Abstract
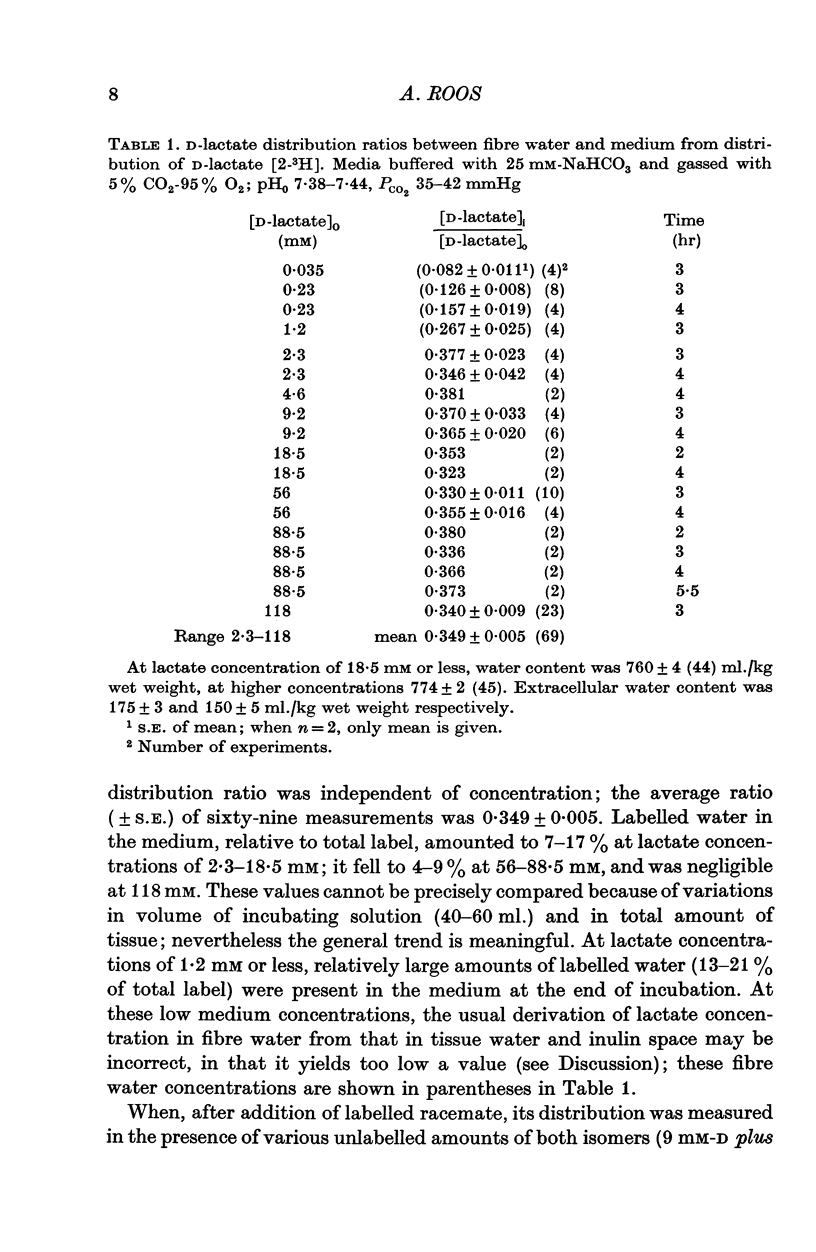
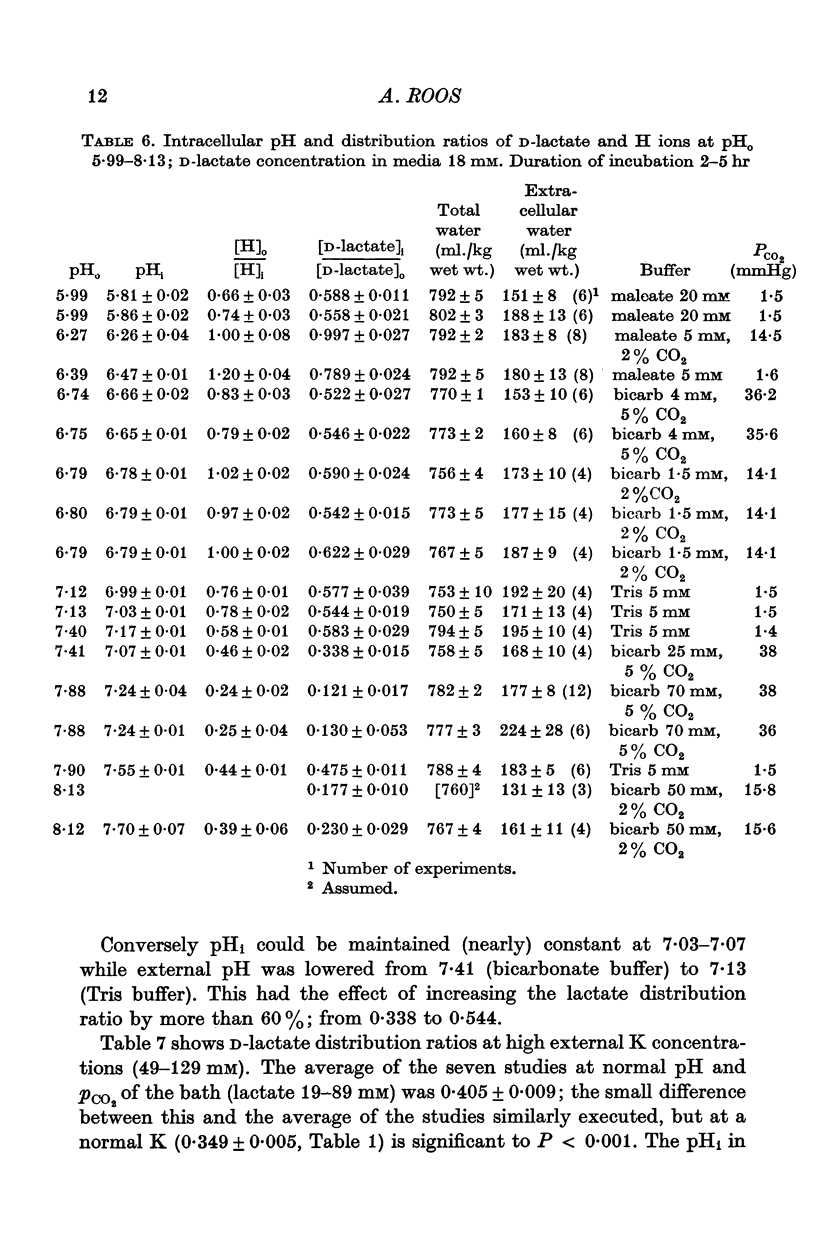
1. The steady-state distribution ratios of D- and L-lactate between fibre water and external fluid were measured in 'intact' rat hemidiaphragm preparations exposed for 2-5 hr to a variety of solutions of normal ionic strength and osmolarity. The studies were designed to minimize the effects, on these distributions, of conversion of lactate and of generation of lactic acid by the muscle. 2. At D-lactate concentrations between 2.3 and 118 mM, at normal pH and PCO2, the D-lactate distribution ratio, obtained from the distribution of [2-(3)H]D-lactate was independent of concentration; it averaged 0.349. As the concentration of D-lactate was reduced below 2.3 mM, its distribution ratio progressively fell to less than 0.1. 3. Radiochromatograms of extracts of incubated muscle showed that the tritium label was not attached to substances other than lactate. 4. At L-lactate concentrations of 59 and 108 mM, at normal pH and PCO2, the average L-lactate distribution ratios, obtained by enzymatic analysis, were respectively 0.395 and 0392. 5. At 19-89 mM D-lactate, depolarizing the muscle fibres by high K(49-127 mM), at normal pH, PCO2, and [K]0[Cl]0 product, only slightly affected the D-lactate distribution ratio which averaged 0.405. 6. The D-lactate distribution ratio and intracellular pH (pHi), obtained with the DMO method (5,5-dimethyl-2,4-oxazolidinedione), were measured in thirty sets of studies after exposure of the muscle to solutions buffered to pH values ranging between 5.99 and 8.13, and containing 18.5-118 mM D-lactate and 6-129 mM-K. 7. The relation between the distribution ratios of D-lactate ([TL]i/[TL]O) and of H ions ([Ho/[H]i) in these studies could be expressed by [TL]i/[T]O = 0.646 [H]o/[H]i+0.056. 8. It was concluded that it is predominantly the undissociated lactic acid molecules, rather than the much more numerous lactate ions, which permeate the fibre membrane; and that the steady-state lactate distribution ratio is determined by the transmembrane pH gradient, and not by membrane potential. 9. The expression of the steady-state lactate distribution ratio as function of relative membrane permeabilities of lactic acid molecule and lactate ion, membrane voltage, and internal and external H ion concentrations indicates that a finite permeability to the ion, three or four orders of magnitude less than that to the molecule, is compatible with the experimental data. When both ion and molecule of any weak acid are permeable, they act as a carrier system for the movement of protons down their electrochemical gradient. 10. Near-maintenance of pHi in the face of high fibre D-lactate (19-44 mM) and DMO (8-42 mM) indicates stimulation of proton extrusion by acid loans. 11. This extrusion is insensitive to ouabain, as judged from the lack of effect of the drug of pHi with acid loading.
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- BRINK F. The role of calcium ions in neural processes. Pharmacol Rev. 1954 Sep;6(3):243–298. [PubMed] [Google Scholar]
- Bergström J., Guarnieri G., Hultman E. Carbohydrate metabolism and electrolyte changes in human muscle tissue during heavy work. J Appl Physiol. 1971 Jan;30(1):122–125. doi: 10.1152/jappl.1971.30.1.122. [DOI] [PubMed] [Google Scholar]
- Butler T. C., Kuroiwa Y., Waddell W. J., Poole D. T. Effects of 5,5-dimethyl-2,4-oxazolidinedione (DMO) on acid-base and electrolyte equilibria. J Pharmacol Exp Ther. 1966 Apr;152(1):62–66. [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J. Nature and significance of concentration relations of potassium and sodium ions in skeletal muscle. Physiol Rev. 1957 Jan;37(1):84–132. doi: 10.1152/physrev.1957.37.1.84. [DOI] [PubMed] [Google Scholar]
- CREESE R. Measurement of cation fluxes in rat diaphragm. Proc R Soc Lond B Biol Sci. 1954 Sep 27;142(909):497–513. doi: 10.1098/rspb.1954.0039. [DOI] [PubMed] [Google Scholar]
- CREESE R., SCHOLES N. W., WHALEN W. J. Resting potentials of diaphragm muscle after prolonged anoxia. J Physiol. 1958 Feb 17;140(2):301–317. doi: 10.1113/jphysiol.1958.sp005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack R. Assay, purification and properties of mammalian D-2-hydroxy acid dehydrogenase. Biochem J. 1969 Oct;115(1):55–64. doi: 10.1042/bj1150055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R. Sodium fluxes in diaphragm muscle and the effects of insulin and serum proteins. J Physiol. 1968 Jul;197(2):255–278. doi: 10.1113/jphysiol.1968.sp008558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Carter N. W. Active transport of 5,5-dimethyl-2,4-oxazolidinedione. Science. 1965 Dec 3;150(3701):1294–1296. doi: 10.1126/science.150.3701.1294. [DOI] [PubMed] [Google Scholar]
- Eggleton M. G., Evans C. L. The lactic acid content of the blood after muscular contraction under experimental conditions. J Physiol. 1930 Oct 31;70(3):269–293. doi: 10.1113/jphysiol.1930.sp002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher W. M. Lactic acid in amphibian muscle. J Physiol. 1907 Mar 27;35(4):247–309. doi: 10.1113/jphysiol.1907.sp001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa K., Kerridge P. M. The hydrogen ion concentration of the muscles of the cat. J Physiol. 1927 Jun 7;63(1):33–41. doi: 10.1113/jphysiol.1927.sp002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler N., Piiper J. Determination of intracellular buffering properties in rat diaphragm muscle. Am J Physiol. 1972 Mar;222(3):747–753. doi: 10.1152/ajplegacy.1972.222.3.747. [DOI] [PubMed] [Google Scholar]
- Heisler N., Piiper J. The buffer value of rat diaphragm muscle tissue determined by P CO2 equilibration of homogenates. Respir Physiol. 1971 Jun;12(2):169–178. doi: 10.1016/0034-5687(71)90050-8. [DOI] [PubMed] [Google Scholar]
- Izutsu K. T. Intracellular pH, H ion flux and H ion permeability coefficient in bullfrog toe muscle. J Physiol. 1972 Feb;221(1):15–27. doi: 10.1113/jphysiol.1972.sp009735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. III. The effect of insulin on pentose uptake by the diaphragm. J Biol Chem. 1957 Feb;224(2):681–693. [PubMed] [Google Scholar]
- MULLINS L. J., NODA K. THE INFLUENCE OF SODIUM-FREE SOLUTIONS ON THE MEMBRANE POTENTIAL OF FROG MUSCLE FIBERS. J Gen Physiol. 1963 Sep;47:117–132. doi: 10.1085/jgp.47.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Some mitochondrial changes in denervated muscle. J Cell Sci. 1968 Mar;3(1):49–54. doi: 10.1242/jcs.3.1.49. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Paillard M. Direct intracellular pH measurement in rat and crab muscle. J Physiol. 1972 Jun;223(2):297–319. doi: 10.1113/jphysiol.1972.sp009848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and buffering power of rat muscle. Am J Physiol. 1971 Jul;221(1):182–188. doi: 10.1152/ajplegacy.1971.221.1.182. [DOI] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and intracellular buffering power of the cat brain. Am J Physiol. 1965 Dec;209(6):1233–1246. doi: 10.1152/ajplegacy.1965.209.6.1233. [DOI] [PubMed] [Google Scholar]
- TUBBS P. K., GREVILLE G. D. The oxidation of D-alpha-hydroxy acids in animal tissues. Biochem J. 1961 Oct;81:104–114. doi: 10.1042/bj0810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLEE C. A., WHITE V. K., HASTINGS A. B. Metabolism of C14 labeled glucose and pyruvate by rat diaphragm muscle in vitro. J Biol Chem. 1952 Mar;195(1):287–297. [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury J. W., Miles P. R. Anion conductance of frog muscle membranes: one channel, two kinds of pH dependence. J Gen Physiol. 1973 Sep;62(3):324–353. doi: 10.1085/jgp.62.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]


