Abstract
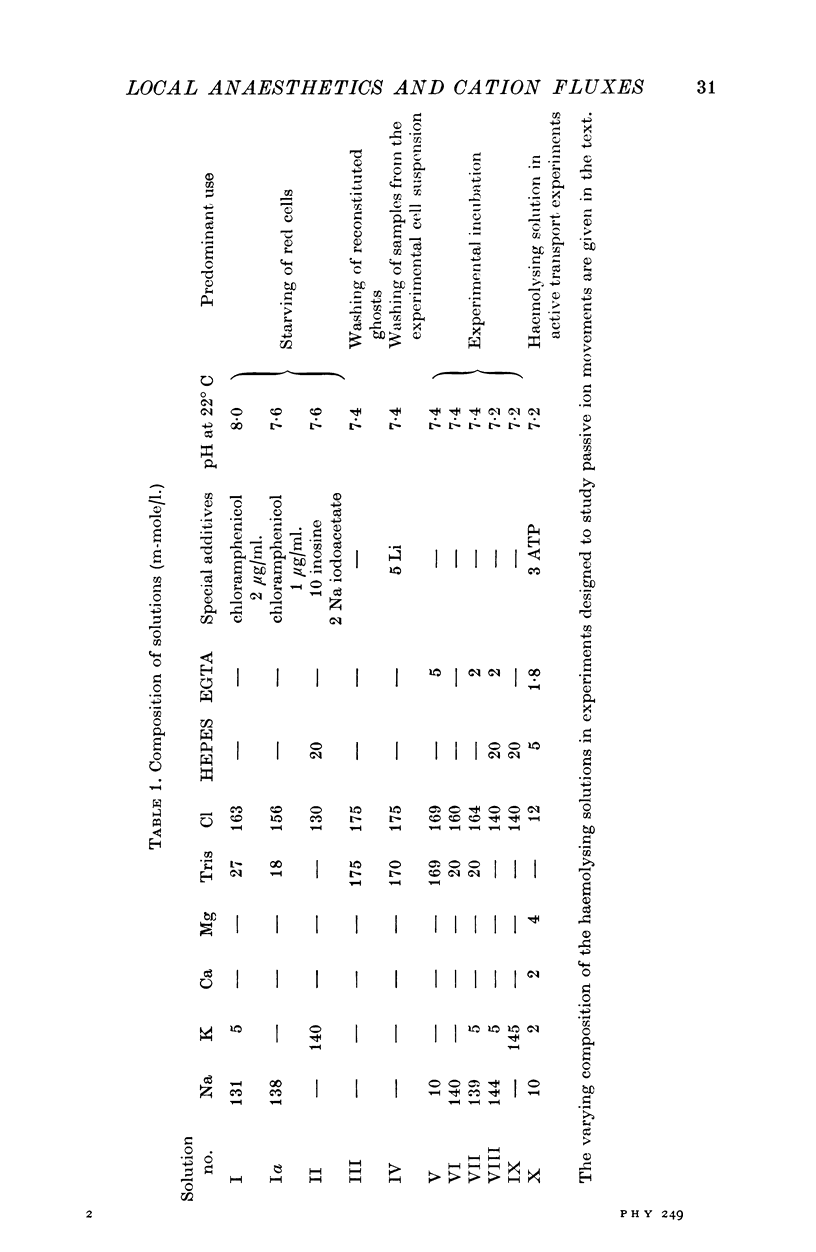
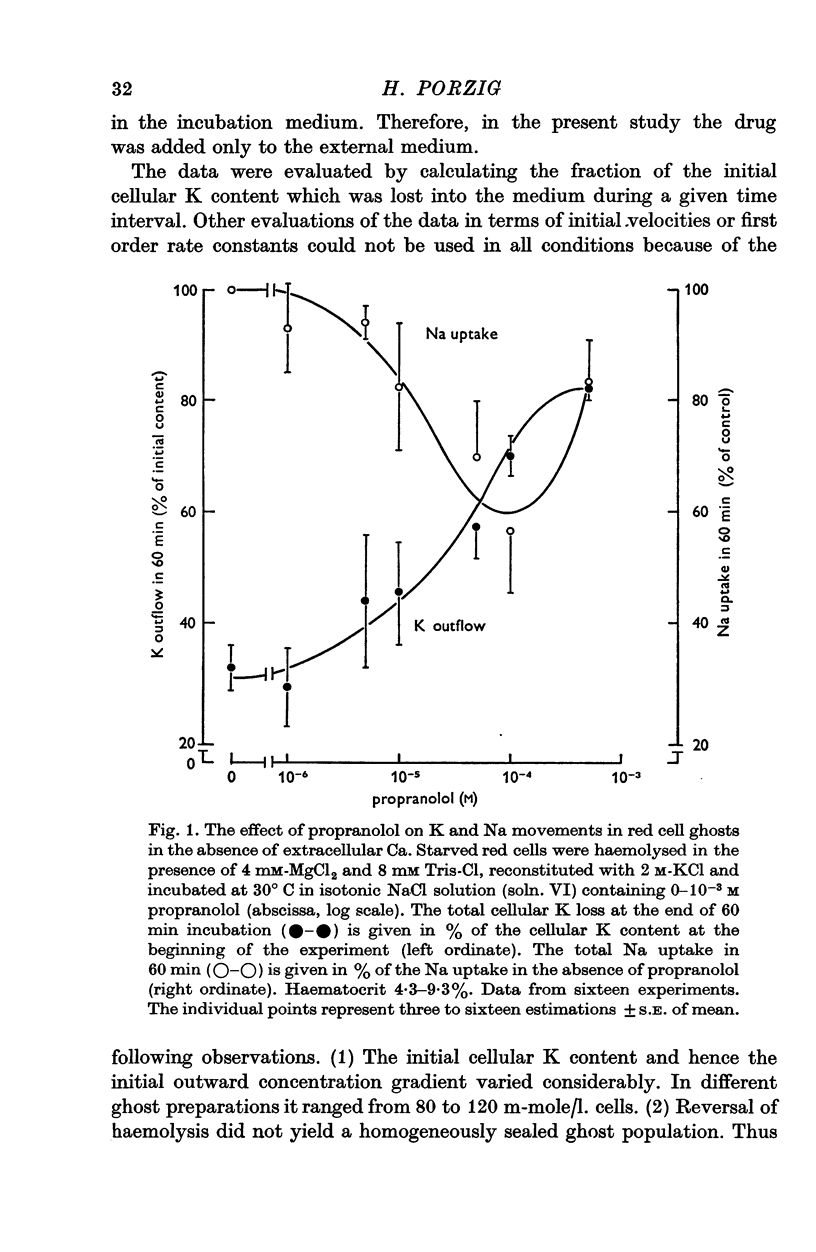
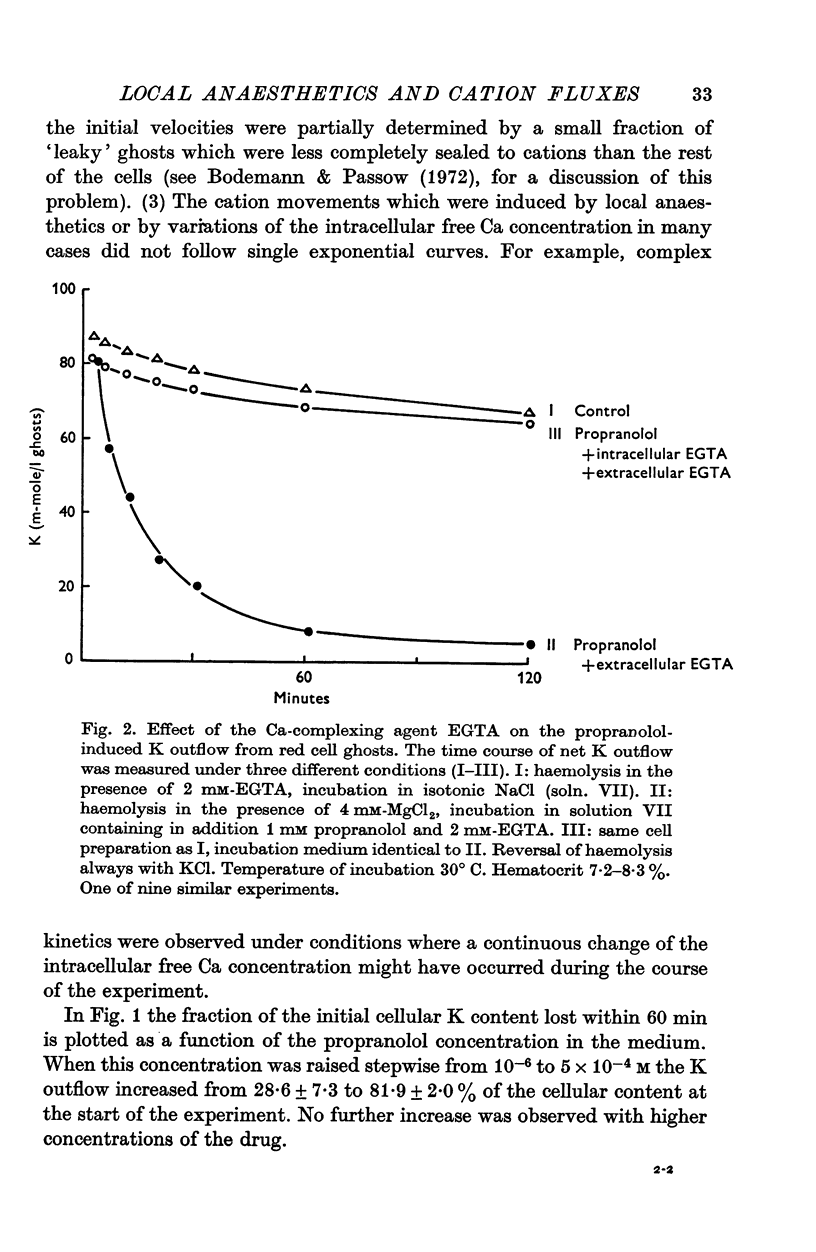
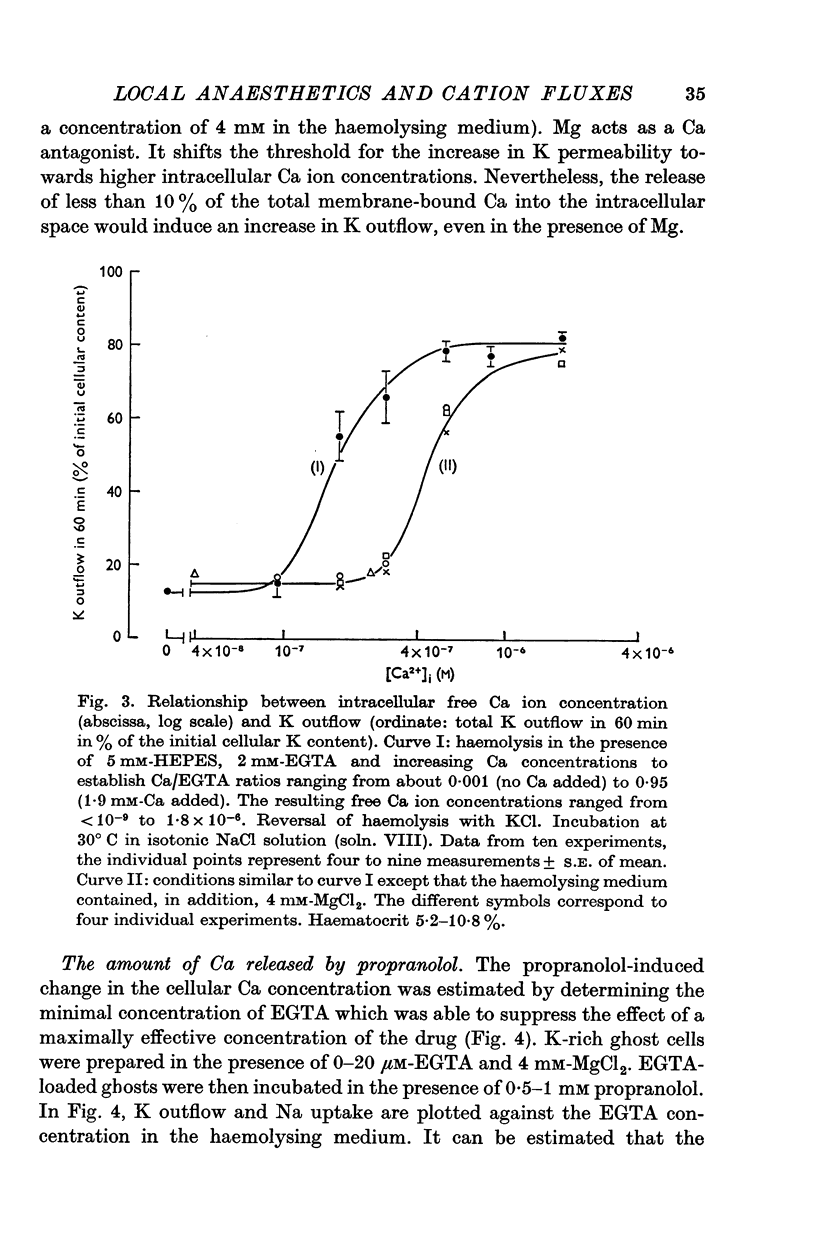
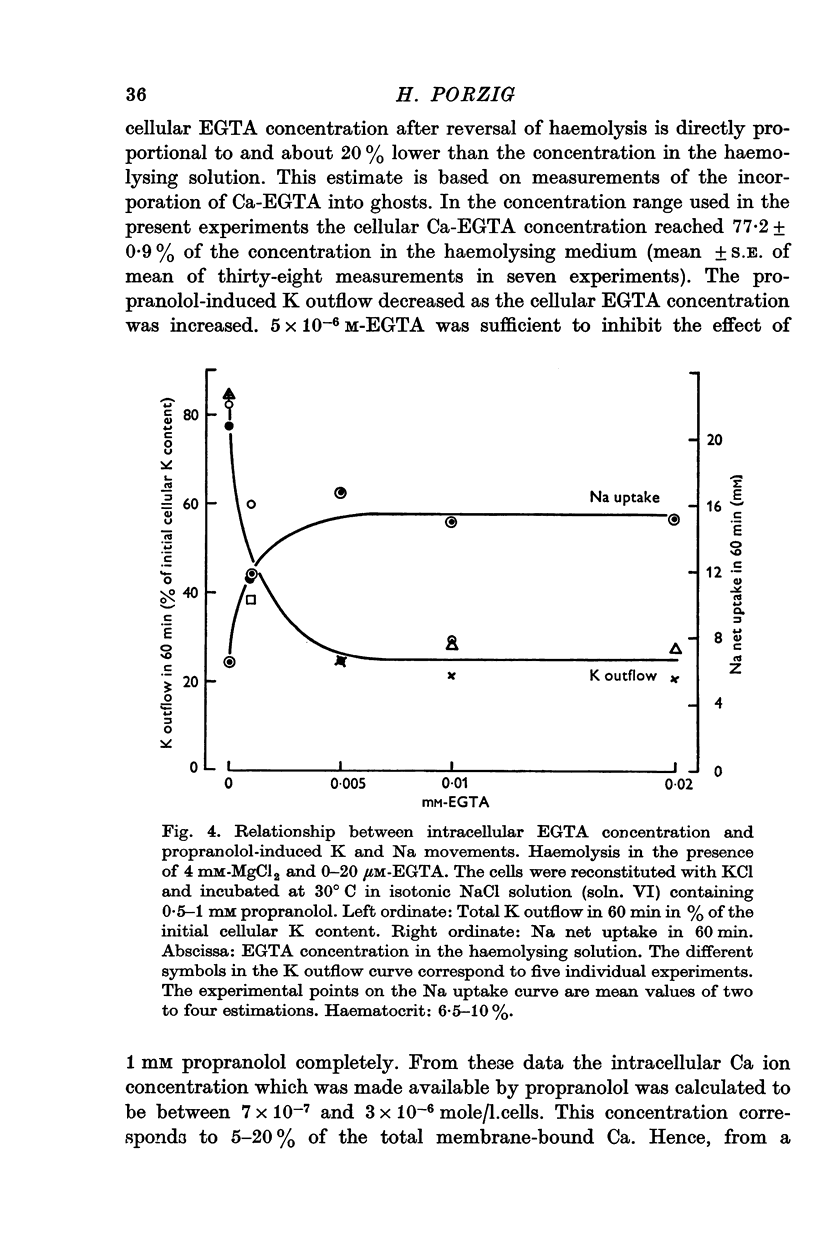
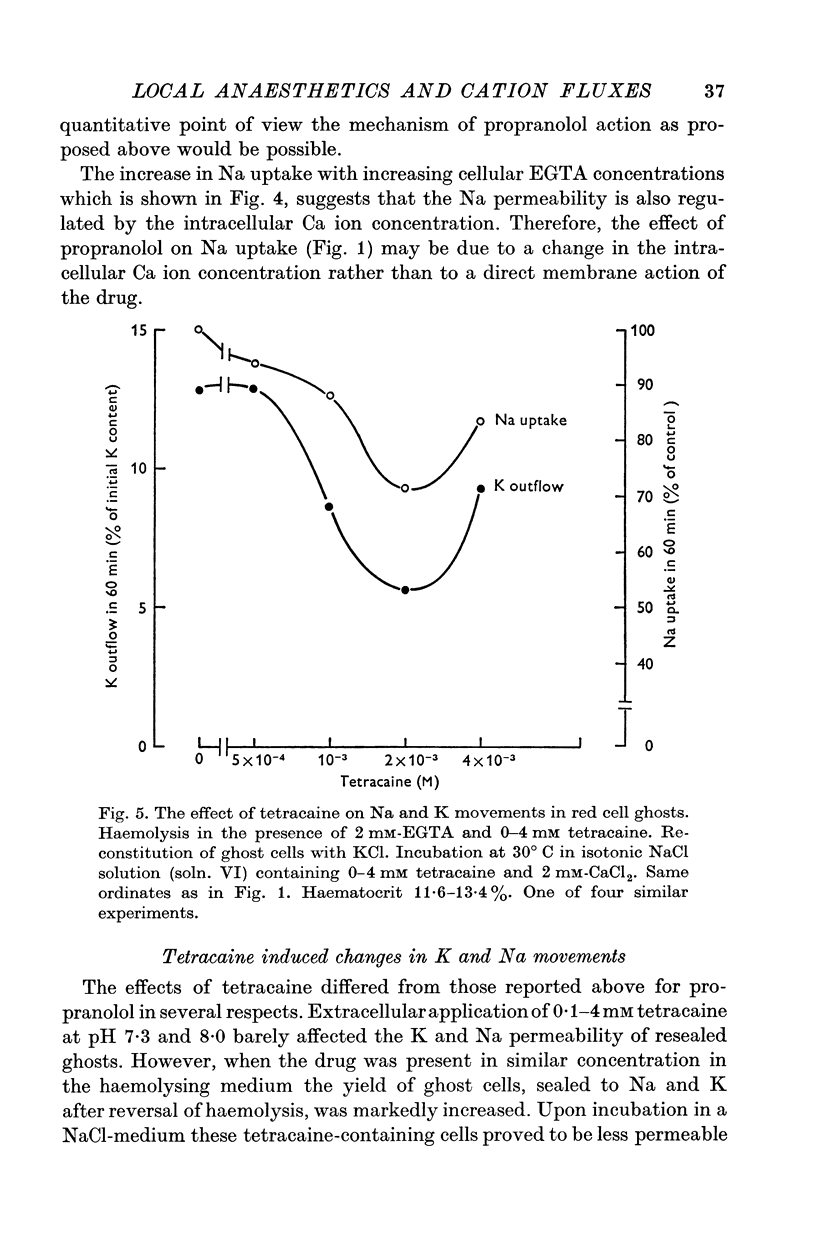
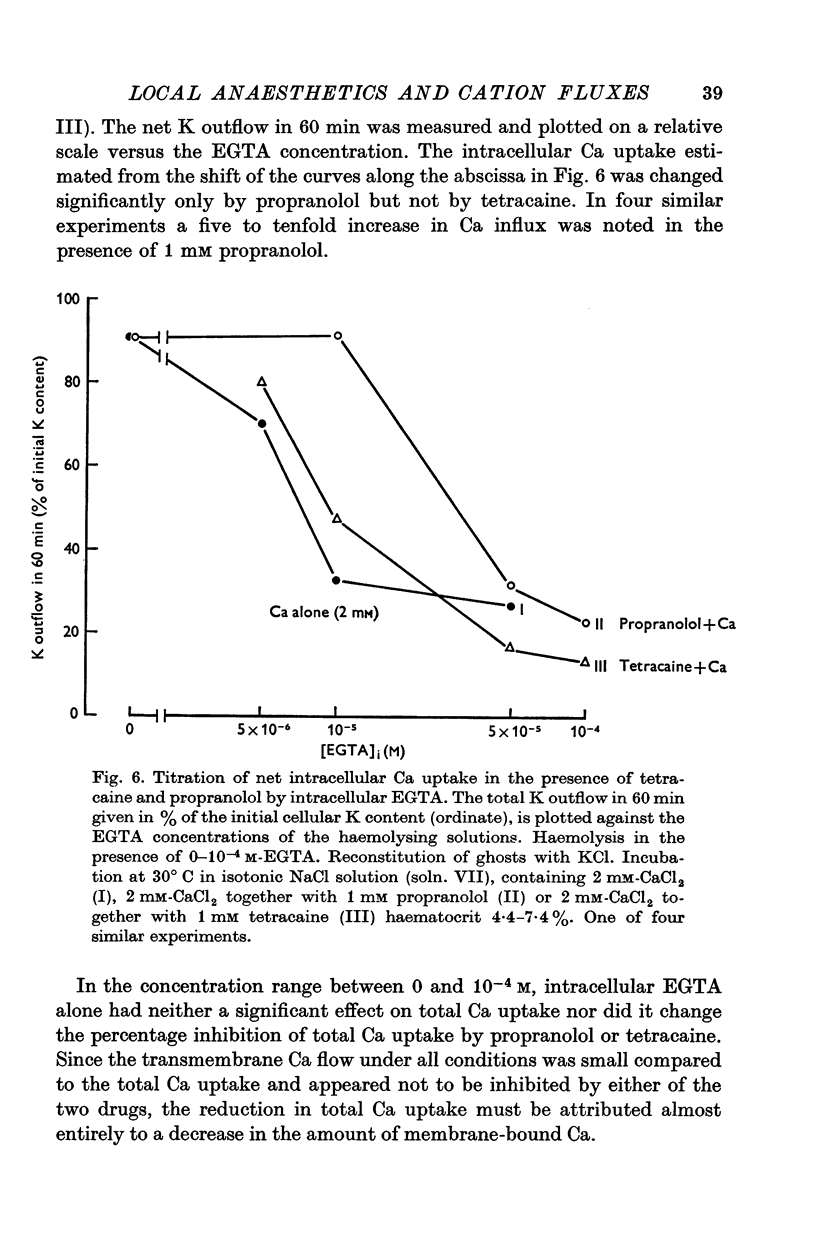
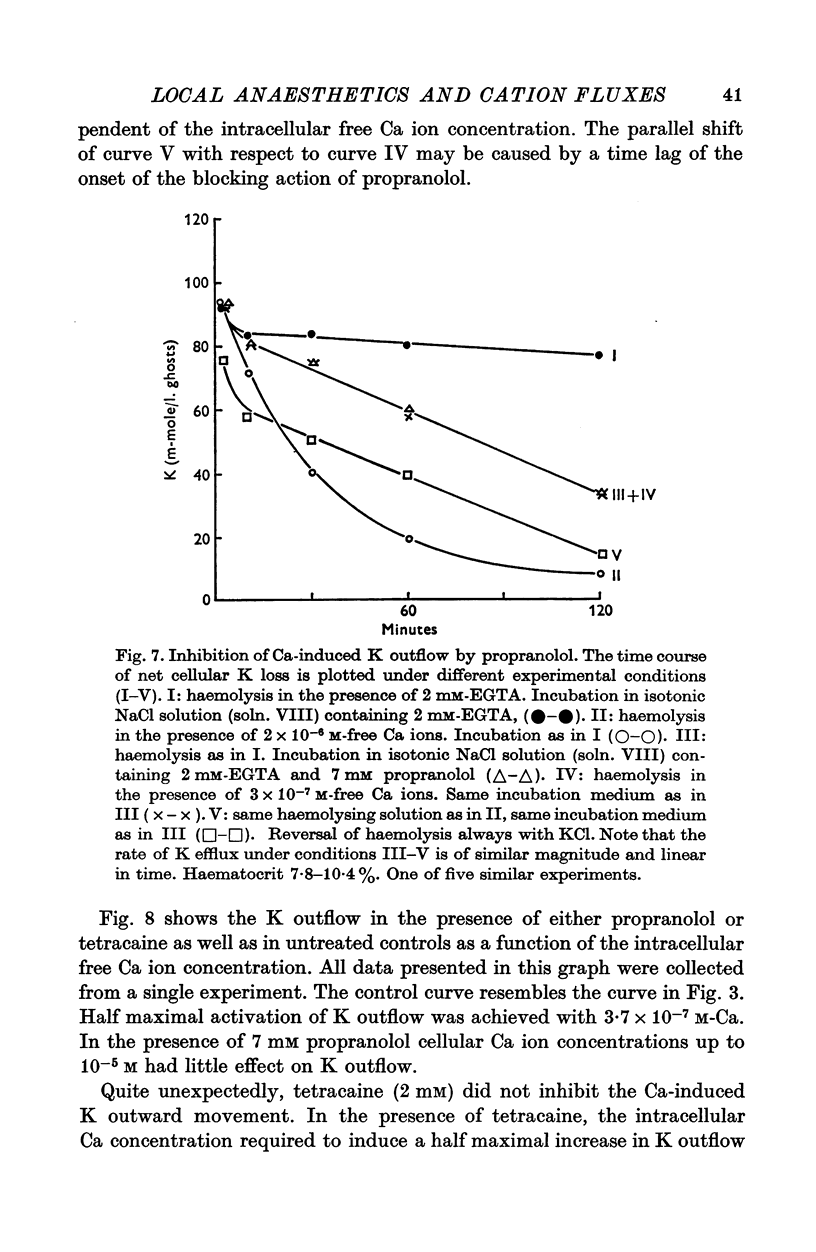
1. The effects of two positively charged local anaesthetic amines, tetracaine and propranolol, on cation permeability were studied in resealed human red cell ghosts prepared from metabolically depleted erythrocytes. 2. The K permeability was reduced by tetracaine but increased by propranolol. The effect of tetracaine was independent of extracellular Ca concentration but was raised to 2-5 x 10(-7) M. The effect of propranolol, which was enhanced when the external Ca concentration was raised, could be completely inhibited by lowering the internal free Ca to less than 10(-7) M. 3. Propranolol, but not tetracaine, increased the intracellular Ca ion concentration by releasing up to 20% of the membrane-bound Ca to the cell interior. This increase in intracellular Ca was sufficient to mediate the observed change in K permeability. 4. Tetracaine and propranolol reduced the Ca binding capacity of the ghost membrane by about 20 and 40% respectively. The Ca permeability was increased by propranolol and was slightly reduced by tetracaine. 5. In high concentrations (2-7 mM) propranolol by itself moderately increased K and Na permeability, but supressed completely the Ca-induced increase in K permeability. Tetracaine in concentrations up to 4 mM enhanced the Ca-induced increase in K permeability. Higher concentrations of the drug caused lysis of the cells. 6. Maximally effective concentrations of tetracaine and propranolol inhibited the ATP-dependent Ca outward transport by 30 and 70% respectively. 7. The effects of tetracaine on K permeability were shared by the local anaesthetics prilocaine and lidocaine, those of propranolol were shared by practolol, a beta-adrenergic antagonist and tetraethylammonium, a ganglionic blocking agent. 8. It is suggested that the differences in the effects of tetracaine and propranolol on cation permeability reflect qualitatively different interactions of the two drugs with Ca binding sites on the inner surface of the membrane.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen N. B. The effect of local anesthetic and pH on sodium and potassium flux in human red cells. J Pharmacol Exp Ther. 1968 Oct;163(2):393–406. [PubMed] [Google Scholar]
- BOLINGBROKE V., MAIZELS M. Calcium ions and the permeability of human erythrocytes. J Physiol. 1959 Dec;149:563–585. doi: 10.1113/jphysiol.1959.sp006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzer H. The effect of quinidine and drugs with quinidine-like action (propranolol, verapamil and tetracaine) on the calcium transport system in isolated sarcoplasmic reticulum vesicles of rabbit skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(3):256–272. doi: 10.1007/BF00501935. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. Competitive action of calcium and procaine on lobster axon. A study of the mechanism of action of certain local anesthetics. J Gen Physiol. 1966 May;49(5):1043–1063. doi: 10.1085/jgp.49.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemann H., Passow H. Factors controlling the resealing of the membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol. 1972;8(1):1–26. doi: 10.1007/BF01868092. [DOI] [PubMed] [Google Scholar]
- Davis L. D., Temte J. V. Effects of propranolol on the transmembrane potentials of ventricular muscle and Purkinje fibers of the dog. Circ Res. 1968 May;22(5):661–677. doi: 10.1161/01.res.22.5.661. [DOI] [PubMed] [Google Scholar]
- Ekman A., Manninen V., Salminen S. Ion movements in red cells treated with propranolol. Acta Physiol Scand. 1969 Mar;75(3):333–344. doi: 10.1111/j.1748-1716.1969.tb04386.x. [DOI] [PubMed] [Google Scholar]
- FEINSTEIN M. B. INHIBITION OF CAFFEINE RIGOR AND RADIOCALCIUM MOVEMENTS BY LOCAL ANESTHETICS IN FROG SARTORIUS MUSCLE. J Gen Physiol. 1963 Sep;47:151–172. doi: 10.1085/jgp.47.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEINSTEIN M. B. REACTION OF LOCAL ANESTHETICS WITH PHOSPHOLIPIDS. A POSSIBLE CHEMICAL BASIS FOR ANESTHESIA. J Gen Physiol. 1964 Nov;48:357–374. doi: 10.1085/jgp.48.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein M. B. Inhibition of contraction and calcium exchange-ability in rat uterus by local anesthetics. J Pharmacol Exp Ther. 1966 Jun;152(3):516–524. [PubMed] [Google Scholar]
- Feinstein M. B., Paimre M. Pharmacological action of local anesthetics on excitation-contraction coupling in striated and smooth muscle. Fed Proc. 1969 Sep-Oct;28(5):1643–1648. [PubMed] [Google Scholar]
- Giotti A., Ledda F., Mannaioni P. F. Effects of noradrenaline and isoprenaline, in combination with - and -receptor blocking substances, on the action potential of cardiac Purkinje fibres. J Physiol. 1973 Feb;229(1):99–113. doi: 10.1113/jphysiol.1973.sp010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Warner A. E. Nature of the calcium dependent potassium leak induced by (+)-propranolol, and its possible relevance to the drug's antiarrhythmic effect. Br J Pharmacol. 1972 Feb;44(2):271–278. doi: 10.1111/j.1476-5381.1972.tb07263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. Cation transport and structure of the red-cell plasma membrane. Circulation. 1962 Nov;26:1202–1213. doi: 10.1161/01.cir.26.5.1201. [DOI] [PubMed] [Google Scholar]
- Harrison D. G., Long C. The calcium content of human erythrocytes. J Physiol. 1968 Dec;199(2):367–381. doi: 10.1113/jphysiol.1968.sp008658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L. A., Wittig J., Wallace A. G. Adrenergic influences on the distal Purkinje system of the canine heart. Circ Res. 1973 Mar;32(3):329–339. doi: 10.1161/01.res.32.3.329. [DOI] [PubMed] [Google Scholar]
- Hellenbrecht D., Lemmer B., Wiethold G., Grobecker H. Measurement of hydrophobicity, surface activity, local anaesthesia, and myocardial conduction velocity as quantitative parameters of the non-specific membrane affinity of nine -adrenergic blocking agents. Naunyn Schmiedebergs Arch Pharmacol. 1973;277(2):211–226. doi: 10.1007/BF00501161. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Divalent cation content of normal and ATP-depleted erythrocytes and erythrocyte membranes. Nouv Rev Fr Hematol. 1972 Nov-Dec;12(6):799–813. [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Frazier T., Yamada M. The site of action and active form of local anesthetics. I. Theory and pH experiments with tertiary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):32–44. [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Pang D. C., Briggs F. N. Mechanism of propranolol inhibition of the cardiac sarcotubule-gamma-AT32P reaction. Biochem Pharmacol. 1973 Jun 1;22(11):1301–1308. doi: 10.1016/0006-2952(73)90304-3. [DOI] [PubMed] [Google Scholar]
- Porzig H. ATP-independent calcium net movements in human red cell ghosts. J Membr Biol. 1972;8(3):237–258. doi: 10.1007/BF01868105. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Greengard P. On the mode of action of local anesthetics. Annu Rev Pharmacol. 1966;6:405–430. doi: 10.1146/annurev.pa.06.040166.002201. [DOI] [PubMed] [Google Scholar]
- Romero P. J., Whittam R. The control by internal calcium of membrane permeability to sodium and potassium. J Physiol. 1971 May;214(3):481–507. doi: 10.1113/jphysiol.1971.sp009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales B., McIntosh D. A. Effects of propranolol and its optical isomers on the radiocalcium uptake and the adenosine triphosphatases of skeletal and cardiac sarcoplasmic reticulum fractions (SRF). J Pharmacol Exp Ther. 1968 Apr;160(2):261–267. [PubMed] [Google Scholar]
- Schatzmann H. J. ATP-dependent Ca++-extrusion from human red cells. Experientia. 1966 Jun 15;22(6):364–365. doi: 10.1007/BF01901136. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Temple D. M., Hasselbach W., Makinose M. The inhibition by beta-adrenoceptor blocking agents of calcium uptake into and efflux from isolated sarcoplasmic vesicles. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(2):187–194. doi: 10.1007/BF00499033. [DOI] [PubMed] [Google Scholar]


