Full text
PDF


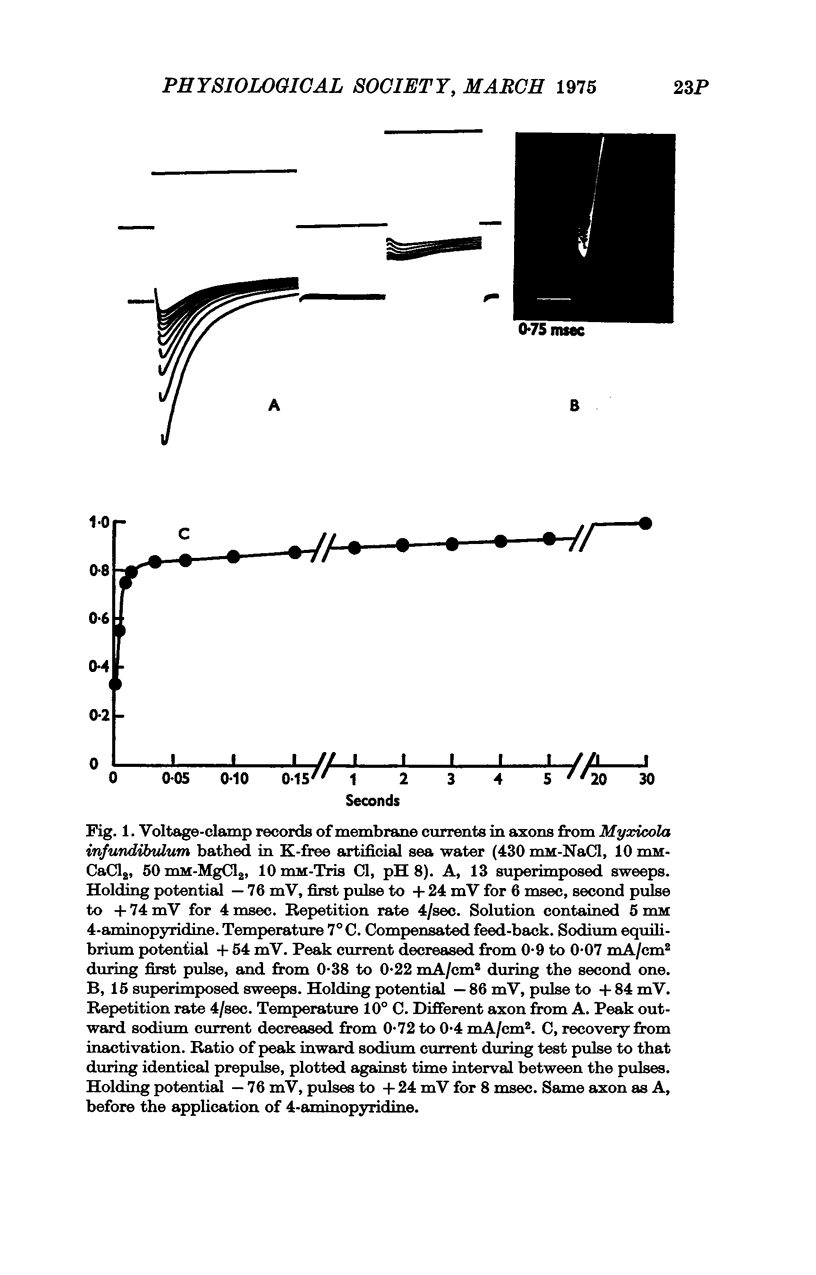















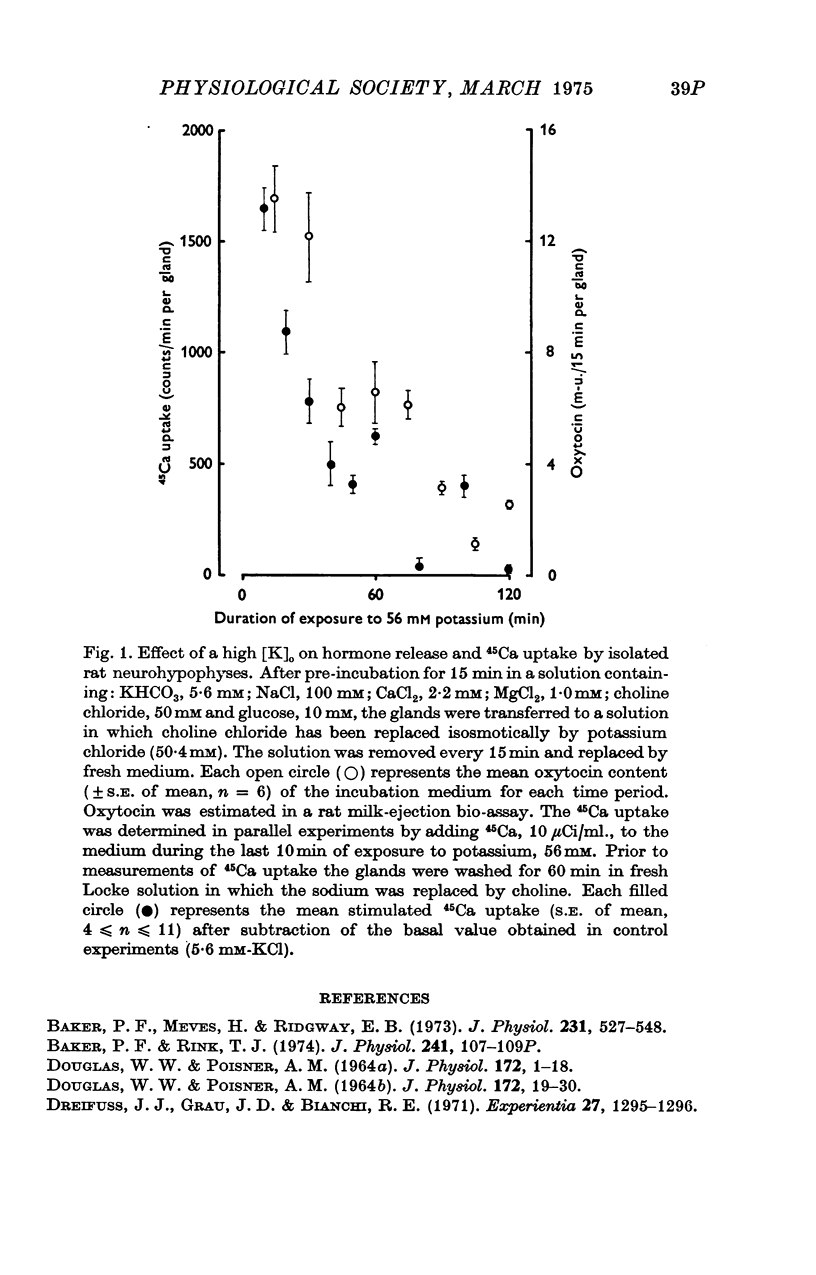

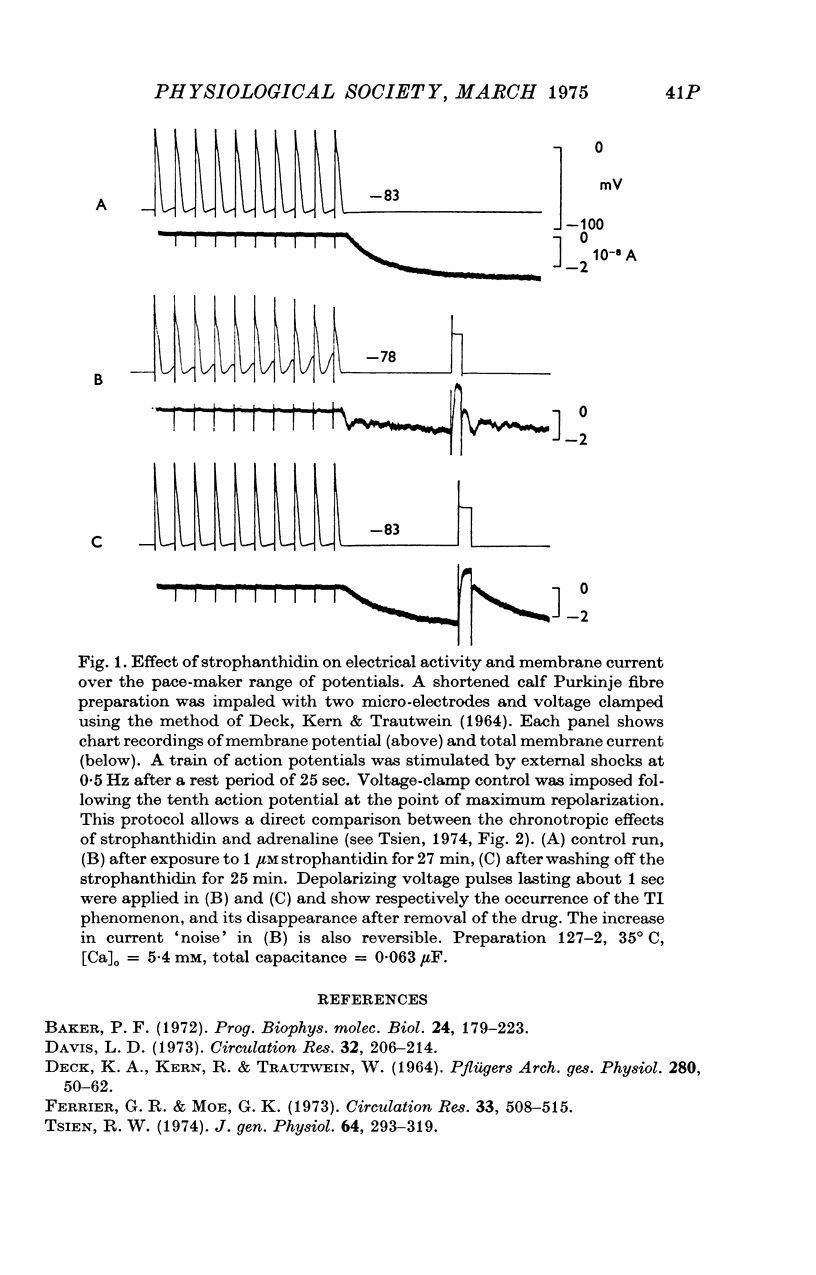

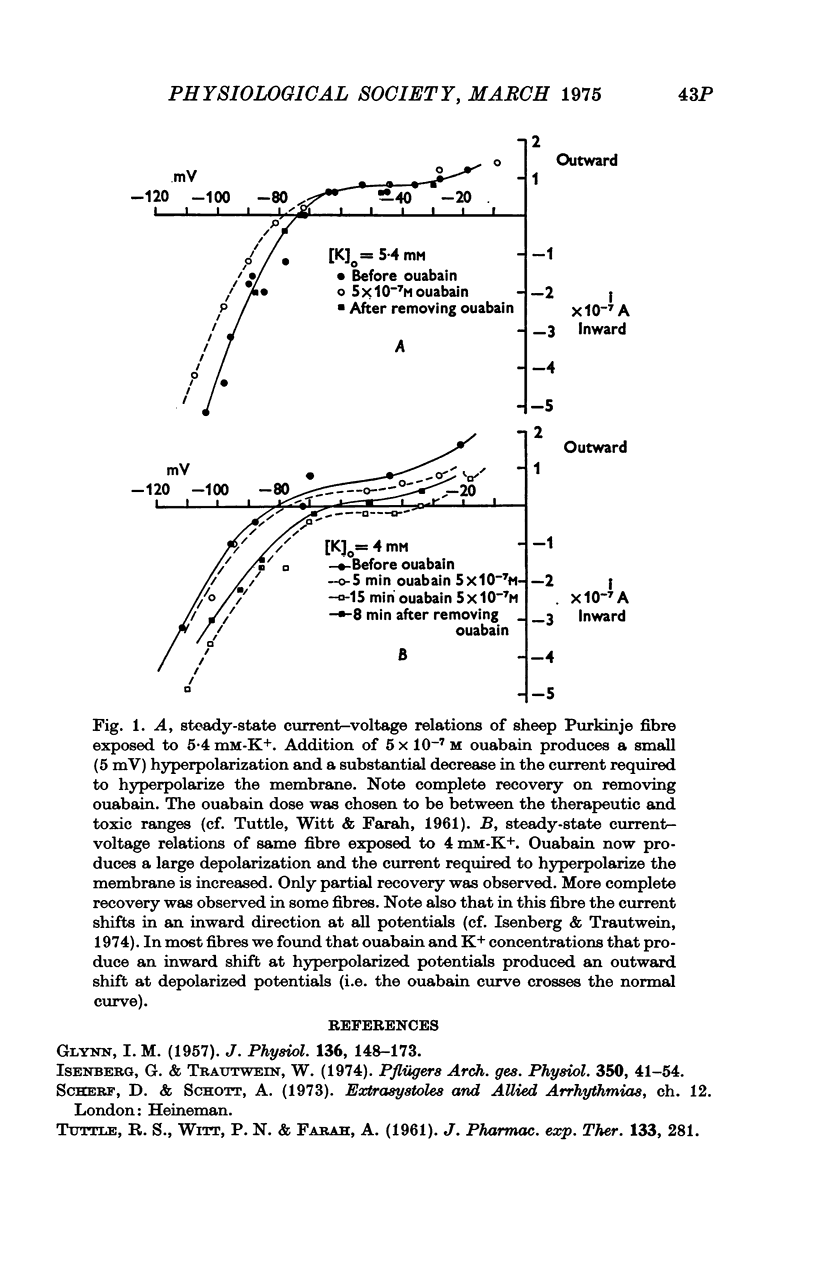












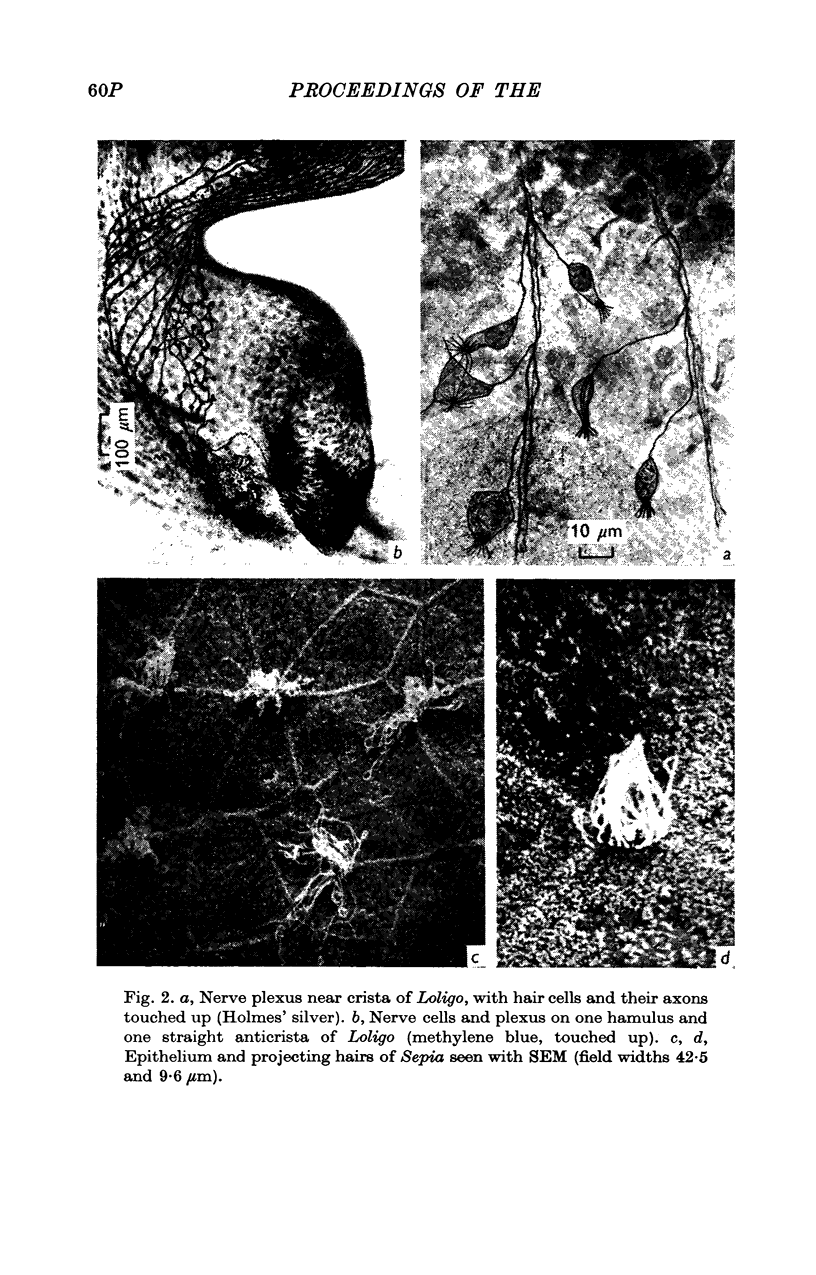









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLETON T. C. AUTORADIOGRAPHY OF SOLUBLE LABELLED COMPOUNDS. J R Microsc Soc. 1964 Sep;83:277–281. doi: 10.1111/j.1365-2818.1964.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Aars H., Akre S. Reflex changes in sympathetic activity and arterial blood pressure evoked by afferent stimulation of the renal nerve. Acta Physiol Scand. 1970 Feb;78(2):184–188. doi: 10.1111/j.1748-1716.1970.tb04654.x. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R., Usherwood P. N. Proceedings: Voltage-clamp studies of the glutamate response at the insect neuromuscular junction. J Physiol. 1974 Oct;242(2):86P–87P. [PubMed] [Google Scholar]
- Armstrong W. M., Lee C. O. Sodium and potassium activities in normal and "sodium-rich" frog skeletal muscle. Science. 1971 Jan 29;171(3969):413–415. doi: 10.1126/science.171.3969.413. [DOI] [PubMed] [Google Scholar]
- Ash A. S., Schild H. O. Receptors mediating some actions of histamine. Br J Pharmacol Chemother. 1966 Aug;27(2):427–439. doi: 10.1111/j.1476-5381.1966.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNTHAL T., WOODCOCK C. C., Jr Responses of the vasomotor center to hypoxia after denervation of carotid and aortic bodies. Am J Physiol. 1951 Jul;166(1):45–54. doi: 10.1152/ajplegacy.1951.166.1.45. [DOI] [PubMed] [Google Scholar]
- BROOKHART J. M., FADIGA E. Potential field initiated during monosynaptic activation of frog motoneurones. J Physiol. 1960 Mar;150:633–655. doi: 10.1113/jphysiol.1960.sp006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Proceedings: Transient release of catecholamines in response to maintained depolarization of bovine adrenal medulla. J Physiol. 1974 Sep;241(2):107P–109P. [PubMed] [Google Scholar]
- Baldwin B. A., Ingram D. L. Effect of heating & cooling the hypothalamus on behavioral thermoregulation in the pig. J Physiol. 1967 Jul;191(2):375–392. doi: 10.1113/jphysiol.1967.sp008256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks B. E., Charlwood K. A., Edwards D. C., Vernon C. A., Walter S. J. Effects of nerve growth factors from mouse salivary glands and snake venom on the sympathetic ganglia of neonatal and developing mice. J Physiol. 1975 May;247(2):289–298. doi: 10.1113/jphysiol.1975.sp010932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli A., Cross B. A., Guz A., Jain S. K., Noble M. I., Trenchard D. W. The effect of carbon dioxide in the airways and alveoli on ventilation; a vagal reflex studied in the dog. J Physiol. 1974 Jul;240(1):91–109. doi: 10.1113/jphysiol.1974.sp010601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian J., Nakajima S. Action potential in the transverse tubules and its role in the activation of skeletal muscle. J Gen Physiol. 1974 Feb;63(2):257–278. doi: 10.1085/jgp.63.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Caputo C., Gonzalez-Serratos H., Venosa R. A. Sodium dependence of the inward spread of activation in isolated twitch muscle fibres of the frog. J Physiol. 1972 Jun;223(2):507–523. doi: 10.1113/jphysiol.1972.sp009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Durant C. J., Ganellin C. R., Parsons E. M. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan R. N., Trevino D. L., Coulter J. D., Willis W. D. Location and somatotopic organization of the cells of origin of the spino-cervical tract. Exp Brain Res. 1973 Apr 30;17(2):177–189. doi: 10.1007/BF00235027. [DOI] [PubMed] [Google Scholar]
- Burns J. K. Relation between blood levels of ACTH and duration of human labour. J Physiol. 1975 Mar;246(2):106P–107P. [PubMed] [Google Scholar]
- Burrill P. H., Sattelmeyer P. A., Lerner J. Effect of theophylline and Na+ on methionine influx in Na+-depleted intestine. Biochim Biophys Acta. 1974 Dec 10;373(2):265–276. doi: 10.1016/0005-2736(74)90150-3. [DOI] [PubMed] [Google Scholar]
- CARLSSON A., FALCK B., FUXE K., HILLARP N. A. CELLULAR LOCALIZATION OF MONOAMINES IN THE SPINAL CORD. Acta Physiol Scand. 1964 Jan-Feb;60:112–119. doi: 10.1111/j.1748-1716.1964.tb02874.x. [DOI] [PubMed] [Google Scholar]
- Costantin L. L. The role of sodium current in the radial spread of contraction in frog muscle fibers. J Gen Physiol. 1970 Jun;55(6):703–715. doi: 10.1085/jgp.55.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T., Snyder S. H. Antiparkinsonian drugs: inhibition of dopamine uptake in the corpus striatum as a possible mechanism of action. Science. 1969 Nov 14;166(3907):899–901. doi: 10.1126/science.166.3907.899. [DOI] [PubMed] [Google Scholar]
- Croughs R. J., Tops C. F., de Jong F. H. Radioimmunoassay of plasma adrenocorticotrophin in Cushing's syndrome. J Endocrinol. 1973 Dec;59(3):439–449. doi: 10.1677/joe.0.0590439. [DOI] [PubMed] [Google Scholar]
- DECK K. A., KERN R., TRAUTWEIN W. VOLTAGE CLAMP TECHNIQUE IN MAMMALIAN CARDIAC FIBRES. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:50–62. doi: 10.1007/BF00412615. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. CALCIUM MOVEMENT IN THE NEUROHYPOPHYSIS OF THE RAT AND ITS RELATION TO THE RELEASE OF VASOPRESSIN. J Physiol. 1964 Jul;172:19–30. doi: 10.1113/jphysiol.1964.sp007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel P. M., Donaldson J., Pratt O. E. A method for injecting substances into the circulation to reach rapidly and to maintain a steady level. With examples of its application in the study of carbohydrate and amino acid metabolism. Med Biol Eng. 1975 Mar;13(2):214–227. doi: 10.1007/BF02477731. [DOI] [PubMed] [Google Scholar]
- Davis L. D. Effect of changes in cycle length on diastolic depolarization produced by ouabain in canine Purkinje fibers. Circ Res. 1973 Feb;32(2):206–214. doi: 10.1161/01.res.32.2.206. [DOI] [PubMed] [Google Scholar]
- Davis L. D. Effect of changes in cycle length on diastolic depolarization produced by ouabain in canine Purkinje fibers. Circ Res. 1973 Feb;32(2):206–214. doi: 10.1161/01.res.32.2.206. [DOI] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Grau J. D., Bianchi R. E. Antagonism between Ca and Na ions at neurohypophysial nerve terminals. Experientia. 1971;27(11):1295–1296. doi: 10.1007/BF02136696. [DOI] [PubMed] [Google Scholar]
- EISENMAN G., RUDIN D. O., CASBY J. U. Glass electrode for measuring sodium ion. Science. 1957 Oct 25;126(3278):831–834. doi: 10.1126/science.126.3278.831. [DOI] [PubMed] [Google Scholar]
- Ebbesson S. O., Campbell C. B. On the organization of cerebellar efferent pathways in the nurse shark (Ginglymostoma cirratum). J Comp Neurol. 1973 Dec 1;152(3):233–254. doi: 10.1002/cne.901520303. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. The mode of action of 5-hydroxytryptophan in facilitating a stretch reflex in the spinal cat. Exp Brain Res. 1975;22(2):145–162. doi: 10.1007/BF00237685. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier G. R., Moe G. K. Effect of calcium on acetylstrophanthidin-induced transient depolarizations in canine Purkinje tissue. Circ Res. 1973 Nov;33(5):508–515. doi: 10.1161/01.res.33.5.508. [DOI] [PubMed] [Google Scholar]
- Flynn S. B., Owen D. A. Proceedings: Vascular histamine receptors in the cat. Br J Pharmacol. 1974 Sep;52(1):122P–122P. [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Gessner K. Active transport potentials, membrane diffusion potentials and streaming potentials across rat kidney proximal tubule. Pflugers Arch. 1974;351(1):85–98. doi: 10.1007/BF00603513. [DOI] [PubMed] [Google Scholar]
- Frömter E., Rumrich G., Ullrich K. J. Phenomenologic description of Na+, Cl- and HCO-3 absorption from proximal tubules of rat kidney. Pflugers Arch. 1973 Oct 22;343(3):189–220. doi: 10.1007/BF00586045. [DOI] [PubMed] [Google Scholar]
- GERTZ K. H. [Transtubular sodium chloride transport and permeability for nonelectrolytes in the proximal and distal convolution of the rat kidney]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;276:336–356. [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. The sphincter pupillae of the guinea-pig: structure of muscle cells, intercellular relations and density of innervation. Proc R Soc Lond B Biol Sci. 1974 Jul 30;186(1085):369–386. doi: 10.1098/rspb.1974.0055. [DOI] [PubMed] [Google Scholar]
- Gladden M. H., Kidd G. L. A technique for mapping intramuscular innervation. J Appl Physiol. 1969 Apr;26(4):507–509. doi: 10.1152/jappl.1969.26.4.507. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry I. A., Stöckel K., Thoenen H., Iversen L. L. The retrograde axonal transport of nerve growth factor. Brain Res. 1974 Mar 15;68(1):103–121. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- Hilton S. M., Jeffries M. G., Vrbová G. Functional specializations of the vascular bed of soleus. J Physiol. 1970 Mar;206(3):543–562. doi: 10.1113/jphysiol.1970.sp009030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman G. D., Naftalin R. J. Galactose transport across the serosal border of rabbit ileum and its role in intracellular accumulation. Biochim Biophys Acta. 1975 Mar 13;382(2):230–245. doi: 10.1016/0005-2736(75)90181-9. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A. S., Coyle J. T., Snyder S. H. Catecholamine uptake by synaptosomes from rat brain. Structure-activity relationships of drugs with differential effects on dopamine and norepinephrine neurons. Mol Pharmacol. 1971 Jan;7(1):66–80. [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Kinzie J. L., Ferrendelli J. A., Alpers D. H. Adenosine cyclic 3':5'-monophosphate-mediated transport of neutral and dibasic amino acids in jejunal mucosa. J Biol Chem. 1973 Oct 25;248(20):7018–7024. [PubMed] [Google Scholar]
- Mekata F. Current spread in the smooth muscle of the rabbit aorta. J Physiol. 1974 Oct;242(1):143–155. doi: 10.1113/jphysiol.1974.sp010698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Holman G. D. The role of Na as a determinant of the asymmetric permeability of rabbit ileal brush-border to D-galactose. Biochim Biophys Acta. 1974 Dec 24;373(3):453–470. doi: 10.1016/0005-2736(74)90025-x. [DOI] [PubMed] [Google Scholar]
- Naftalin R., Curran P. F. Galactose transport in rabbit ileum. J Membr Biol. 1974;16(3):257–278. doi: 10.1007/BF01872418. [DOI] [PubMed] [Google Scholar]
- Nellans H. N., Frizzell R. A., Schultz S. G. Coupled sodium-chloride influx across the brush border of rabbit ileum. Am J Physiol. 1973 Aug;225(2):467–475. doi: 10.1152/ajplegacy.1973.225.2.467. [DOI] [PubMed] [Google Scholar]
- Nijima A. Afferent discharges from arterial mechanoreceptors in the kidney of the rabbit. J Physiol. 1971 Dec;219(2):477–485. doi: 10.1113/jphysiol.1971.sp009673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. A., Parsons M. E. Proceedings: Histamine receptors in the cardiovascular system of the cat. Br J Pharmacol. 1974 May;51(1):123P–124P. [PMC free article] [PubMed] [Google Scholar]
- Parker J. C. Metabolism of external adenine nucleotides by human red blood cells. Am J Physiol. 1970 Jun;218(6):1568–1574. doi: 10.1152/ajplegacy.1970.218.6.1568. [DOI] [PubMed] [Google Scholar]
- Paul D. H., Roberts B. L. Responses of neurones in the cerebellar corpus of the dogfish (Scyliorhinus canicula). J Physiol. 1975 Jan;244(1):47P–49P. [PubMed] [Google Scholar]
- RAMIREZ V. D., SAWYER C. H. ADVANCEMENT OF PUBERTY IN THE FEMALE RAT BY ESTROGEN. Endocrinology. 1965 Jun;76:1158–1168. doi: 10.1210/endo-76-6-1158. [DOI] [PubMed] [Google Scholar]
- Reinking R. M., Stuart D. G. Servo-regulated electromagnetic system for muscle stretch and vibration. Am J Phys Med. 1974 Feb;53(1):1–28. [PubMed] [Google Scholar]
- Richardson D., Stella A., Leonetti G., Bartorelli A., Zanchetti A. Mechanisms of renal release of renin by electrical stimulation of the brainstem in the cat. Circ Res. 1974 Apr;34(4):425–434. doi: 10.1161/01.res.34.4.425. [DOI] [PubMed] [Google Scholar]
- Ruf K. B., Younglai E. V., Holmes M. J. Induction of precocious sexual maturation in female rats by electrochemical stimulation of the brain. Brain Res. 1974 Oct 4;78(3):437–446. doi: 10.1016/0006-8993(74)90926-3. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Albuquerque E. X. Differences in the tubocurarine antagonism of the activation of muscle spindle afferents by siccinylcholine, acetylcholine and nicotine. J Pharmacol Exp Ther. 1967 Jun;156(3):573–584. [PubMed] [Google Scholar]
- Stöckel K., Paravicini U., Thoenen H. Specificity of the retrograde axonal transport of nerve growth factor. Brain Res. 1974 Aug 23;76(3):413–421. doi: 10.1016/0006-8993(74)90818-x. [DOI] [PubMed] [Google Scholar]
- TUTTLE R. S., WITT P. N., FARAH A. The influence of ouabain on intracellular sodium and potassium concentrations in the rabbit myocardium. J Pharmacol Exp Ther. 1961 Sep;133:281–287. [PubMed] [Google Scholar]
- Tsien R. W. Effects of epinephrine on the pacemaker potassium current of cardiac Purkinje fibers. J Gen Physiol. 1974 Sep;64(3):293–319. doi: 10.1085/jgp.64.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Voigtlander P. F., Moore K. E. Turning behavior of mice with unilateral 6-hydroxydopamine lesions in the striatum: effects of apomorphine, L-DOPA, amanthadine, amphetamine and other psychomotor stimulants. Neuropharmacology. 1973 May;12(5):451–462. doi: 10.1016/0028-3908(73)90061-0. [DOI] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibitory of glycine on spinal neurons in the cat. J Neurophysiol. 1968 Jan;31(1):81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]
- el-Awqati Q., Cameron J. L., Greenough W. B., 3rd Electrolyte transport in human ileum: effect of purified cholera exotoxin. Am J Physiol. 1973 Apr;224(4):818–823. doi: 10.1152/ajplegacy.1973.224.4.818. [DOI] [PubMed] [Google Scholar]
- von Baeyer H., von Conta C., Haeberle D., Deetjen P. Determination of transport constants for glucose in proximal tubules of the rat kidney. Pflugers Arch. 1973 Nov 8;343(4):273–286. doi: 10.1007/BF00595815. [DOI] [PubMed] [Google Scholar]
- von D5auring M., Andres K. H., Iravani J. The fine structure of the pulmonary stretch receptor in the rat. Kidney Int. 1974 May;5(5):215–222. doi: 10.1007/BF00525771. [DOI] [PubMed] [Google Scholar]






