Abstract
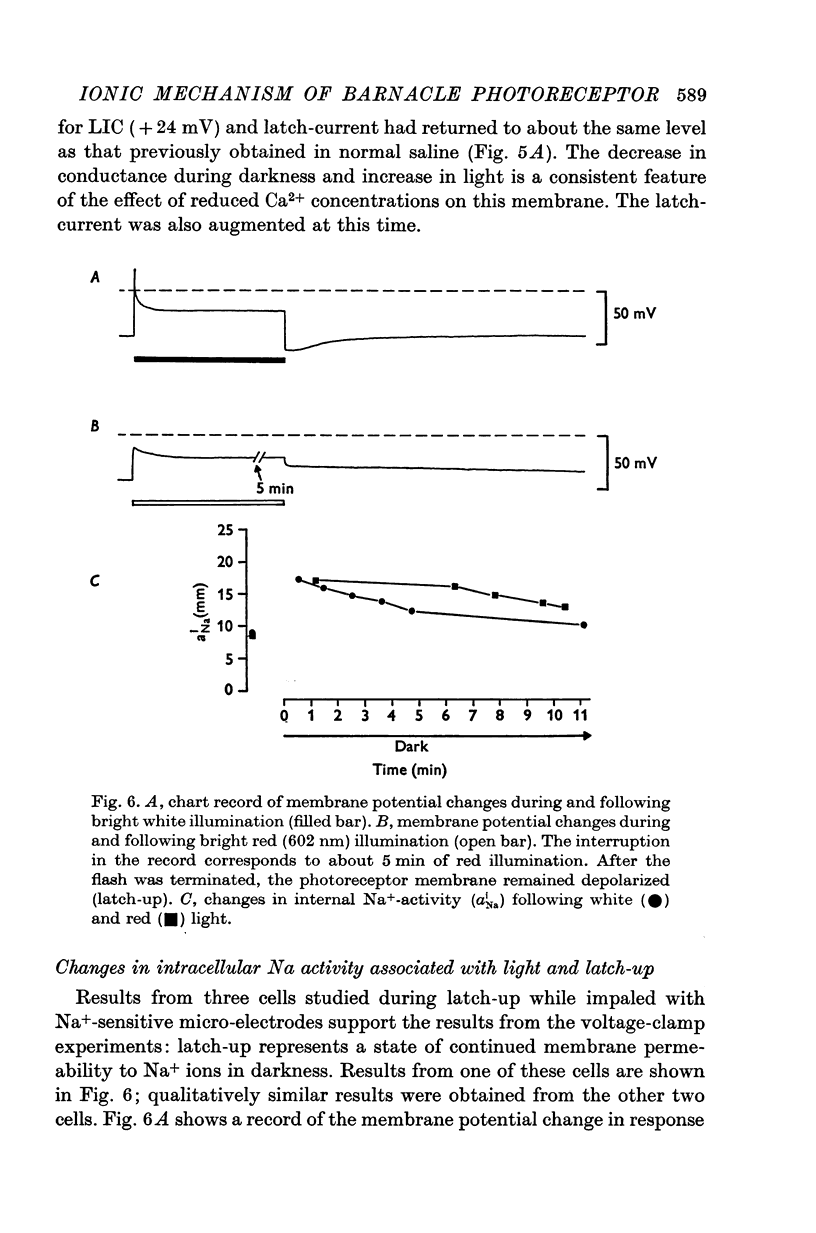
1. The membrane mechanism of a quasi-stable membrane depolarization (latch-up) that persists in darkness following red light was examined in barnacle photoreceptor with micro-electrode techniques including voltage-clamp and Na+-sensitive micro-electrodes. 2. Current-voltage (I-V) relations of the membrane in darkness following red light (latch-up) and in darkness following termination of latch-up with green light, indicate that latch-up is associated with an increase of membrane conductance. 3. The latch-current (membrane current in darkness following red light minus membrane current in darkness following a gree flash that terminates latch-up) was inward at the resting potential, reversed sign at about +26mV (mean of six cells), and became outward at more positive membrance potentials. 4. Current-voltage relations of the membrane during green light (no latch-up) closely resembled those during latch-up. The light-induced current (LIC) elicited by green ligh (membrane current during the light flash minus membrane current in darkness following the light flash) was inward from the resting potential to +26mV (mean of six cells), then reversed sign and became outward. 5. The latch-current and LIC were both augmented in reduced Ca2+ solutions and decreased as Na-+ was reduced at a fixed Ca2+ concentration. 6. Both LIC and latch-current reversed sign at a more negative membrane potential (increment V equals 14mV) in solutions containing one quarter the normal amount of Na+. 7. The internal Na-+ activity (a-iNa) of a photoreceptor increased from about 10-18 mM upon illumination with long steps of intense red or white illumination. Five minutes in darkness after white light, a-iNa had recovered significantly, whereas a-iNa remained elecated following red illumination. 8. Latch-up seems to be a persistence in darkness of the same membrane mechanism that normally occurs during illumination; i.e. a conductance increase to Na+ ions. Ca2+ ions act primarily to suppress this current. There is evidence for a net Na+ influx during illumination that is sustained in darkness during latch-up.
Full text
PDF

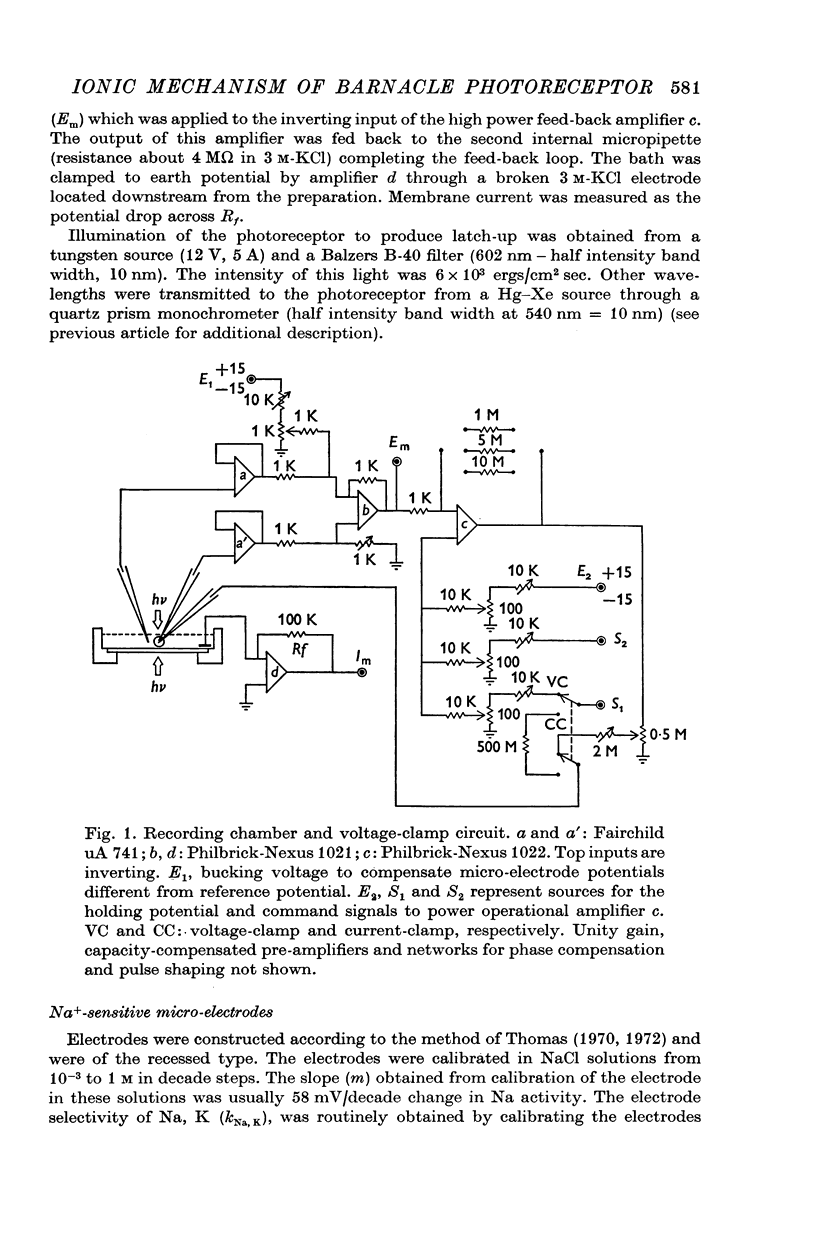

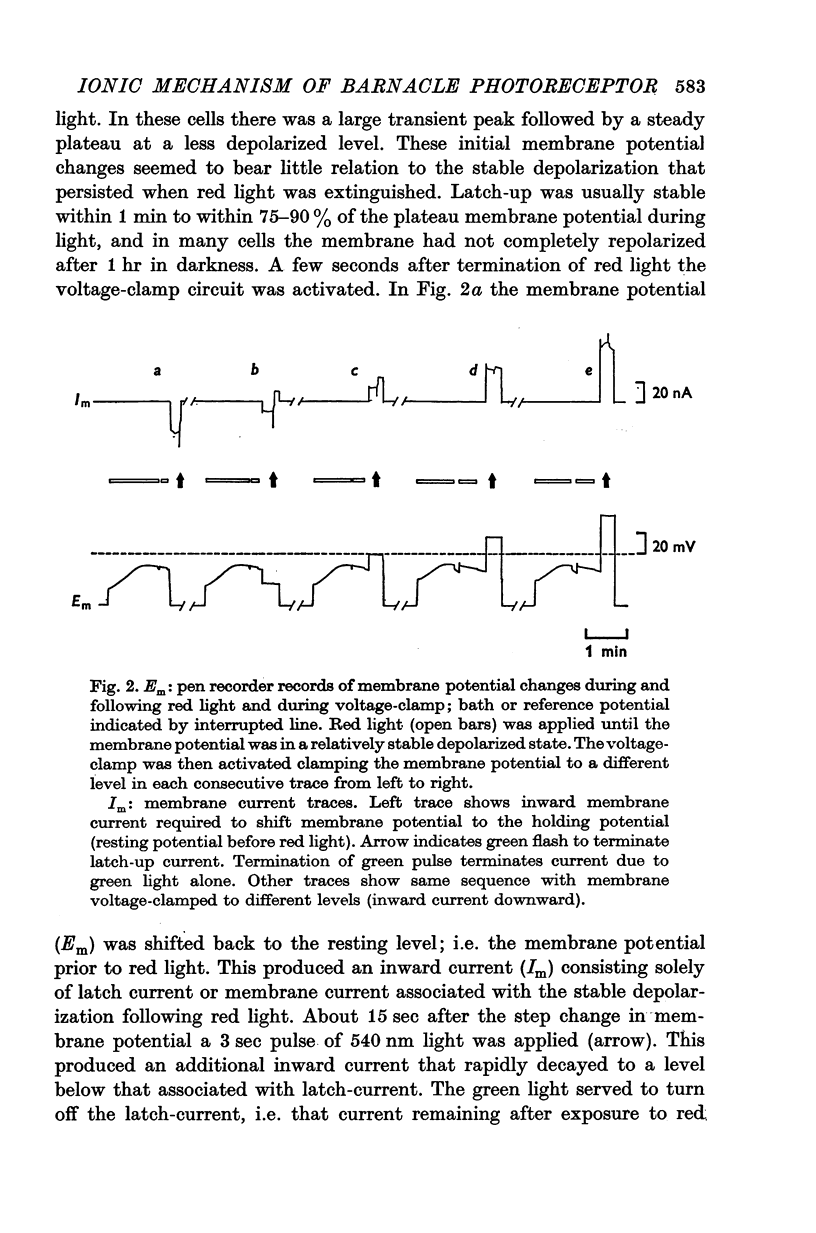
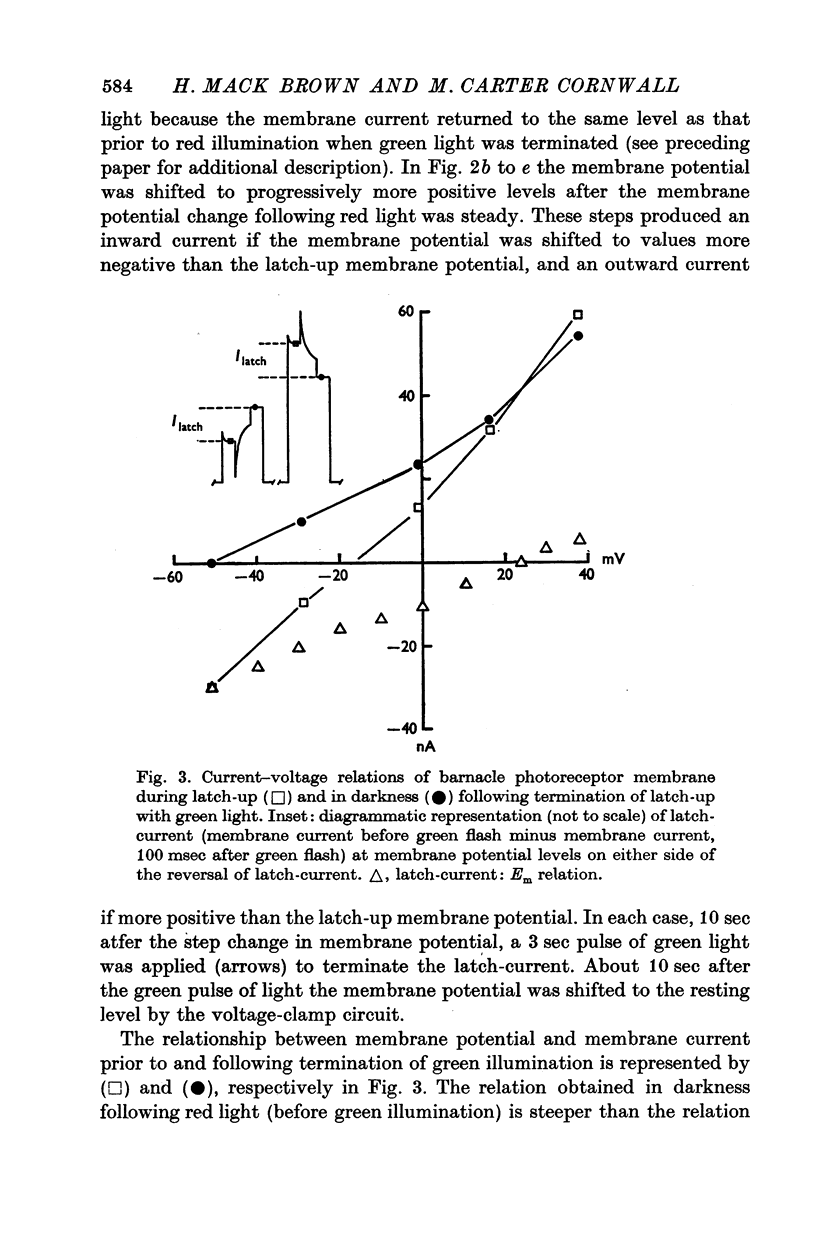

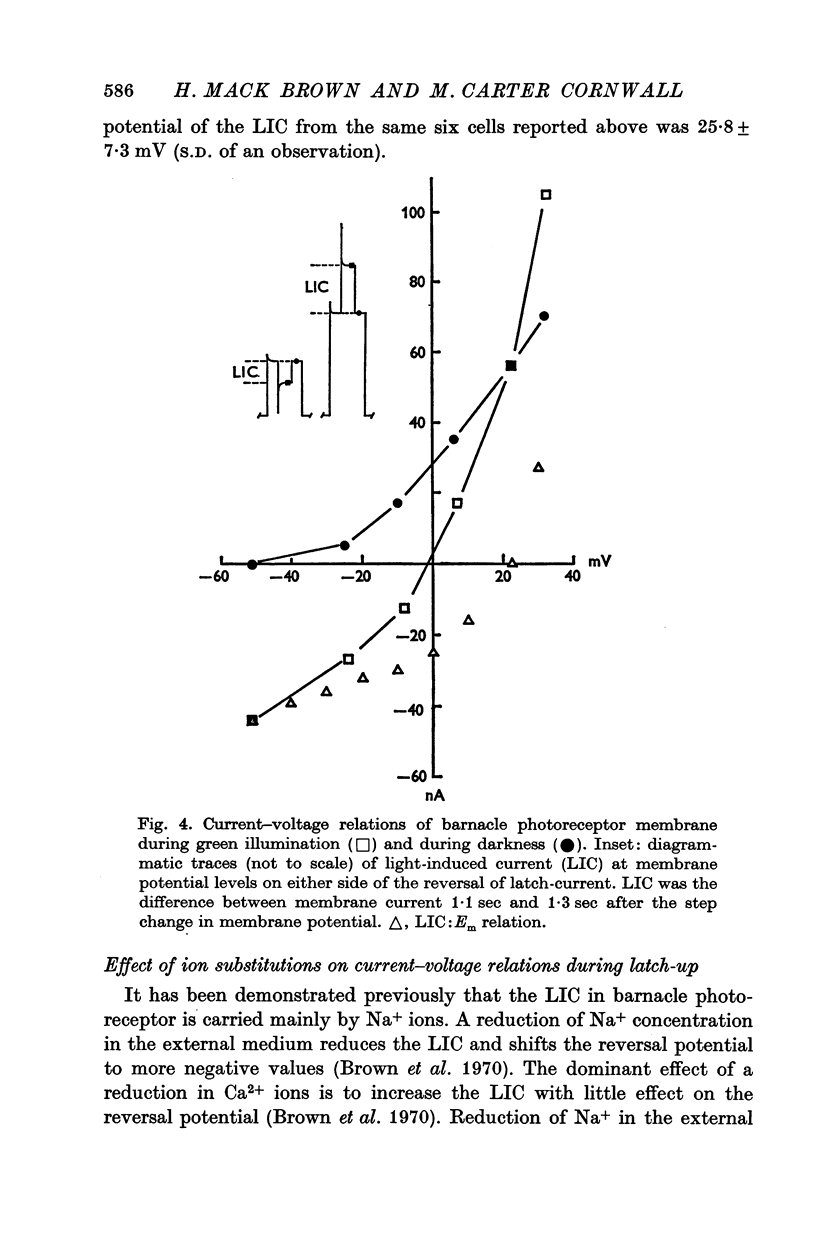
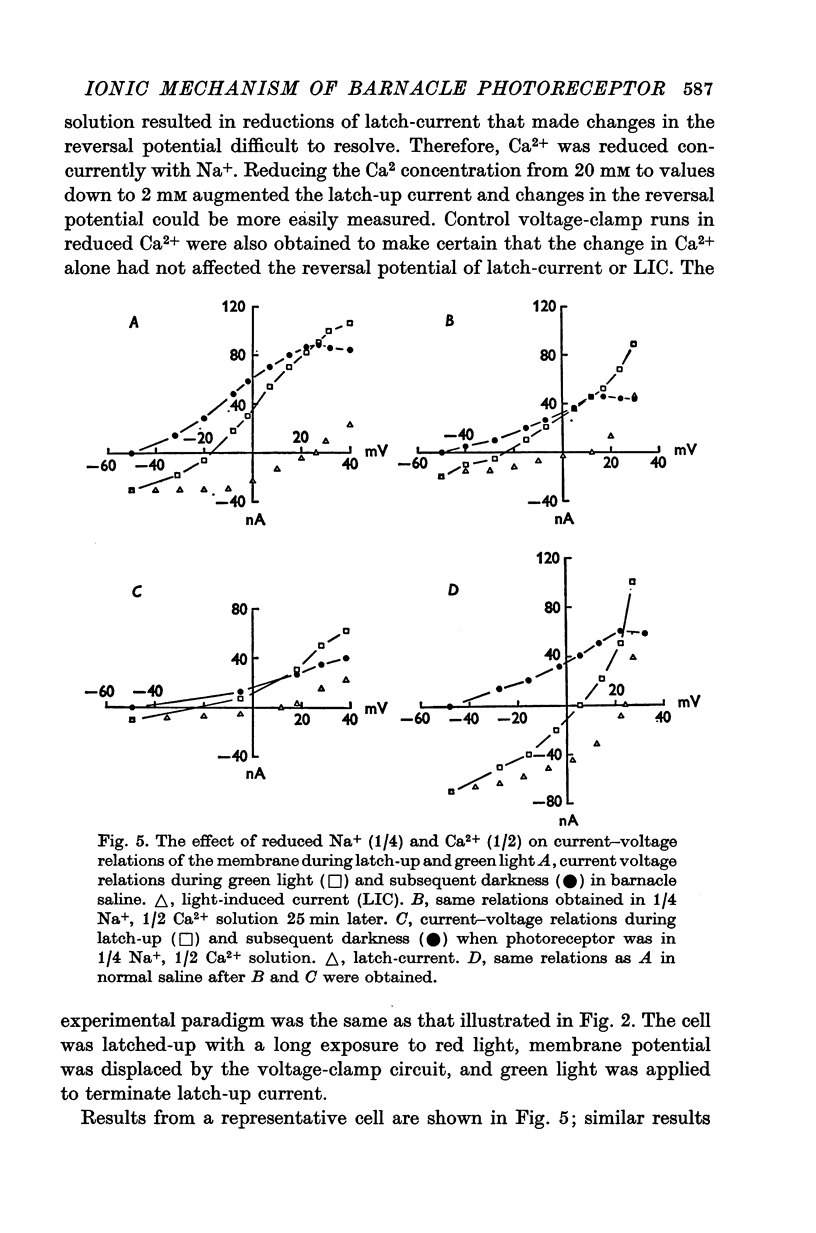





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann F., Hadjilazaro B. A depolarizing aftereffect of intense light in the drone visual receptor. Vision Res. 1972 Jan;12(1):17–31. doi: 10.1016/0042-6989(72)90134-4. [DOI] [PubMed] [Google Scholar]
- Brown H. M., Cornwall M. C. Spectral correlates of a quasi-stable depolarization in barnacle photoreceptor following red light. J Physiol. 1975 Jul;248(3):555–578. doi: 10.1113/jphysiol.1975.sp010988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. W. Electrical characteristics of a barnacle photoreceptor. Fed Proc. 1971 Jan-Feb;30(1):69–78. [PubMed] [Google Scholar]
- Brown H. M., Meech R. W., Koike H., Hagiwara S. Current-voltage relations during illumination: photoreceptor membrane of a barnacle. Science. 1969 Oct 10;166(3902):240–243. doi: 10.1126/science.166.3902.240. [DOI] [PubMed] [Google Scholar]
- Hillman P., Hochstein S., Minke B. A visual pigment with two physiologically active stable states. Science. 1972 Mar 31;175(4029):1486–1488. doi: 10.1126/science.175.4029.1486. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Minke B., Hillman P. Antagonistic components of the late receptor potential in the barnacle photoreceptor arising from different stages of the pigment process. J Gen Physiol. 1973 Jul;62(1):105–128. doi: 10.1085/jgp.62.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Brown H. M., Hagiwara S. Hyperpolarization of a barnacle photoreceptor membrane following illumination. J Gen Physiol. 1971 Jun;57(6):723–737. doi: 10.1085/jgp.57.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972 Jun;59(6):701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKA K. I. Recording of retinal action potentials from single cells in the insect compound eye. J Gen Physiol. 1961 Jan;44:571–584. doi: 10.1085/jgp.44.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte J., Brown J. E. Electrophysiological properties of cells in the median ocellus of Limulus. J Gen Physiol. 1972 Feb;59(2):167–185. doi: 10.1085/jgp.59.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte J., Brown J. E., Smith T. G., Jr A hyperpolarizing component of the receptor potential in the median ocellus of Limulus. Science. 1968 Nov 8;162(3854):677–679. doi: 10.1126/science.162.3854.677. [DOI] [PubMed] [Google Scholar]
- Nolte J., Brown J. E. Ultraviolet-induced sensitivity to visible light in ultraviolet receptors of Limulus. J Gen Physiol. 1972 Feb;59(2):186–200. doi: 10.1085/jgp.59.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. New design for sodium-sensitive glass micro-electrode. J Physiol. 1970 Sep;210(2):82P–83P. [PubMed] [Google Scholar]


