Abstract
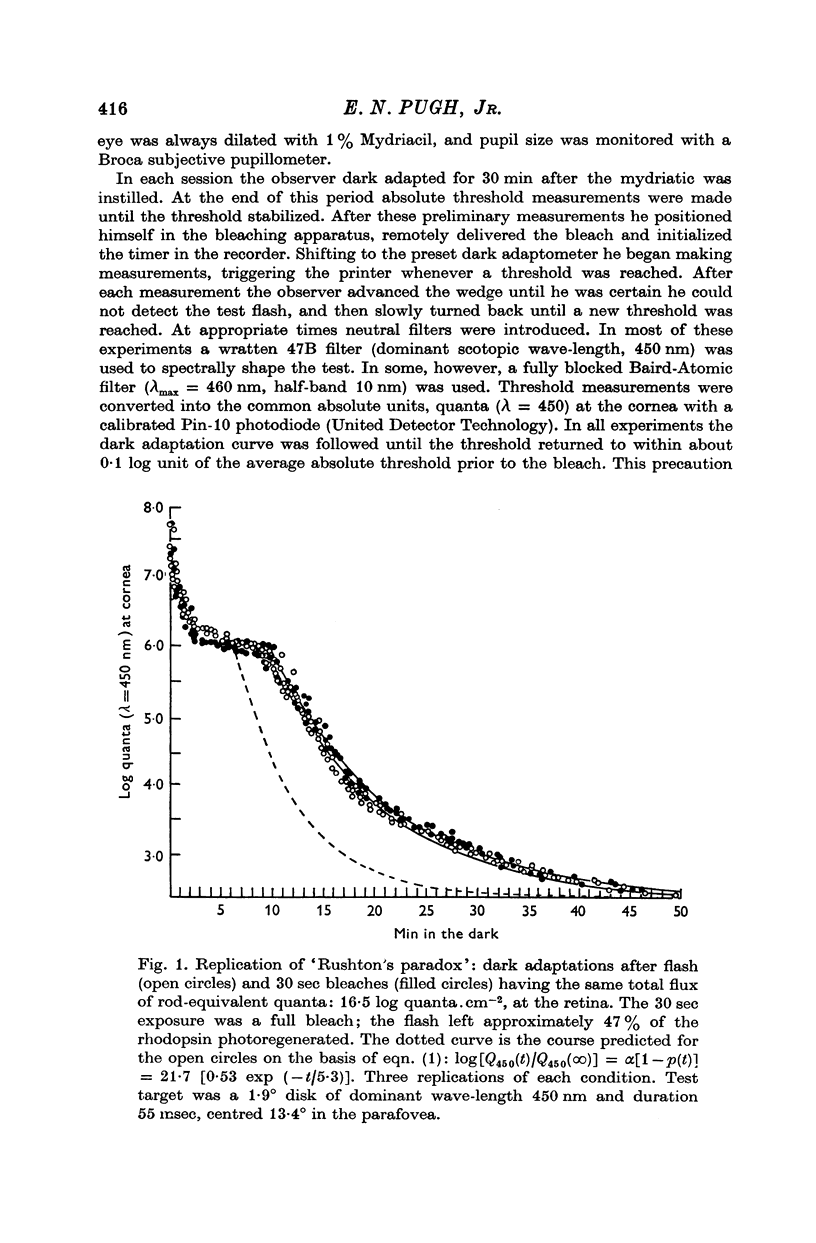
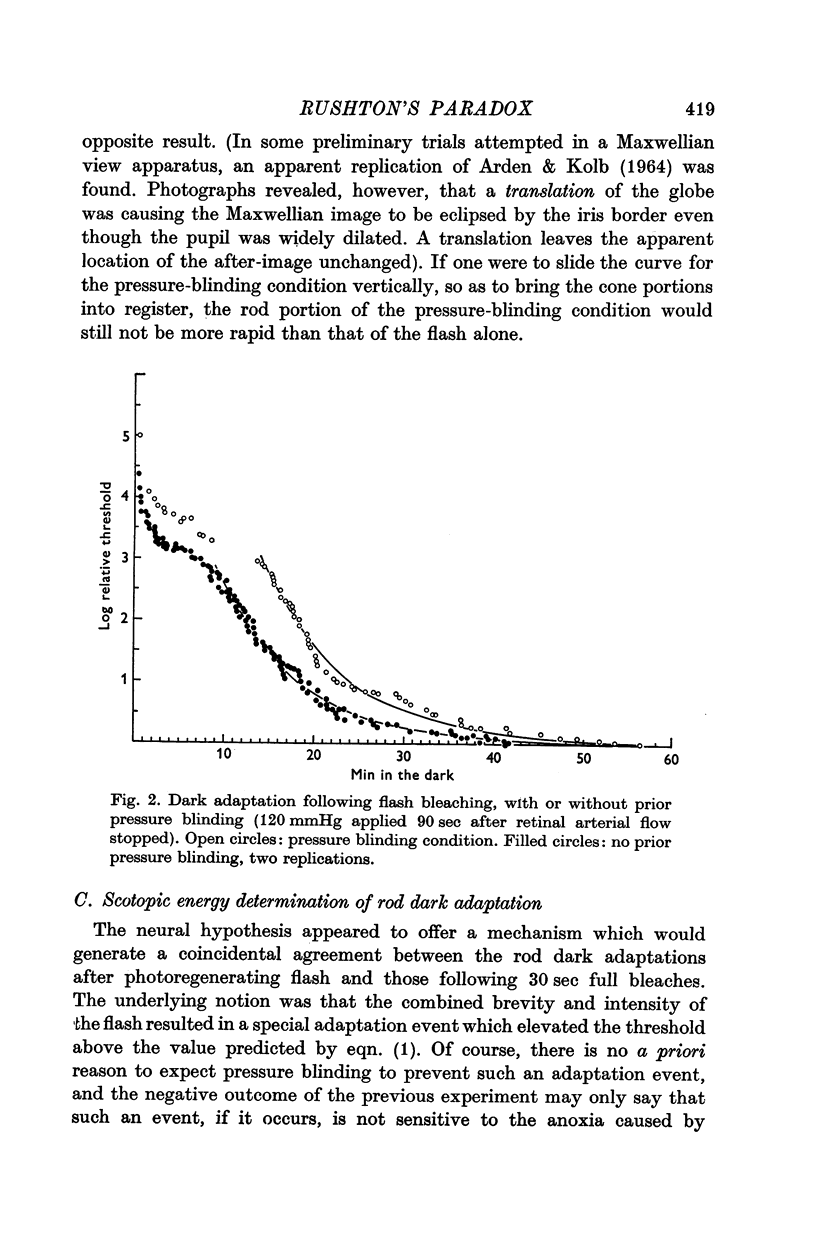
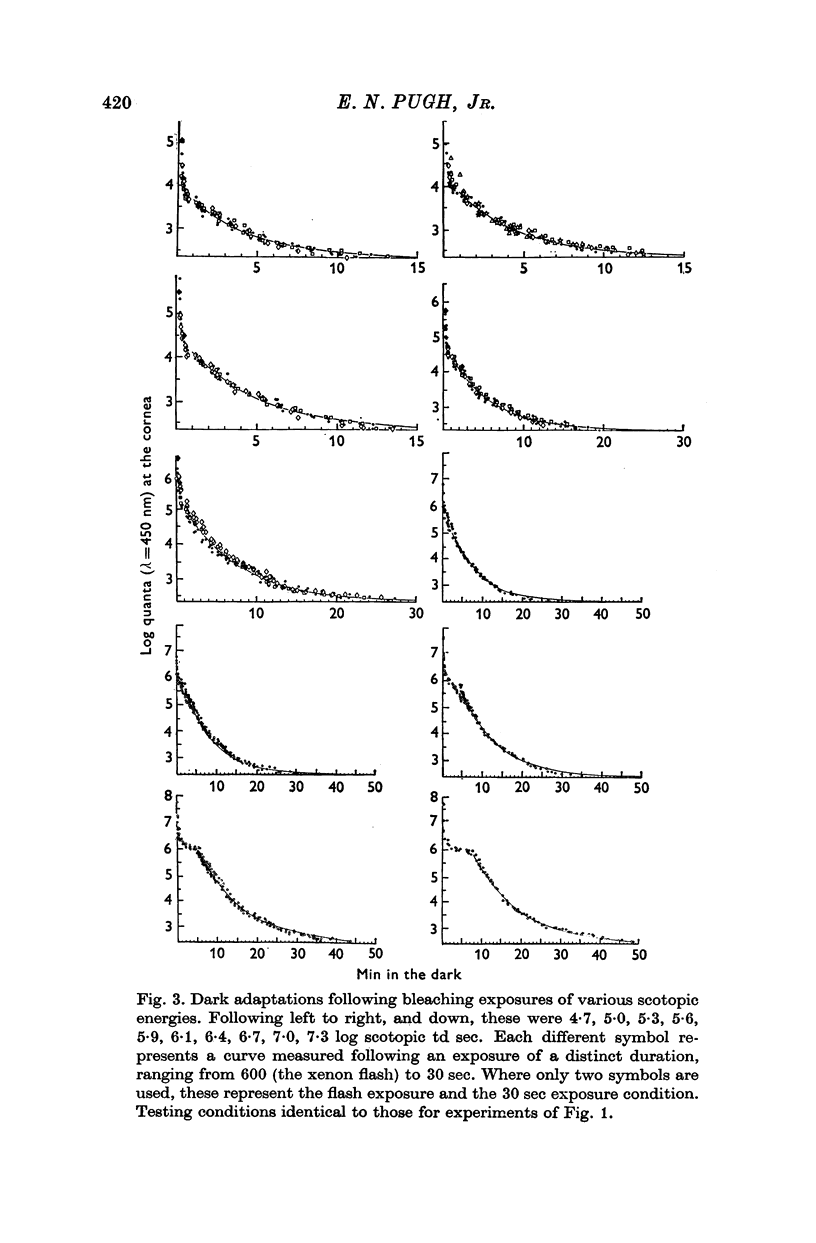
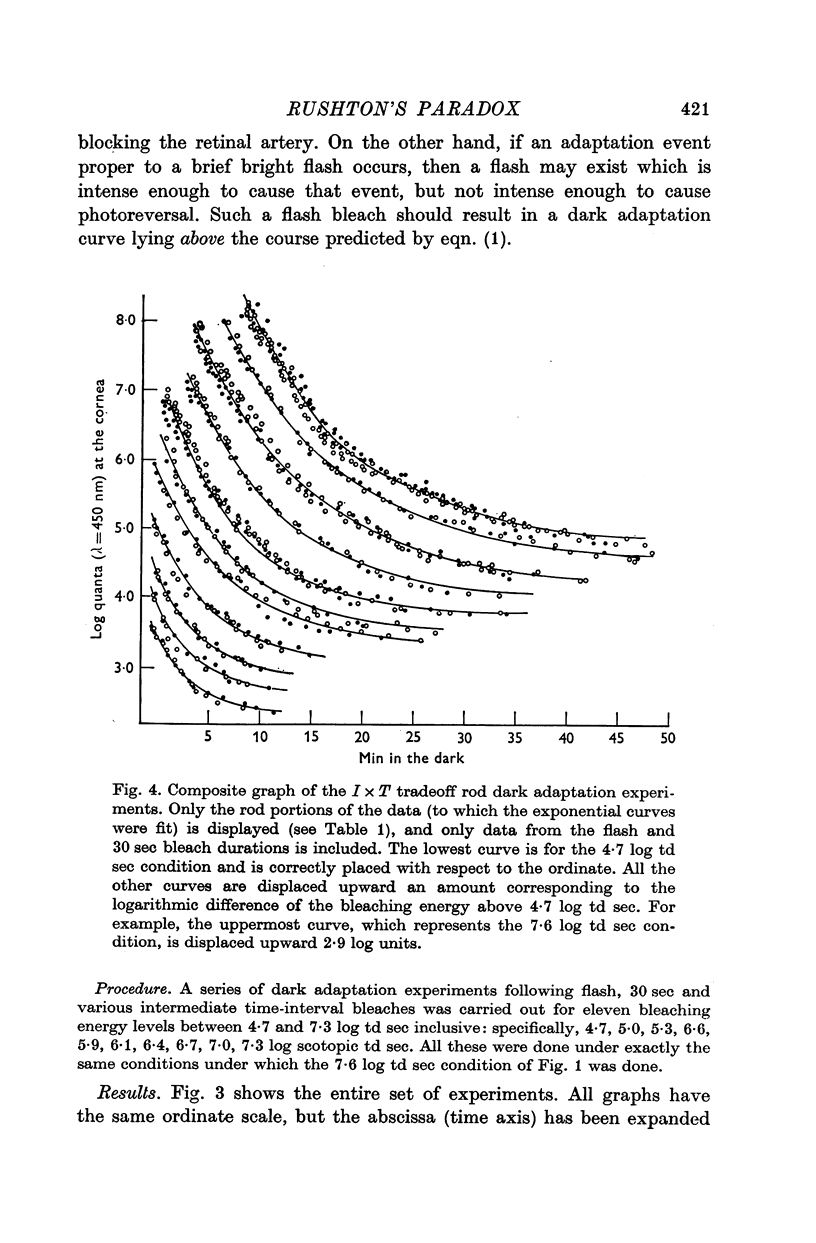
1. Rod dark adaptations after a photoregenerating flash and quantum-equivalent 30 sec bleach are found to be in exact agreement, while the measured rhodopsin regenerations are grossly different. This finding confirms and clarifies "Rushton's paradox', the failure of the Dowling-Rushton equation (linking log sensitivity linearly with unregenerated rhodopsin) to account for human rod dark adaptation after flash photolysis. 2. The hypothesis that the agreement between rod dark adaptation curves after a photoregenerating flash and after a quantum-equivalent 30 sec bleach is coincidental is rejected on the basic of two classes of experiments. 3. Rod "bleaching' adaptation is demonstrated to be entirely determined by the number of rhodopsin molecules which absorb at least one quantum in a temporal period T, whose range includes the time interval 600 musec less than or equal T less than or equal 30 sec. This generalization obtains over the entire scotopic energy range (congruent to 3 log units) where rod dark adaptations has been studied. 4. Thus, the state of "bleaching' adaptation is determined by some by-product of the normal chain of events in scotopic excitation. About this by-product three important deductions are made: (i) its production is a monotonic function of the initial effective quantum absorptions; (ii) its production occurs before the metarhodopsin I leads to to metarhodopsin II dark reaction; (iii) it cannot be any photoproduct of the rhodopsin cycle.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES A., 3rd, GURIAN B. S. Effects of glucose and oxygen deprivation on function of isolated mammalian retina. J Neurophysiol. 1963 Jul;26:617–634. doi: 10.1152/jn.1963.26.4.617. [DOI] [PubMed] [Google Scholar]
- Alpern M. Effect of a bright light flash on dark adaptation of human rods. Nature. 1971 Apr 9;230(5293):394–396. doi: 10.1038/230394a0. [DOI] [PubMed] [Google Scholar]
- Alpern M., Pugh E. N., Jr The density and photosensitivity of human rhodopsin in the living retina. J Physiol. 1974 Mar;237(2):341–370. doi: 10.1113/jphysiol.1974.sp010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern M. Rhodopsin kinetics in the human eye. J Physiol. 1971 Sep;217(2):447–471. doi: 10.1113/jphysiol.1971.sp009580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. T. The eclectroretinogram: its components and their origins. Vision Res. 1968 Jun;8(6):633–677. doi: 10.1016/0042-6989(68)90041-2. [DOI] [PubMed] [Google Scholar]
- Def Webster H., Ames A. REVERSIBLE AND IRREVERSIBLE CHANGES IN THE FINE STRUCTURE OF NERVOUS TISSUE DURING OXYGEN AND GLUCOSE DEPRIVATION. J Cell Biol. 1965 Sep 1;26(3):885–909. doi: 10.1083/jcb.26.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. Visual adaptation of the rhodopsin rods in the frogs retina. J Physiol. 1968 Nov;199(1):59–87. doi: 10.1113/jphysiol.1968.sp008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. S-potentials in the skate retina. Intracellular recordings during light and dark adaptation. J Gen Physiol. 1971 Aug;58(2):163–189. doi: 10.1085/jgp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Visual adaptation in the retina of the skate. J Gen Physiol. 1970 Oct;56(4):491–520. doi: 10.1085/jgp.56.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S., Haig C., Chase A. M. THE INFLUENCE OF LIGHT ADAPTATION ON SUBSEQUENT DARK ADAPTATION OF THE EYE. J Gen Physiol. 1937 Jul 20;20(6):831–850. doi: 10.1085/jgp.20.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollins M., Alpern M. Dark adaptation and visual pigment regeneration in human cones. J Gen Physiol. 1973 Oct;62(4):430–447. doi: 10.1085/jgp.62.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTE F. A., RIOPELLE A. J. The effect of varying the intensity and the duration of pre-exposure upon subsequent dark adaptation in the human eye. J Comp Physiol Psychol. 1953 Feb;46(1):49–55. doi: 10.1037/h0062117. [DOI] [PubMed] [Google Scholar]
- Pugh E. N. Rhodopsin flash photolysis in man. J Physiol. 1975 Jun;248(2):393–412. doi: 10.1113/jphysiol.1975.sp010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. Blue light and the regeneration of human rhodopsin in situ. J Gen Physiol. 1957 Nov 20;41(2):419–428. doi: 10.1085/jgp.41.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. EFFECT OF INSTANTANEOUS FLASHES ON ADAPTATION OF THE EYE. DARK ADAPTATION AFTER EXPOSING THE EYE TO AN INSTANTANEOUS FLASH. Nature. 1963 Sep 7;199:971–972. doi: 10.1038/199971a0. [DOI] [PubMed] [Google Scholar]
- Ripps H., Weale R. A. Flash bleaching of rhodopsin in the human retina. J Physiol. 1969 Jan;200(1):151–159. doi: 10.1113/jphysiol.1969.sp008686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton W. A., Powell D. S. The early phase of dark adaptation. Vision Res. 1972 Jun;12(6):1083–1093. doi: 10.1016/0042-6989(72)90099-5. [DOI] [PubMed] [Google Scholar]
- Rushton W. A., Powell D. S. The rhodopsin content and the visual threshold of human rods. Vision Res. 1972 Jun;12(6):1073–1081. doi: 10.1016/0042-6989(72)90098-3. [DOI] [PubMed] [Google Scholar]


