Abstract
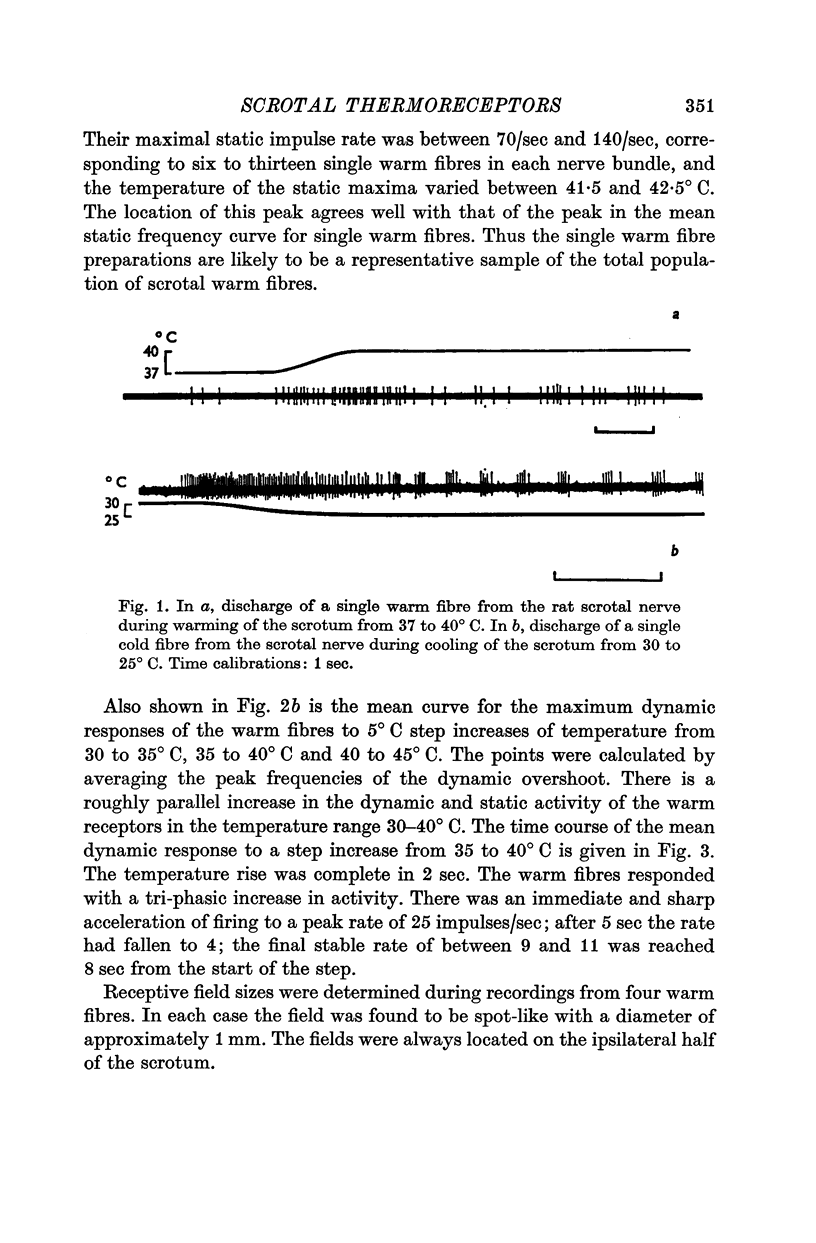
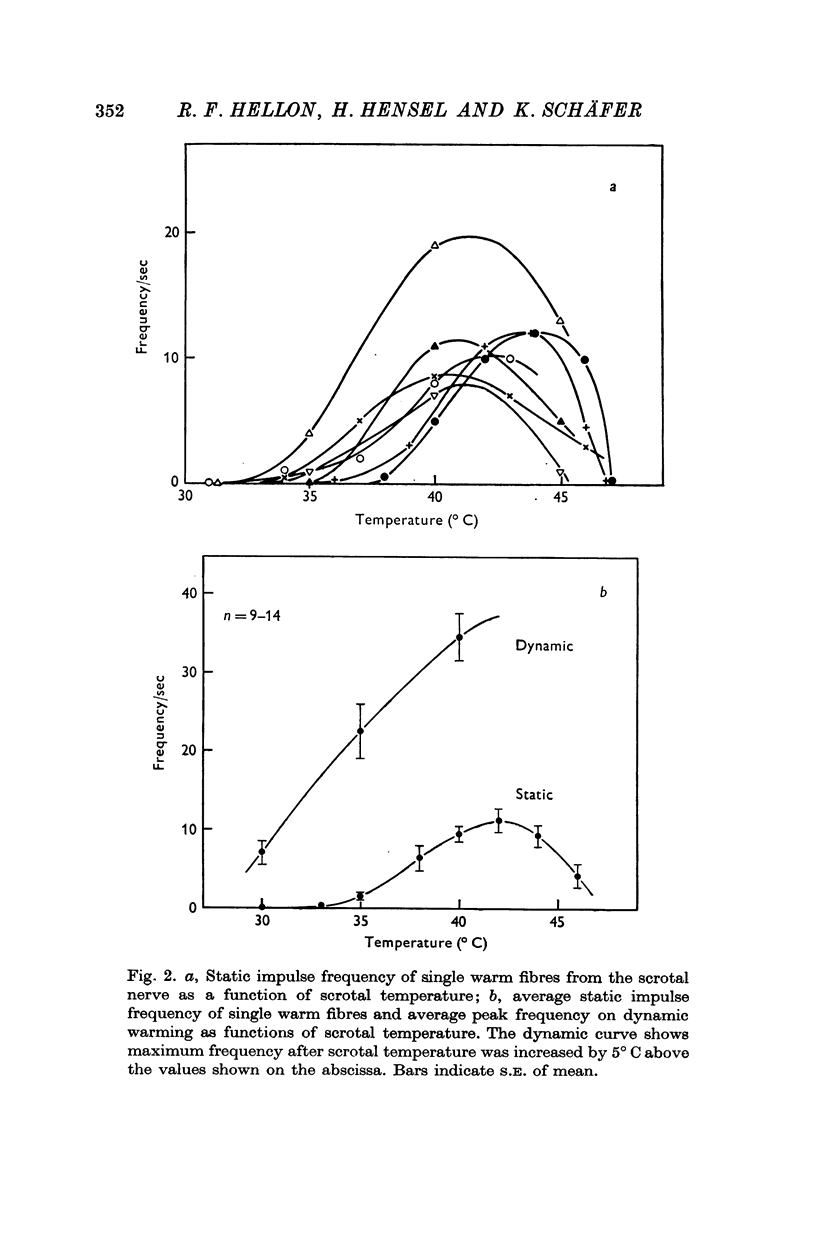
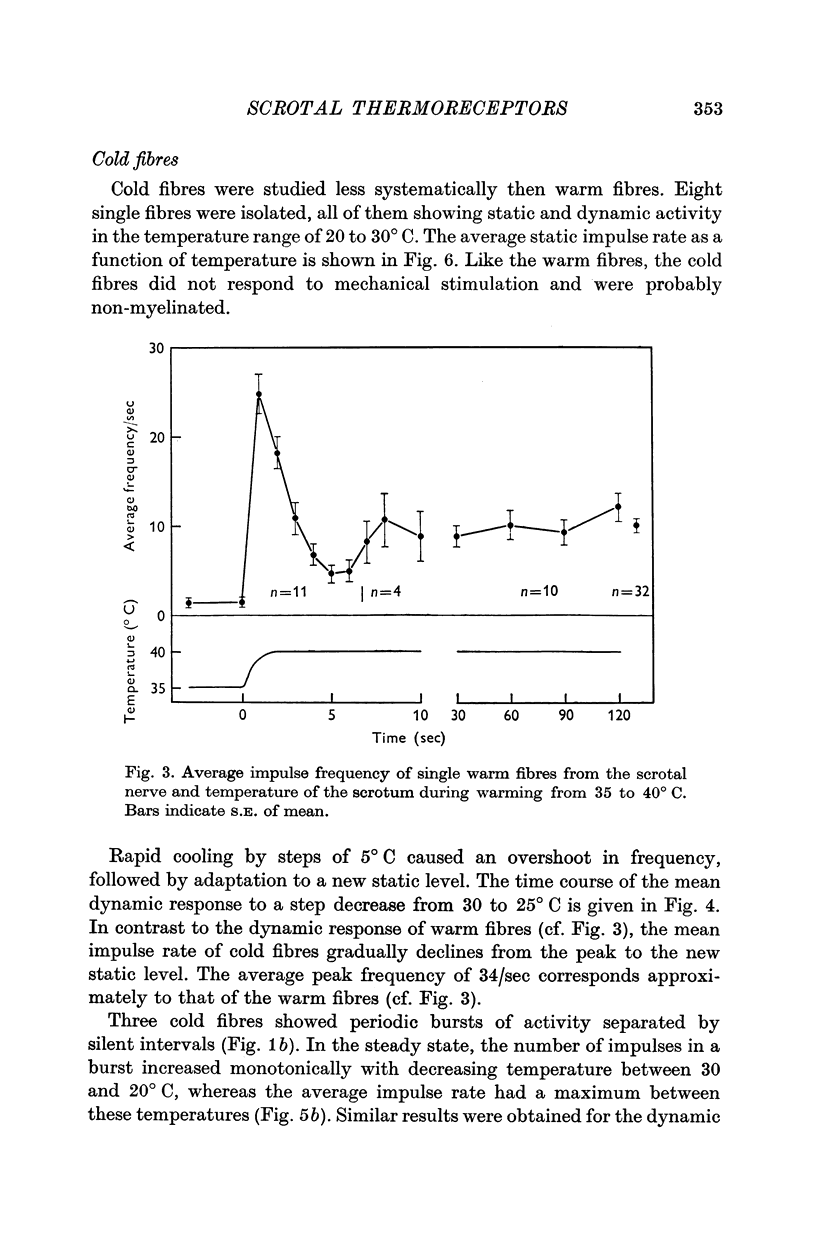
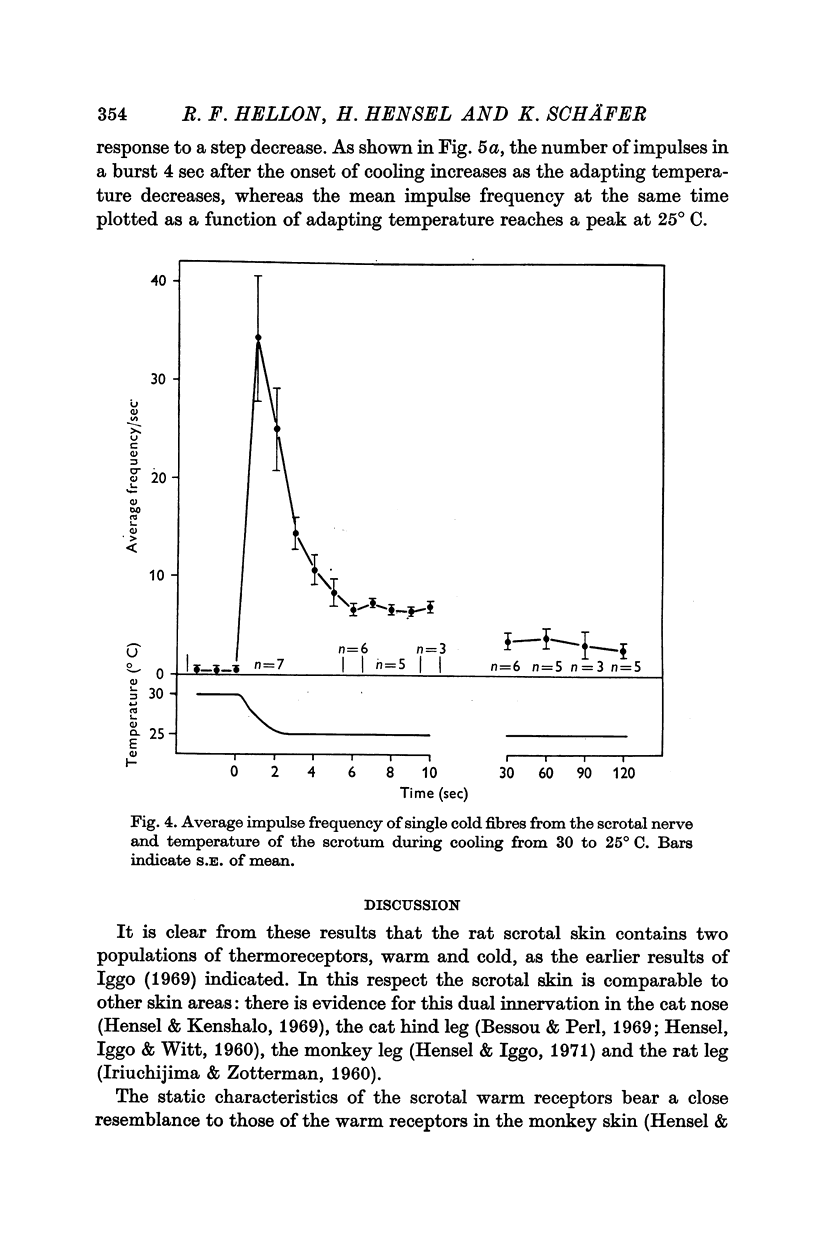
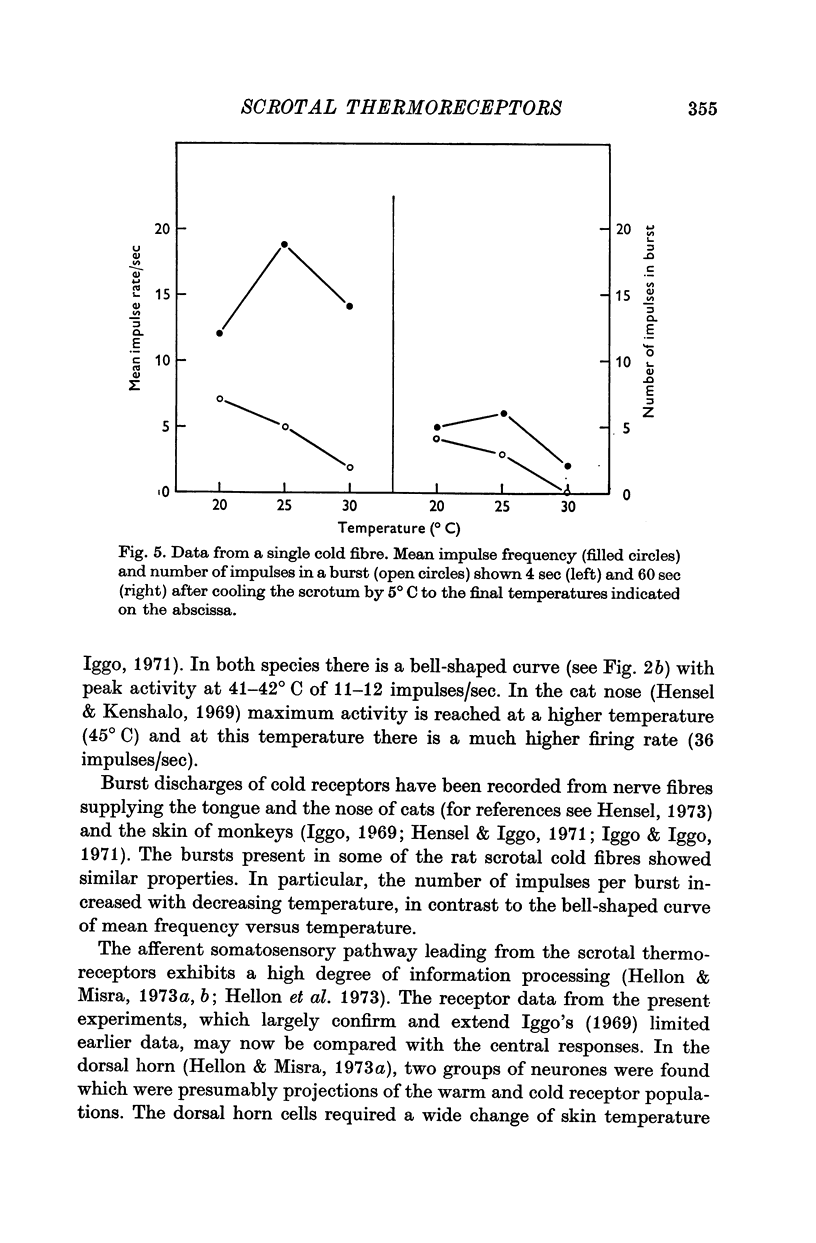
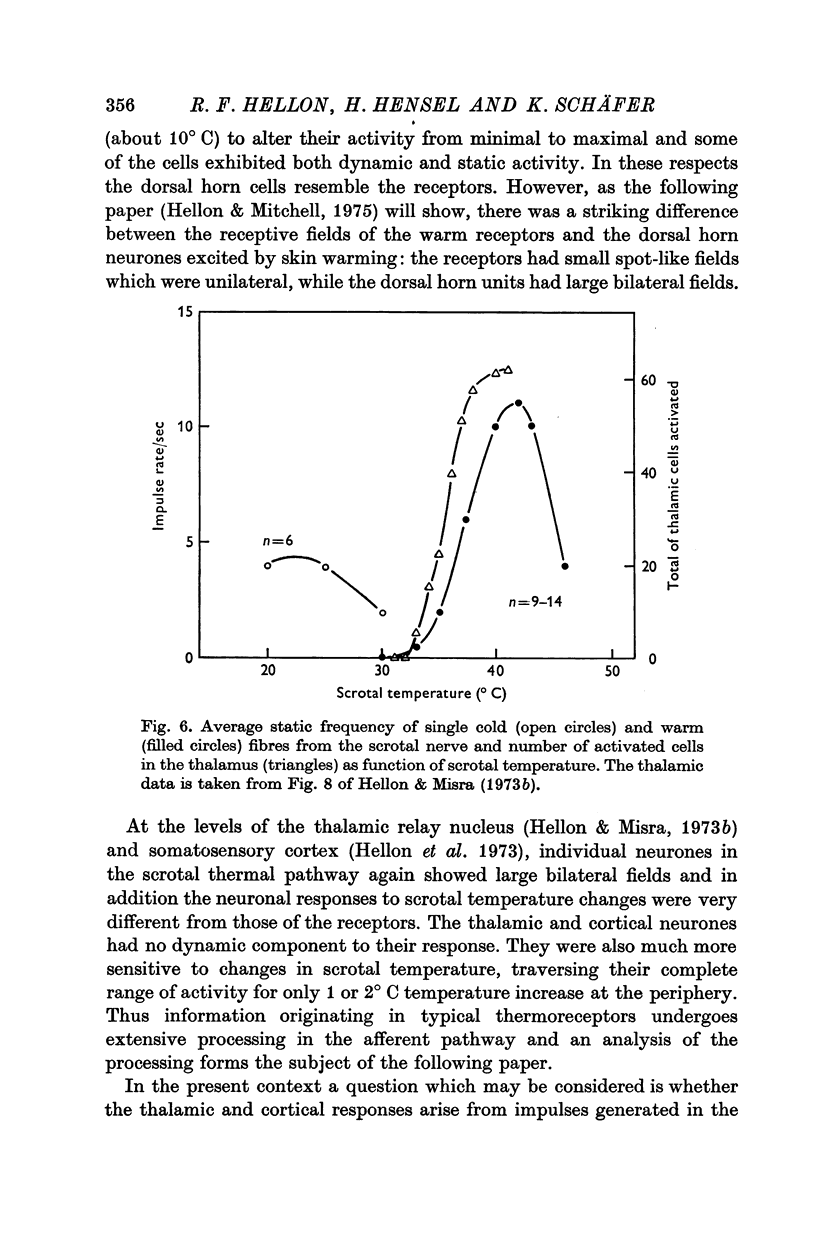
1. The technique of single fibre dissection has been used to study the warm and cold thermoreceptors in the rat scrotum. 2. The warm receptors showed dynamic activity during increases of scrotal temperature and static activity when temperature was constant. The static activity/temperature curve was bell-shaped, with minima at 31 and 45 degrees C and a peak at 42 degrees C. 3. The cold receptors also showed dynamic and static responses to reductions of temperature. At steady temperatures the impulses from some receptors were grouped in bursts. The number of impulses in each burst increased from zero at 30 degrees C to four at 20 degrees C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessou P., Perl E. R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- HENSEL H., IGGO A., WITT I. A quantitative study of sensitive cutaneous thermoreceptors with C afferent fibres. J Physiol. 1960 Aug;153:113–126. doi: 10.1113/jphysiol.1960.sp006522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K. Neurones in the dorsal horn of the rat responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):375–388. doi: 10.1113/jphysiol.1973.sp010275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K. Neurones in the ventrobasal complex of the rat thalamus responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):389–399. doi: 10.1113/jphysiol.1973.sp010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Mitchell D. Convergence in a thermal afferent pathway in the rat. J Physiol. 1975 Jun;248(2):359–376. doi: 10.1113/jphysiol.1975.sp010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel H., Iggo A. Analysis of cutaneous warm and cold fibres in primates. Pflugers Arch. 1971;329(1):1–8. doi: 10.1007/BF00586896. [DOI] [PubMed] [Google Scholar]
- Hensel H., Kenshalo D. R. Warm receptors in the nasal region of cats. J Physiol. 1969 Sep;204(1):99–112. doi: 10.1113/jphysiol.1969.sp008901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRIUCHIJIMA J., ZOTTERMAN Y. The specificity of afferent cutaneous C fibres in mammals. Acta Physiol Scand. 1960 Jul 15;49:267–278. doi: 10.1111/j.1748-1716.1960.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Iggo A. Cutaneous thermoreceptors in primates and sub-primates. J Physiol. 1969 Feb;200(2):403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Iggo B. J. Impulse coding in primate cutaneous thermoreceptors in dynamic thermal conditions. J Physiol (Paris) 1971 May;63(3):287–290. [PubMed] [Google Scholar]
- Mitchell D., Hellon R. F. Latencies in a thermosensitive pathway. Experientia. 1974 Oct 15;30(10):1159–1161. doi: 10.1007/BF01923664. [DOI] [PubMed] [Google Scholar]



