistance for multidrug-resistant isolates of Mycobacterium tuberculosis from a major reference hospital in Assiut, Egypt. J. Clin. Microbiol. 39:2330-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dale, J. W., D. Brittain, A. A. Cataldi, D. Cousins, J. T. Crawford, J. Driscoll, H. Heersma, T. Lillebaek, T. Quitugua, N. Rastogi, R. A. Skuce, C. Sola, D. van Soolingen, and V. Vincent. 2001. Spacer oligonucleotide typing of bacteria of the Mycobacterium tuberculosis complex: recommendations for standard nomenclature. Int. J. Tuber. Lung Dis. 5:216-219. [PubMed] [Google Scholar]
- 3.Eisenach, K. D., M. D. Cave, J. H. Bates, and J. T. Crawford. 1990. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J. Infect. Dis. 161:977-981. [DOI] [PubMed] [Google Scholar]
- 4.el Moghazy, E. 1997. National tuberculosis program report: epidemiological review and action plan. Egyptian Ministry of Health, Cairo.
- 5.Garcia-Monco, J. C. 1999. Central nervous system tuberculosis. Neurol. Clin. 17:737-759. [DOI] [PubMed] [Google Scholar]
- 6.Girgis, N. I., J. E. Sippel, M. E. Kilpatrick, W. R. Sanborn, I. A. Mikhail, E. Cross, M. W. Erian, Y. Sultan, and Z. Farid. 1993. Meningitis and encephalitis at the Abbassia Fever Hospital, Cairo, Egypt, from 1966 to 1989. Am. J. Trop. Hyg. 48:97-107. [DOI] [PubMed] [Google Scholar]
- 7.Girgis, N., Y. Sultan, Z. Farid, M. Mansour, M. Erian, L. Hanna, and A. Mateczun. 1998. Tuberculous meningitis, Abbassia Fever Hospital-Naval Medical Research Unit No. 3-Cairo, Egypt, from 1976 to 1996. Am. J. Trop. Med. Hyg. 58:28-34. [DOI] [PubMed] [Google Scholar]
- 8.Hermans, P. W., F. Messadi, H. Guebrexabher, D. van Soolingen, P. E. de Haas, H. Heersma, H. de Neeling, A. Ayoub, F. Portaels, and D. Frommel. 1995. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia, and The Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J. Infect. Dis. 171:1504-1513. [DOI] [PubMed] [Google Scholar]
- 9.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveid, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology: a guide for the level III laboratory. U.S. Department of Health and Human Services publication no.86-8230., p. 159-184. U. S. Department of Health and Human Services, Washington, D.C.
- 11.Leonard, J. M., and R. M. Des Prez. 1990. Tuberculous meningitis. Infect. Dis. Clin. N. Am. 4:769-787. [PubMed] [Google Scholar]
- 12.Pfyffer, G. E. 1999. Nucleic acid amplification for mycobacterial diagnosis. J. Infect. 39:21-26. [DOI] [PubMed] [Google Scholar]
- 13.Plikaytis, B. B., R. H. Gelber, and T. M. Shinnick. 1990. Rapid and sensitive detection of Mycobacterium leprae using a nested-primer gene amplification assay. J. Clin. Microbiol. 28:1913-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soini, H., X. Pan, L. Teeter, J. M. Musser, and E. A. Graviss. 2001. Transmission dynamics and molecular characterization of Mycobacterium tuberculosis isolates with low copy numbers of IS6110. J. Clin. Microbiol. 39:217-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sreevatsan, S., P. Escalante, X. Pan, D. Gillies, S. Siddiqui, C. Khalaf, B. Kreiswirth, P. Bifani, L. Adams, T. Ficht, V. Perumaalla, M. Cave, J. van Embden, and J. Musser. 1996. Identification of a polymorphic nucleotide in oxyR specific for Mycobacterium bovis. J. Clin. Microbiol. 34:2007-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, and T. M. Shinnick. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Soolingen, D., P. W. M. Hermans, P. E. W. De Haas, D. R. Soll, and J. D. A. van Embden. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Soolingen, D., M. W. Borgdorff, P. E. W. de Haas, K. Kremer, J. Veen, M. G. G. Sebek, J. Veen, M. Dessens, K. Kremer, and J. D. A. van Embden. 1999. Molecular epidemiology of tuberculosis in the Netherlands: a nationwide study during 1993 through 1997. J. Infect. Dis. 180:726-736. [DOI] [PubMed] [Google Scholar]
- 19.van Soolingen, D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J. Intern. Med. 249:1-26. [DOI] [PubMed] [Google Scholar]
- 20.Zuger, A., and F. D. Lowy. 1991. Tuberculosis of the central nervous system, p. 425-455. In W.M. Sheld, R. J. Whitley, and D. T. Durack (ed.), Infections of the central nervous system. Raven Press, Ltd., New York, N.Y.